Antimalaria and Anti-Inflammatory Activites New Chloroquine and Primaquine Hybrids Targeting the Blockade of Malaria Parasite Transmission
Received: 01-Sep-2023 / Manuscript No. wjpt-23-112292 / Editor assigned: 04-Sep-2023 / PreQC No. wjpt-23-112292(PQ) / Reviewed: 18-Sep-2023 / QC No. wjpt-23-112292 / Revised: 22-Sep-2023 / Manuscript No. wjpt-23-112292(R) / Accepted Date: 29-Sep-2023 / Published Date: 29-Sep-2023 DOI: 10.4172/wjpt.1000208
Abstract
Malaria is a disease that necessitates the development of new treatments not only to combat Plasmodium but also to alleviate infection symptoms such as fever and inflammation. Chloroquine (CQ) and Primaquine (PQ) were coupled to the pharmacophoric group found in phenylacetic anti-inflammatory medicines to create a sequence of 21 hybrid molecules. These chemicals were created with a dual purpose in mind: to kill Plasmodium while also acting on the inflammatory process caused by malaria infection. Nine different biological approaches were used to test the substances. In vitro, the carbonylated CQ derivative was more effective than CQ, reduced parasitemia in P. berghei by up to 37% on day 7. PQ derivative 17 was slightly carbonylated. PQ is less powerful. In mosquitoes, the gem-difluoro PQ derivative showed a high level of transmission blocking of the malaria sporogonic cycle. Compounds 6 and 20 lowered No generation and suppressed TNF production in LPS-stimulated J774A.1 macrophages in a dose-dependent manner. Our findings suggest a plausible and intriguing strategy for developing new chemical entities that operate as transmission-blocking medications for treating malaria caused by Plasmodium falciparum and Plasmodium vivax, as well as the anti-inflammatory mechanism associated with the condition. We introduce a new family of hybrid compounds made up of the anti-plasmodial medicines primaquine and chloroquine. To yet, no treatment has been found to be effective against all phases of Plasmodium’s life cycle. We devised and synthesized a new-generation molecule including both primaquine and chloroquine components from accessible precursors, with the goal of developing medicines with bioactivity against different stages of the parasite’s life cycle. The hybrid molecule 3 has activity against asexual and sexual P. falciparum blood stages, as well as P. berghei sporozoites and liver stages, in vitro. The hybrid is active against P. berghei liver and blood stages in vivo. The concept of using one chemical to combine distinct mechanisms of action that attack different Plasmodium stages in the mammalian host was successfully validated by our findings. It is our hope that the pathogen will be outwitted by the new design of such chemicals in the spread of drug resistance. The chemical is accessible in a smooth and adaptable manner according to the streamlined synthesis process, and it is open to additional molecular modification.
Keywords
Chloroquine; Primaquine; Hybrid molecules; Plasmodium; Multistage; Anti-inflammatory
Introduction
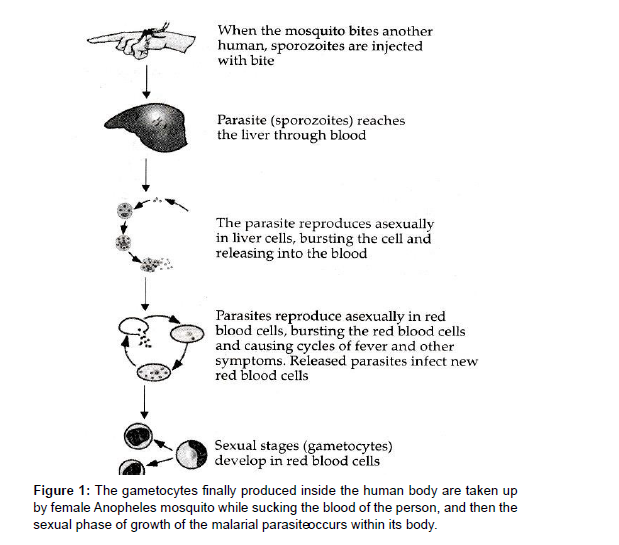
Quinolines are one of the most commonly prescribed malaria medications. Quinine was the first quinoline derivative to be used to treat malaria, followed by synthetic quinoline derivatives including Chloroquine (CQ) and Primaquine (PQ). CQ was the first choice of treatment for malaria since it is one of the safest, most inexpensive, and most effective medications against numerous forms of plasmodia. However, due to abuse, Chloroquine Resistant Strains (CQR) have emerged, rendering it useless in many regions of the world. There is widespread parasite resistance, putting the efficacy of malaria medicine treatments in jeopardy. This resistance is characterized as a delay in parasite elimination after treatment with artemisinin derivatives, which justifies the development of these drugs. Novel antimalarial drug like 8-aminoquinoline PQ and the recently approved Tafenoquine are the only medications approved to treat relapsing P. vivax malaria, and they both act as antimicrobials despite their side effects. The primary purpose of all potential antimalarial medicines is to kill the malaria parasite. The most deadly form of the disease is Cerebral Malaria (CM), which is linked to elevated levels of cytokines and chemokines as mediators of inflammation. P. vivax is the most widely distributed malaria species, accounting for malaria cases in America in 2010, with cases reported in Brazil. Since 1946, Chloroquine has been the welltolerated treatment of choice for acute vivax malaria. The drug relieves fever and parasitemia within 72 hours of the first dose and is rapidly absorbed and slowly eliminated, primarily as the parent drug and as a metabolite of approximately 3: 1 Desethychloroquine [DCQ] in a ratio of about the plasma half-life is about 50 hours and therapeutic levels against vivax malaria persist in the blood until the 21st to 35th day after treatment [1, 2 ]. Due to recurrence of parasitemia via asexual transmission, CQ-resistant P. vivax favor recurrence. After treatment with blood schizontocides, parasites in the blood stage. In an ideal world, determining drug concentrations and their most relevant active metabolites in the blood would confirm in vivo CQ Resistance [CR]. When Australians who immigrated