Application of LUS to Treat Acute Respiratory Distress Syndrome (ARDS) in a Critically Ill Patient with Severe COVID-19
Received: 01-May-2023 / Manuscript No. jpcm-23-98404 / Editor assigned: 03-May-2023 / PreQC No. jpcm-23-98404 (PQ) / Reviewed: 17-May-2023 / QC No. jpcm-23-98404 / Revised: 22-May-2023 / Manuscript No. jpcm-23-98404 (R) / Accepted Date: 27-May-2023 / Published Date: 29-May-2023 DOI: 10.4172/2165-7386.1000530
Abstract
Background: Rapid development and a high death rate characterise the acute respiratory distress syndrome (ARDS), a condition that is highly frequent in intensive care units (ICUs). Infections brought on by the novel SARSCoV-2 coronavirus can quickly proceed in individuals who are already critically unwell to ARDS. It's crucial to diagnose patients quickly and accurately, and to check for ARDS while they're being treated. Computed tomography (CT) examination is not always feasible due to the special characteristics of COVID-19 patients, and chest radiographs have a low sensitivity and specificity for the detection of lung illnesses. As a result, bedside lung ultrasonography (LUS) can be utilised as a novel method for ARDS diagnosis in COVID-19 patients. Bilateral non-uniform B lines can be seen in the pulmonary field that is not gravity-dependent. The B lines are denser and even present as "white lung" in the dorsal pulmonary area. Areas of consolidation with a static or dynamic air bronchogram sign are often observed in the dorsal pulmonary field, particularly in the basilar section. The "lung slip" typically lessens or vanishes in the fused B-line region. The pleural line is rough, thicker, uneven, and contains numerous tiny consolidations. Both primary and secondary ARDS had identical pulmonary ultrasonography results.
Case description: In the setting described above, we provide our experience with treating a significant COVID-19 case and a literature evaluation. An 81-year-old man patient with COVID-19-related ARDS. LUS was used to direct the implementation of prone ventilation, and we discovered that the pulmonary edoema in the gravity-dependent region did lessen over time. The posterior consolidation started to open after nine hours of prone breathing. The transition from fragment sign to B line