from Papua New Guinea failed to obtain normal treatment, P.vivax CR was discovered. Evidence of the occurrence exists in South America, however there is a scarcity of data. No recurrent parasitemia was detected in certain trials conducted in different locales within 28 days [9 -11] or 30 days after the combined CQ and primaquine PQ therapy failed in P. vivax malaria acquired by Canadian visitors in GUYANA. P. vivax CR has been recorded in three cases in 177 patients in Colombia, resulting in the proper 28-day follow-up of 109 P. vivax patients who were only provided CQ [PQ prescription postponed to 28 days] in 2007. After plasmatic CQ dose to confirm 10.1 percent resistance Although PQ possesses schizonticidal activity against P. vivax, it is commonly utilized as a hypnozoiticidal medication. In vitro evidence of synergy between PQ and CQ against P. falciparum schizonts has been found. For the asexual blood stage of P. vivax, however, there is no indication of synergy between these two medicines. In patients with uncomplicated Vivax malaria, treatment efficacy after 28 days did not differ substantially between the CQ monotherapy group and the group receiving CQ plus PQ for 14 days, according to the available data [3, 4]. These facts, however, solely apply to Asian tribes and cannot be easily generalized to Latin America. The reasoning for extended parasitemia due to treatment resistance explains particularly severe anaemia; nevertheless, no individual individuals have been identified. Because CQ is no longer utilized in most of their locations, P. vivax CR who develop anaemia are well described [5, 6 ]. We measured in vivo CQ resistance in patients with uncomplicated P. vivax from western BRAZIL using CQ [standard dose of 25 mg/kg over the first 3 days estimated first 7 days] and PQ [0.5 mg/kg / Day over the first 3 days estimated first 7 days], as well as the dynamics of haemoglobin levels over the follow-up period in both resistant and sensitive groups. Malaria is a leading source of morbidity and mortality, with antimicrobials in use [ 7 ] and novel antimicrobials being discovered all over the world. The malarial parasite, Plasmodium, requires two hosts to complete its life cycle. In humans, the asexual phase of growth occurs, which can be represented as follows (Figure 1, 2, 3)
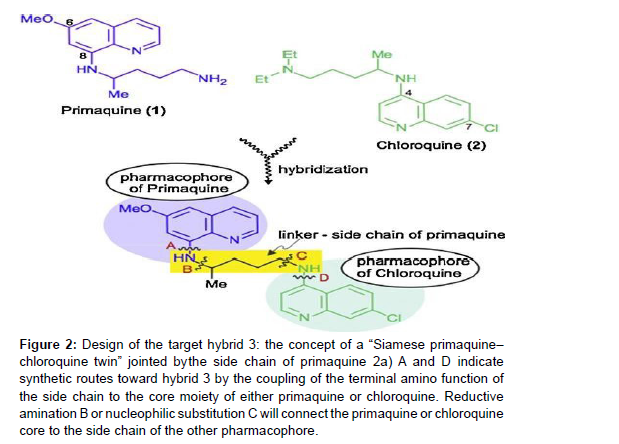
Figure 2:Design of the target hybrid 3: the concept of a “Siamese primaquine–
chloroquine twin” jointed by the side chain of primaquine 2a) A and D indicate
synthetic routes toward hybrid 3 by the coupling of the terminal amino function of
the side chain to the core moiety of either primaquine or chloroquine. Reductive
amination B or nucleophilic substitution C will connect the primaquine or chloroquine
core to the side chain of the other pharmacophore.
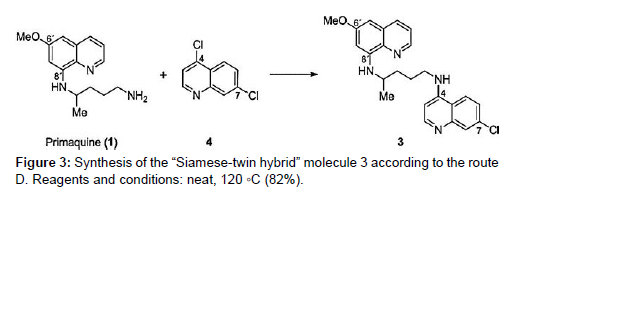
It is a well-tolerated 4-aminoquinoline antimalarial drug that is active against blood schizonts and has been used as a standard therapy for decades. Because of widespread resistance, the effectiveness of Chloroquine has been gradually declining since the early 1990s. At the same time, the official malaria treatment policy was altered to a two-drug combination therapy. There is some evidence, however, that the removal of Chloroquine from the market led in a fall of Chloroquine resistant Plasmodium species due to their reduced fitness relative to wild-type parasites. These findings point to the possibility of reintroducing Chloroquine (2) into malarial combination medication therapy [8 ].
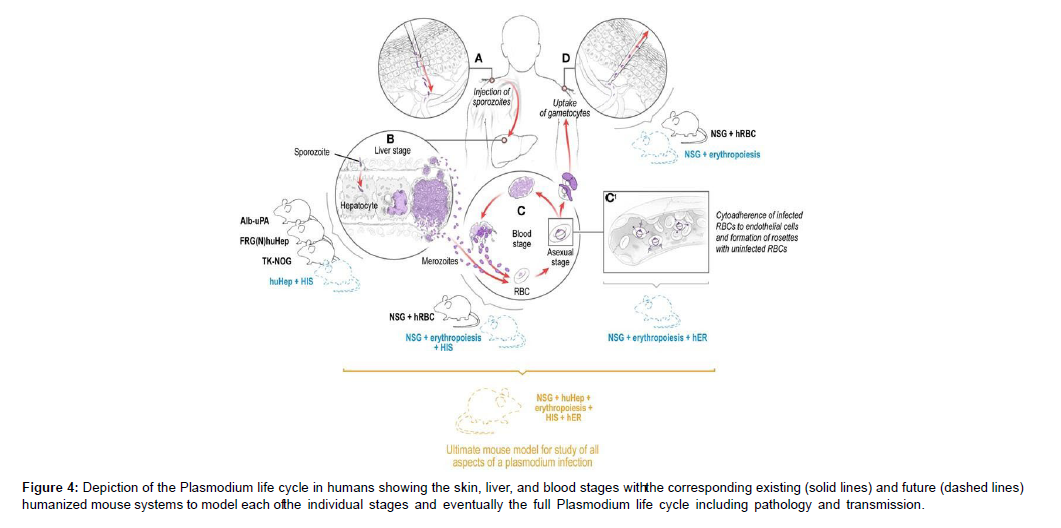
Due to their potential side effects, two additional antimalarial medicines – 8-aminoquinolines pamaquine and primaquine (1) – have been overlooked in recent years. Methemoglobinemia and haemolytic anaemia in patients with glucose-6-phosphate dehydrogenase deficiency. This is unfortunate because primaquine, an 8- aminoquinoline, is effective against liver-stage schizonts and is the only drug that kills hypnozoites. The conjugate’s increased lipophilicity relative to that of its parent chemicals was predicted to ensure appropriate membrane permeability. Furthermore, the planned hybrid structure contained nucleophilic nitrogen, which was critical for protonation and accumulation of the molecule in digesting vacuoles. Furthermore, we expected structure 3 to be a non-substrate for the P. falciparum Chloroquine-Resistant Transporter (PfCRT), which is responsible for Plasmodium’s decreased sensitivity to chloroquinelike chemicals. These considerations backed up our theory that hybrid 3 could be a promising therapeutic candidate [9 , 10 ].. The freshly synthesised hybrid 3 was put to the test. In vitro assays and in vivo tests in rats were used to determine its activity against all stages of Plasmodium in the mammalian host. Both in vitro and in vivo, the chemical displayed considerable inhibitory effects against Plasmodium liver and blood-stage parasites (Figure 4)
Figure 4: Depiction of the Plasmodium life cycle in humans showing the skin, liver, and blood stages witht he corresponding existing (solid lines) and future (dashed lines) humanized mouse systems to model each oft he individual stages and eventually the full Plasmodium life cycle including pathology and transmission.
Infection is initiated when a female Anopheles mosquito injects saliva-containing sporozoites into the skin. Sporozoites traverse dermal cells and gain access to the blood. The highly motile sporozoites transit to the liver where each sporozoite infects a single hepatocyte. One to two weeks after hepatocyte invasion, merozoites exit the liver and begin a 48-h cycle of Red Blood Cell (RBC) invasion, replication, RBC rupture, and new merozoite release. During RBC infection, the parasite expresses variant surface antigens on the surface of the infected red blood cell, which interacts with Human Endothelial Receptors (hER), thus mediating the binding of infected RBCs to the microvascular endothelium of various organs. A small number of blood-stage parasites differentiate into sexual gametocytes, which are taken up by mosquitoes in blood meals to continue the transmission to new human hosts [11 , 12 ].
Assays for parasite inhibition in vitro
10,000 isolated salivary gland sporozoites were treated with the hybrid 3 for 15 minutes at 4 C before being transferred to albumincoated eight-well slides for the gliding-motility experiment. Sporozoites were allowed to glide for 15 minutes at 37°C before being fixed for 10 minutes with 4% paraformaldehyde (AppliChem). The slides were then cleaned in PBS/1 percent FCS before being blocked in PBS/10 percent FCS for 15 minutes at 37 degrees Celsius. Monoclonal anti P. berghei CSP antibody was used to visualise sporozoites. anti-mouse (Invitrogen). The wells were mounted in PBS with 10% glycerol and embedded in nail polish. Experiments were carried out in triplicate, with each well containing 100 sporozoites. The unpaired student’s t-test was used to determine statistical significance. Sporozoite invasion experiments were carried out using a modified approach previously reported. 30,000 sporozoites, in a nutshell were pre-incubated with the hybrid compound 3 for 15 minutes at 4 degrees Celsius before being transferred to confluent human hepatoma HuH7 cells for 90 minutes at 37 degrees Celsius in the presence of the compound [13 ].
Cells were washed, fixed, and sporozoites were stained with doublestaining to distinguish extracellular from intracellular sporozoites after incubation with sporozoites. Experiments were carried out in triplicate, with each well containing 100 sporozoites. An unpaired student’s t-test was used to determine statistical significance. As previously stated, exoerythrocytic stages were developed in a typical experimental method. 8-well chamber slides (Nunc) were plated with 3 104 HuH7 cells and grown to con-fluency at 37 C one day before sporozoite infection. Purified sporozoites suspended in complete medium were placed in the chambers and allowed to invade for a period of time. 90 minutes. The medium was replenished, and 1 M-10 nM of compound 3 was added. After that, medium was replenished on a daily basis [14]. Parasites were frozen, permeabilized, and stained by immune fluorescence antibody staining using hybridoma culture supernatant anti-HSP70 antibody after 24 or 48 hours of liver stage development, as described previously. For the strains K1 and K2, activity against P. falciparum blood stages was shown. The K1 strain of P. falciparum was used to test antiplasmodial activities (resistant to chloroquine and pyrimethamine).
The assay was modified from the [3 H]-hypoxanthine incorporation assay. Infected human red blood cells were subjected to serial drug dilutions in microtiter plates in RPMI 1640 media with 5% Albumax II [15 ]. After a 48-hour incubation period at 37°C in a low-oxygen environment, 0.5 Ci [3 H]- hypoxanthine was added to each sample. After another 24 hours of incubation, the cultures were harvested onto glass-fiber filters and rinsed with distilled water. A Beta plate TM liquid scintillation counter was used to count the radioactivity. The results were represented as a percentage of the untreated controls and were recorded as Counts Per Minute (CPM) per well at each medication concentration. The IC50 values were determined using the sigmoidal inhibition curves. The averages of four data from two independent experiments carried out in duplicate are used to calculate IC50 values. Malstat reported activity against blood stages for the strains 3D7 and Dd2. Assay The assay was used to determine the viability of the parasite. P. falciparum 3D7 or Dd2 synchronized ring stages were plated at 1% parasitemia and 5% parasitemia in RPMI 1640 medium with Albumax II. (5 g/L) on microtiter plates with 96 wells. The hybrid molecule was dissolved in a final concentration of 0.5 percent DMSO and added in serial dilutions (640 pM-50 M) [16]. The plate was incubated for 72 hours at 37 degrees Celsius in a humidified, airtight incubator with 5% CO2 and 5% O2 in N2. In a 96-well microtiter plate, parasites were cultured in vitro for 72 hours, resuspended, and aliquots of 20 L were taken and added to 100 L of Malstat reagent. A 20 L mixture of NBT (Nitro Blue Tetrazolium)/Diaphorase (1:1; 1 mg/mL stock each) was added to the Malstat reaction to determine PLDH activity. Optical densities were measured in an ELISA reader at 630 nm after the plates were agitated for 30 minutes at room temperature [17 ]. The tests were carried out in threes. A negative control of 0.5 percent aqueous DMSO was employed, as well as dilution series of chloroquine (2) and primaquine (1) as further controls. The IC50 values were derived from variable-slope sigmoidal dose-response curves using the Graph Pad Prism programme version 4 after each chemical was tested 2–3 times. For each chemical, the average IC50 value was computed (Table 1).
| Assay | % | Oocyst Number (Mean | % | Compounds (Dose) | Oocyst Number (Mean | % | % |
|---|---|---|---|---|---|---|---|
| Parasitemia (Gametocythemia) | ± SD) | Mosquitoes Infection | ± SD | Inhibition Mosquitoes Infection | Oocyst Reduction | ||
| 1 | 10.3 (42%) | 256±180 | 95 | 20(50mg/kg) 20(25mg/kg) PQ(15mg/kg) | 64 ± 64 | 5 | 81.3 |
| 7.3 (49%) | 137± 100 | 90 | 142±118 | 10 | 0 | ||
| 7.0% (45%) | 117 ± 72 | 90 | 0 ± 0 | 15 | 100 |
Table 1: Oocyst number of P. gallinaceum in Ae. fluviatilis. Mosquitoes were allowed to blood feed on chickens before and after treatment with compound 20 and PQ.
Microsomes: Determination of the hybrid’s metabolic stability in rat liver
The hybrid 3’s phase I metabolism was examined using cytochrome-P450 dependent monooxygenase. Microsomes were extracted from untreated female Sprague Dawley rats and male rats treated for three days with corn oil, beta-Naphthoflavone (bNF; 100 mol/kg/days), Phenobarbital (PB; 400 mol/kg/days), or both bNF + PB. The day after the last treatment, the animals were slaughtered and microsomes were prepared: the liver was homogenised in 0.25 M sucrose with 0.1 mM EDTA (pH 7.4) and centrifuged for 20 minutes at 10,000 g followed by 1 hour at 100,000 g. All of the steps were completed on ice. Microsomes were prepared and kept at 80 degrees Celsius until needed. Incubation methods for microsomal incubations in their entirety (final volume 1000 L) As a NADPH-generating system, the hybrid 3 (100 M), rat liver microsomal protein (1 mg/mL), 0.1 M phosphate buffer (pH7.4), and -nicotinamide adenine dinucleotide phosphate (NADPH,1 mM) were added. Isocitrate (10 mM), isocitrate dehydrogenase (0.05 U), MgCl2 (4 mM), and NADP (1 mM) were used to make the NADPH-generating system, which was pre-incubated for 5 minutes at 37 C before being added to the incubation system. For 15, 30, 60, and 90 minutes, the entire incubation system was incubated. After the incubation period, 8-hydroxyquinoline was added for quantification purposes, the reaction was stopped immediately, and 500 L ethyl acetate was extracted. The residues were diluted in 50 percent methanol/water (v/v) and submitted to ion pair HPLC with UV detection after the solvent had evaporated (255 nm). Incubations in the control group were carried out under the same conditions as the experimental group. Heat-deactivated microsomes were subjected to the same circumstances. A reversed phase column (Symmetry C18, 3.9 mm 150 mm, 5 m;Waters) was used for HPLC analysis. Mobile phase A: methanol/water (1:10, v/v) with added phosphoric acid (0.01 mM) and hexanesulfonate (5 mM); B: methanol; linear gradient: 100% A to 100% B in 30 minutes at 0.65 mL/min injection volume 10 L The metabolism of the hybrid 3 was quantitatively described as relative peak areas (peak area hybrid/peak area internal standard) [18, 19].
Antimalarial medication development status and new developments
Malaria remains a severe hazard in developing countries, with more than 1 million clinical episodes and 3000 deaths per day. Malaria killed between 150 and 300 million people in the last century, accounting for 2–5% of all deaths. Approximately 40% of the world’s population currently lives in malaria-infected areas. Young children and pregnant women get the most severe symptoms of the condition. Despite the fact that malaria is native to most tropical locations, Sub-Saharan Africa accounts for 90 percent of disease-related death. Antimalarial medications are the only therapy option because an approved vaccine for malaria has yet to be developed. Despite the fact that Chloroquine was the first antimalarial drug to be synthesized, for more than 30 years, Chloroquine has been a near-magical cure, but the advent and spread of Chloroquine resistant parasites has rendered it virtually worthless in most parts of the world [20]. Artemisinin, a plant derived antimalarial, is now the only medicine available that is effective against the parasite internationally. Despite the fact that various new medications have been launched in the last 30 years, widespread or isolated examples of resistance suggest that their efficacy will be limited. As a result, novel treatments and regimens for malaria control are urgently needed. This paper provides a review of current antimalarial treatment choices as well as current efforts to create new medications based on both recent technology developments and adjustments to existing treatments, as well as combination therapies. The majorities of antimalarial medications have been discovered and developed using traditional drug discovery techniques, while drug design based on pathogen and host genomic and proteomic data is still in its early stages. The parasite food vacuole, apicoplast, and mitochondrion have been identified as the key organelles for therapeutic targeting based on their significance in parasite development and survival [21, 22]. Several components of the metabolic process are also being studied as therapeutic targets. Some of the medications already in use or in research have clear mechanisms of action; however, the exact modes of action of many pharmaceuticals have yet to be determined. It will be feasible to build target specific medications with improved safety and efficacy using genome and proteome information. Chloroquine, a four-aminoquinoline, was first synthesised chemically in 1934 as a quinine alternative. Chloroquine is deposited specifically in the parasite’s feeding vacuole, where it works as an antimalarial by blocking the polymerization of the harmful haem. The parasite produces histidine-rich protein 2, which catalyses the conversion of haem into the non-toxic and insoluble haemozoin [7, 8]. Resistance to chloroquine in P. falciparum is linked to changes in a feeding vacuole transport protein rather than a shift in haem processing [9 ]. Chloroquine-resistant parasites emerged from four unique founder events that happened in various geographic areas, according to a genome-wide microsatellite analysis. Chloroquine resistance became widespread as a result of this spread [10]. Chloroquine resistance appears to be independent of changes in this locus in P. vivax [11]. When the newly formed World Health Organization (WHO) declared war on malaria and dedicated to its universal eradication in the early 1950s, Chloroquine rose to prominence.
Primaquine, an 8-aminoquinilone, is highly effective against hypnozoites, a dormant type of P. vivax liverstage parasites. Its one-of-a-kind characteristic makes it appropriate for treating P. vivax infections alone or in conjunction with other antimalarials.
Primaquine is effective against the parasite’s sexual stages and has been used successfully to eradicate malaria from the Vanuatu archipelago in the southwest Pacific [37]. In clinical trials in Indonesia, Colombia, and Papua New Guinea, primaquine was found to be exceedingly effective. Soldiers on jungle patrol in Colombia were given primaquine every day for 17 weeks in field studies. When compared to a placebo, daily dosage of primaquine was 94 percent effective [38 ]. It is 85% efficient against P. falciparum and 85% effective against P. vivax. Methaemoglobinemia and haemolysis in Glucose6-Phosphate Dehydrogenase (G6PD)- deficient people are two serious possible side effects of primaquine treatment. Minor gastrointestinal effects, such as stomach ache, are also linked to primaquine use, however these are normally minimised when the medicine is taken with food. In a recent field experiment, 2% of participants were unable to tolerate daily primaquine usage [39].
In malaria-endemic areas, multidrug-resistant parasites have become a serious treatment issue for healthcare providers. The development of drug resistance is the expected selective response of a microorganism to lifethreatening situations, in broad evolutionary terms. As a result, parasite resistance to all currently available antimalarial drugs is almost certain to emerge. in the near future In the field of antibacterial medications, cases of Staphylococcus resistant to vancomycin, the most powerful antibiotic, have begun to show up in emergency rooms across the United States. New medications will be needed to replace those that have lost their effectiveness as long as malaria remains a worldwide health problem [23, 24 ]. The bulk of currently available antimalarial medications have their origins in herbal remedies used by traditional healers. With the entire genomic sequence of the parasite, its host (human), and vector (Anopheles), new targets could be developed utilising rational drug design and other emerging technologies in the near future. Genome and proteome data, for example, have been crucial in the discovery of new diseases. Several parasite proteins are essential for the plastid to function properly. The identification of the type II fatty acid synthesis pathway in Plasmodium, as well as other plastid-related processes that differ significantly from their human counterparts, has opened up a new set of targets. The plastid’s prokaryotic origin will also serve as a predictor of which bacterial inhibitors might be effective antimalarial. The majority of the current targets were previously targeted for other human diseases. There is already a substantial body of scientific data and libraries of chemicals that can be used to combat these targets. A structural genomics project is presently ongoing with the goal of identifying the structure of prospective therapeutic targets in a large number of protozoan species. When these elements are joined, they form a synergy [25, 26]. Two lines of investigation will provide a strong impetus for the discovery of compounds with potent antimalarial activity. However, because similar targets are encoded in the human genome, developers of these compounds must ensure that the inhibitors have a high degree of selectivity towards the parasite enzyme. This is particularly important in the case of malaria, as more than 90% of malaria deaths occur in children under the age of five, an age group in which safety is vital. Malaria also affects some of the world’s poorest countries, where healthcare services similar to those seen in the Western world do not exist. Any antimalarial medicine developed in this situation would need to have a brief curative regimen, be effective with single-daily dose, and be cost-effective. affordably priced The majority of antimalarial drugs work by reducing or eliminating asexual erythrocytic-stage parasites in the infected host; however, none of these drugs are designed to treat the pathogenesis of severe malaria and cerebral malaria, which are responsible for the majority of malaria-related deaths in African children. An antimalarial medication that selectively targets malaria pathogenesis without requiring total parasite eradication could drastically reduce fatalities in young children while also addressing the problem of antimalarial treatments losing or losing efficacy [27 , 28]. In the absence of a viable malaria vaccine, new medications that are effective against all stages of malaria parasites, including gametocytes, will be required to eradicate the disease completely a case of malaria Through effective public–private collaborations, several for-profit and non-profit organizations are spearheading efforts to alleviate the burden of this devastating disease in endemic nations by discovering, producing, and providing novel inexpensive antimalarial medications. The MMV, a public and private collaboration that is now supporting research for the development of roughly two dozen novel chemicals for malaria therapy, is leading the charge in this area. These efforts are crucial in keeping the pipeline of antimalarial drugs alive. Resistance to PQ (primaquine) and CQ (chloroquine) in P. falciparum. The research looked into more than just PQ. However, there are various equivalents. 8-amino-2- methylquinolinecompound 1’a derivatives of 8-amino-2-methylquinoline comprising the amino-alkyl side a chain of PQ [compound-2’]and carboxy-primaquine [CPQ], which is a key metabolite of PQ PQ and compound 2 had weak effect against blood stages parasites, as expected, whereas analogues with no aminoalkyl side chain [compound 1 and CPQ] had no detectable activity at all. TQ also demonstrated only sporadic activity against parasites that live inside erythrocytes. Compound 2 lowered the IC-50 of CQ in the CQ-resistant K1 parasite strain from 390Nm to 40Nm at subinhibitory concentrations, almost identical to the CQ-sensitive D10 strain. [39Nm] PQ and TQ, compound 2, were studied in depth, and it was discovered that all three compounds had a substantial synergistic interaction with CQ in the resistant K1 strain, but have no influence on the IC-50 of the CQ-sensitive D10 strain [30].
The resistance reversing effect of PQ appears to be connected to its ability to induce CQ accumulation in parasites expressing a CQresistant of the pfcrt gene. Analogues missing the amino-alkyl side chain of PQ showed no resistance reversing activity. FV [FOOD VACCULE] membrane protein Plasmodium falciparum Chloroquine Resistances Transporter [PFCRT] is encoded by this gene. The effect of PQ and CQ accumulation in Q-resistant parasites is caused by changes in pfcrt, which was used to trans infect a CQ-sensitive parasite strain with a CQ-resistant pcfrt obtained from the Dd2 strain. The presence of the mutant PFCRT Protein is the only difference between the parasites and the sensitive strain. When compared to the identical parasite strain trans-infected with its own CQ-sensitive pfcrt, these parasites accumulated about a fourth of the CQ. In C3Dd12, PQ was found to entirely restore CQ accumulation in a dose-dependent manner, but CQ accumulation was unaffected in C2GC03. PQ was found to be even more effective than the well-known CQ-resistances reverse [verapamil] at recovering CQ accumulation in C3Dd2 [31, 32].
P. falciparum parasites, for example, appeared to be responsive to quinine in vitro sites of the ICEMRS will be used to track the emergence and spread of resistance. Vivax malaria is also endemic in several ICEMRS, and EX vivo drug tests for P. vivax are available are also being carried out, but the assays are hampered by the challenges of P. vivax culture and the recognition that certain drug assays [CHLOROQUINE] necessitate a high proportion of parasites in the ring stages and a high parasitemia.
Hope for new antimalarial medications stems from the discovery of novel antimalarial drug targets
Malaria is a severe global danger that claims the lives of over 2 million people every year. Due to the growth of drug-resistant parasites, the lack of a viable vaccine, and the expansion of insecticide resistant vectors, treating malaria is becoming increasingly challenging. As a result, new chemotherapeutic techniques are required for malaria treatment, necessitating the search for new drug targets. Different ways to identifying novel antimalarial drug targets are discussed here. In order to generate fresh, rationally designed lead compounds, we have also paid close attention to the existing proven targets. Some of the most significant parasite proteins have been proposed as targets; however, more in vitro or in vivo structure and function investigations of these proteins are required to confirm their suitability as targets [33]. The investigation of the interactome between the apicoplast, mitochondrion, and genomic DNA will be valuable in finding key pathways or proteins that regulate critical pathways for parasite growth and survival, and could be attractive targets. Molecules involved in parasite invasion of host erythrocytes, as well as infected erythrocyte ion channels, which are required for parasite intra-erythrocyte survival and stage progression, are becoming more appealing targets. This review will go through the present state of knowledge on prospective antimalarial drug targets that could be used to build new pathways or proteins influencing critical processes for parasite development and survival, apicoplast and mitochondrion analyses could be promising targets. Molecules involved in parasite invasion of host erythrocytes, as well as infected erythrocyte ion channels, which are required for parasite intra-erythrocyte survival and stage progression, are becoming more appealing targets. This review will go through the present state of knowledge on prospective antimalarial drug targets that could be used to build new antimalarial [34, 35].
Treatment with 8-aminoquinoline
Primaquine is the most widely used drug in this class for primary (causal) and terminal (post-exposure) malaria prophylaxis, radical cure of Plasmodium vivax and Plasmodium ovale (elimination of hypnozoites, the parasites’ dormant liver forms), and as a singledose gametocytocidal agent in Plasmodium falciparum infections [118] . Tafenoquine was discovered in 1978 [119], but it has taken a long time to reach the clinic, and it is currently in phase 3 clinical studies. Although its PK properties differ from those of Primaquine, if authorised, it is expected to have similar clinical indications. In its main role in terminal prophylaxis and radical treatment, Primaquine is usually given as a 14-day course. This regimen has a number of drawbacks, including hemolysis in individuals with Glucose- 6-Phosphate Dehydrogenase (G6PD) deficiency, dose-related gastrointestinal side effects, and so on. Methemoglobinemia is a risk, and because to its complexity, it has a low compliance rate [120]. Pretreatment screening for G6PD deficiency, drug administration with food, and monitoring for cyanosis and respiratory symptoms are all measures that can be used to reduce adverse effects, all of which are more likely if G6PD-normal patients are given abbreviated highdose regimens to improve compliance [121]. PK/PD investigations of different short-course regimens should be investigated, especially when models based on known single-dose PK data show that they should be safe, because toxicity may be depending on characteristics such as age and race/ethnicity [122]. Because intermediate and poor metabolizers have more relapses, the efficacy of Primaquine against liver forms of P. vivax may be connected to the activity of the CYP2D6 enzyme [123]. Models based on known single-dose PK data show that they should be safe, because toxicity may be depending on characteristics such as age and race/ethnicity. Furthermore, Lumefantrine is a recognised 2D6 inhibitor, coadministration of the ACT artemether lumefantrine and Primaquine may reduce the Primaquine component’s efficacy. PK/PD investigations are needed to investigate these interactions in greater depth, especially since the formation of temporary active phenolic metabolites may kill hypnozoites while potentially promoting toxicity [124]. Primaquine might thus be a pro-drug that needs to be broken down in the body before it can be used. The inclusion of a 5-(3-trifluoromethyl)-phenoxy group in tafenoquine, but not Primaquine, is thought to be the explanation for Tafenoquine’s longer elimination t12 (14 days vs. 4–6 h) and reduced proclivity for methemoglobinemia [119]. A single 300 mg dose regimen, based on PK/PD and efficacy data from studies of a variety of dose regimens given in combination with CQ, appears to be the most effective. A standard 14 days course of Primaquine 15 mg/day appears to be at least as effective in avoiding P. vivax relapses as a mg Tafenoquine dosage, with a similar frequency of side effects [125,126]. There is growing evidence that, like primaquine, CYP2D enzyme system function is required for antimalarial action [127]. Concerns about Tafenoquine’s renal and ocular damage appear to be unfounded [128]. Although data from ongoing phase 3 trials could provide further information. In conclusion, 8-aminoquinoline medicines play an important role in the treatment and control of malaria. The use of PK/PD has helped with Tafenoquine dose optimization, but further research is needed to investigate the interactions between the two medicines in this class’s metabolism, efficacy, and toxicity. The protracted process of elimination because prolonged and severe hemolysis can occur in G6PD-deficient people who are accidentally treated with this medicine, t12 of Tafenoquine has implications for the necessity of effective G6PD screening. The feasibility and cost-effectiveness of using CYP2D6 metabolizer status as a predictor of parasitological response and adverse effects is unknown at this time, but it could be the topic of future research.
Novel antimalarial medications
The threat of Artemisinin resistance has heightened the urgency to discover novel antimalarial treatments that are both efficacious and well tolerated [129]. The spiroindolone class is arguably the most clinically relevant. These medications stop parasites from making proteins [130]. A potential drug resistance mutation has also been postulated as a specific molecular target for the spiroindolones in the gene encoding the P-type Cationtransporter ATPase4 (PfATP4). The medications may disrupt wild-type PfATP4, causing major rheological changes in parasitized erythrocytes, which are subsequently quickly removed by the reticulo-endothelial system [131]. The spiroindolone cipargamin has advanced from dose-finding and safety research to human malaria testing. A 3-day regimen of 30 minutes each day was developed based on volunteer data [132] and allometric scaling from animal research. A PK and early effectiveness investigation of initial parasite clearance in small groups of individuals with falciparum or vivax malaria was conducted at a dose of mg/day [133]. The parasite clearance was rapid, with a t12 of 21 hours, justifying daily dosage. Hepatic dysfunction, which occurred in 14 percent of malaria patients in the two human investigations [133], was a possible safety signal that needs to be investigated in larger-scale comparative trials. The history of antimalarial treatment over the last 60 years or so, during which the therapeutic armamentarium has been gradually depleted due to parasite drug resistance, provides compelling evidence for using quantitative pharmacology tools in the development or validation of dose regimens. Recent advancements in sample schedules, assay technology, and PK/PD modeling have resulted in more evidencebased treatments, particularly for higher risk groups including children and pregnant women. PK/PD investigations may become part of routine monitoring of efficacy in phase 4 rather than merely at earlier stages of drug development as technology becomes more cost effective. This could make early detection of treatment failure easier, limiting the potential for significant human and social harm. Malariarelated morbidity and mortality have an impact. Antimalarial therapy advancements should not, however, be used to replace other parts of malaria control, such as the use of insecticide-impregnated bed nets and vector control methods.
Expert opinions
The growing use of highly sensitive LC-MS/MS assays and more convenient sample regimens (low blood volume and sparse time points post-dose), in combination with robust population PK/PD analyses that include Using existing data, researchers have identified patient categories that may be underdosed. Young children given IV Artesunate for severe disease and oral artemether lumefantrine for uncomplicated malaria, as well as pregnant women given CQ, SP, Lumefantrine, and Piperaquine as part of ACT, are good examples. In these instances, newly recommended dosage mg/kg regimens (which can be based on simulations from PK/PD models) should ideally be evaluated further to confirm enhanced efficacy without an increase in undesirable effects [40]. Because there are signs that Artemisinin derivative doses may reach a toxicity-related ceiling without reversing the recently observed Artemisinin resistance or tolerance (delayed initial parasite clearance rather than increased late recrudescence), modelling suggests that the role of the longer-acting partner drug is critical in preventing higher-grade infections. A failure of treatment However, with the loss of the ACT components’ mutual protection, there is a frightening prospect of widespread clinically significant ACT failure in the nottoo distant future [41]. As a result, new schizonticidal medicines with a fast onset of action are required eliminate parasites quickly and could thus become a viable alternative to Artemisinin medicines when used in conjunction with a longer-acting partner treatment. More research is needed to assess their efficacy, acceptability, and safety, as well as any potential PK/PD interactions with potential partners such as Lumefantrine, Piperaquine, or Naphthoquine. In the context of HIV treatment, potential medication interactions with ACT components have been discovered, as well as with pharmaceuticals that are known to alter ventricular repolarization. These could be helpful in the event of HIV to reduce the incidence of recurrent malaria, but there’s also a chance that ART interactions will reduce ACT’s efficacy by reducing drug exposure. PK/PD studies are critical for determining the right dose while maximising the potential for benefit. Because electrocardiographic monitoring may not be possible where health care facilities are limited, it is critical to reduce the risk of adverse cardiovascular outcomes when antimalarial drugs that prolong the QTc are used, by adhering to recommended dose regimens and avoiding additional pharmacotherapy with agents that have the same effect (such as macrolide antibiotics). In this context, detailed in vitro and in vivo assessment of the potential for 4- Aminoquinolines and similar medicines to produce malignant dysrhythmias is also necessary so that relative risks may be able to be measured. Halofantrine and Lumefantrine, for example, are chemically similar but have different cardiotoxicity profiles. Primaquine is still the sole treatment for P. vivax infections that is both effective and safe. gametocytocidal agent with the highest efficacy. Tafenoquine, a chemically similar 8-amminoquinoline molecule, has taken a long time to create. Because Tafenoquine has a substantially longer (14-day) elimination t12 than Primaquine, persistent hemolysis in G6PD-deficient patients is a major problem. The new development of point of care testing for G6PD status may aid in the practical application of Tafenoquine, which may have a larger potential than Primaquine for reducing the consequences of repeated vivax relapses on the risk of anaemia and local malaria transmission. It is important to remember that improvements in antimalarial therapy are greatest when other components of control are addressed, such as the use of insecticide-impregnated bed nets and vector-reduction strategies. Because of this, the WHO’s goal of eradication has been reintroduced such a well-thought-out strategy [42, 43].
Novel antimalarial medications
The threat of Artemisinin resistance has heightened the urgency to discover novel antimalarial treatments that are both efficacious and well tolerated. The spiroindolone class is arguably the most clinically relevant. These medications stop parasites from making proteins. A potential drug resistance mutation has also been postulated as a specific molecular target for the spiroindolones in the gene encoding the P-type Cation Transporter ATPase4 (PfATP4). The medications may disrupt wild-type PfATP4, causing major rheological changes in parasitized erythrocytes, which are subsequently quickly removed by the reticuloendothelial system. The spiroindolone cipargamin has advanced from dose finding and safety research to human malaria testing. A 3-day regimen of 30 minutes each day was developed based on volunteer data. Allometric scaling from animal research. A PK and early effectiveness investigation of initial parasite clearance in small groups of individuals with falciparum or vivax malaria was conducted at a dose of mg/day. The parasite clearance was rapid, with a t12 of 21 hours, justifying daily dosage. Hepatic dysfunction, which occurred in 14 percent of malaria patients in the two human investigations , was a possible safety signal that needs to be investigated in larger-scale comparative trials.
Outcome
The history of antimalarial treatment over the last 60 years or so, during which the therapeutic armamentarium has been gradually depleted due to parasite drug resistance, provides compelling evidence for using quantitative pharmacology tools in the development or validation of dose regimens [45, 46]. Recent advancements in sample schedules, assay technology, and PK/PD modeling have resulted in more evidence based treatments, particularly for high risk groups including children and pregnant women. PK/PD investigations may become part of routine monitoring of efficacy in phase 4 rather than merely at earlier stages of drug development as technology becomes more cost-effective. This could make early detection of treatment failure easier, limiting the potential for significant human and social harm. Malaria related morbidity and mortality have an impact. Antimalarial therapy advancements should not, however, be used to replace other parts of malaria control, such as the use of insecticide-impregnated bed nets and vector control methods [47].
Expert opinions
The growing use of highly sensitive LC-MS/MS assays and more convenient sample regimens (low blood volume and sparse time points post-dose), in combination with robust population PK/PD analyses that include Using existing data, researchers have identified patient categories that may be under dosed. Young children given IV Artesunate for severe disease and oral artemether Lumefantrine for uncomplicated malaria, as well as pregnant women given CQ, SP, Lumefantrine, and Piperaquine as part of ACT, are good examples. In these instances, newly recommended dosage mg/kg regimens (which can be based on simulations from PK/PD models) should ideally be evaluated further to confirm enhanced efficacy without an increase in undesirable effects [48].
Because there are signs that Artemisinin derivative doses may reach a toxicity-related ceiling without reversing the recently observed Artemisinin resistance or tolerance (delayed initial parasite clearance rather than increased late recrudescence), modelling suggests that the role of the longer-acting partner drug is critical in preventing highergrade infections. A failure of treatment However, with the loss of the ACT components’ mutual protection, there is a frightening prospect of widespread clinically significant ACT failure in the nottoo distant future [49].
As a result, new schizonticidal medicines with a fast onset of action are required spiroindolones eliminate parasites quickly and could thus become a viable alternative to Artemisinin medicines when used in conjunction with a longer-acting partner treatment. More research is needed to assess their efficacy, acceptability, and safety, as well as any potential PK/PD interactions with potential partners such as Lumefantrine, Piperaquine, or Naphthoquine [50, 51].
In the context of HIV treatment, potential medication interactions with ACT components have been discovered, as well as with pharmaceuticals that are known to alter ventricular repolarization. These could be helpful in the event of HIV. Reducing the incidence of recurrent malaria, but there’s also a chance that ART interactions will reduce ACT’s efficacy by reducing drug exposure. PK/PD studies are critical for determining the right dose while maximising the potential for benefit [51, 52 ]. Because electrocardiographic monitoring may not be possible where health-care facilities are limited, it is critical to reduce the risk of adverse cardiovascular outcomes when antimalarial drugs that prolong the QTc are used, by adhering to recommended dose regimens and avoiding additional pharmacotherapy with agents that have the same effect (such as macrolide antibiotics). In this context, detailed in vitro and in vivo assessment of the potential for 4-aminoquinolines and similar medicines to produce malignant dysrhythmias is also necessary so that relative risks may be calculated. This can be measured. Halofantrine and lumefantrine, for example, are chemically similar but have different cardio toxicity profiles [53V].
Primaquine is still the sole treatment for P. vivax infections that is both effective and safe. Gametocytocidal agent with the highest efficacy. Tafenoquine, a chemically similar 8-amminoquinoline molecule, has taken a long time to create. Because Tafenoquine has a substantially longer (14-day) elimination t12 than Primaquine, persistent hemolysis in G6PD-deficient patients is a major problem. The new development of point-of-care testing for G6PD status may aid in the practical application of Tafenoquine, which may have a larger potential than Primaquine for reducing the consequences of repeated vivax relapses on the risk of anaemia and local malaria transmission [54].
It is important to remember that improvements in antimalarial therapy are greatest when other components of control are addressed, such as the use of insecticide-impregnated bed nets and vectorreduction strategies. Because of this, the WHO’s goal of eradication has been reintroduced such a well-thought-out strategy.
A five-year perspective
Given recent advancements in sample, assay, and population modelling technology, the possibility of more convenient, less expensive, and collaborative PK/PD investigations is real. This should lead to the creation of more sensible antimalarial treatment regimens adapted to the clinical setting, such as for the very young, pregnant women, and those with coexisting diseases like HIV [55, 56]. Although conventional Therapeutic Drug Monitoring (TDM) with immediate clinical application is problematic in resource-constrained environments, DBS sampling and a rapid turnover LC-MS/MS assay in a central facility, along with current information technology, can help. Technology has the potential to improve the everyday management of individual patients in remote locations while also providing a rich source of pharmacoepidemiological data. When frequent TDM is not practicable due to cost or logistical constraints, this technique could be utilised to see if sub therapeutic drug concentrations are to blame for clinical treatment failures that occur from time to time or when drug quality is in question. In order to maximize mutual protection against parasite resistance, researchers should evaluate the tolerance, efficacy, and toxicity of new combinations of existing medications, such as employing more than two drugs together or more frequent dosage throughout the same treatment period [57, 58]. This could apply to antimalarial medications that have been on the shelf for a while due to prior resistance but are now being used again. Parasite sensitivity has reappeared, according to in vitro and other evidence (Table 2).
| Assay | Compound | % Reduction (Mean Parasitemia ± SD)* | Survival (Mean ± SD) |
|---|---|---|---|
| 5th 7th | |||
| 4.6 ± 2 31 ± 6.4 | 13 ± 3 | ||
| Non treated CQ** | 99% (0.1 99% | ||
| 6 | ± 0) (0.4 ± | 22 ± 6 | |
| 0.4) | |||
| 1 | 0% (4.9 ± | ||
| 1.7) 11% | 16 ± 3 | ||
| (27.2 ± | |||
| 0% (5 ± 6.8 | |||
| 1.7) 2% (30 | 20 ± 7 | ||
| 8 | ± 16.2) |
Table 2: Antimalarial activity of synthetic compounds in mice infected with P. berghei treated with daily doses of 25 mg/kg body weight for three consecutive days.
Plan of Work
Antimalaria medications are used to treat and prevent malaria infection. The majority of antimalaria treatments target the erythrocytic phases of malaria infection, which is the stage of infection that causes symptoms. The fundamental goal of treatment is to remove the diseasecausing parasites from a patient’s bloodstream as quickly as possible, in order to prevent an uncomplicated case of malaria from progressing to severe disease or death. Antimalaria drugs function by destroying malaria parasites in their early stages of development in the liver and red blood cells. The life cycle of malaria parasites is the goal of this project.
• Antimalaria medications now in use
• Chloroquine-resistant and multidrug-resistant malaria
• Recent breakthroughs in antimalaria drug therapy – Analogs of existing agents, Natural products, Compounds Used in combination therapies involved in the fight against different diseases , Active compounds against newer targets (Table 3, 4, 5).
| Compound | IC50 (M) | ||
|---|---|---|---|
| 1 | |||
| 2 | |||
| Hybrid 3 | |||
| 1 and 2 | 3D7 | Dd2 | K1 |
| 3.11 ± 1.536 | 1.12 ± 0.351 | 46 ± 0.08 | |
| 0.03 ± 0.002 | 0.26 ± 0.126 | 146 ± 0.02 | |
| 0.64 ± 0.046 | 58 ± 0.185 | 0.08 ± 0.0048 | |
| 0.03 ± 0.012 | 0.19 ± 0.035 | 0.169 ± 0.055 | |
Table 3: Antiplasmodial activity of the hybrid compound 3 on the asexual blood stages of three P. falciparum strains. Infected human red blood cells were incubated with serial compound dilutions for a total exposure time of 72 h. Endpoint reading was done by Malstat (3D7 and Dd2) or by incorporation od titrated hypoxanthine (K1).
| Compound | Relative gametocytemia (%) |
|---|---|
| 1 | 0.2* ± 0.05 |
| 2 | 0.9 ± 0.07 |
| Hybrid DMSO control | 3 0.7* ± 0.04 |
| 1.0 ± 0.19 |
Table 4: Gametocytogenesis inhibition assay in P. falciparum. P. falciparum NF54 gametocytes were incubated with hybrid 3 for 48 h. After 7 days of compound-free cultivation gametocytemia (gametocytes stages IV and V) was determined on Giemsa-stained blood films. 0.5% aqueous DMSO served as negative control and a dilution series (640 pM–50 M) of chloroquine (2) and primaquine (1) as additional controls were used. The hybrid compound 3 was added in a serial dilution of 640 pM–50 M dissolved in 0.5% aqueous DMSO.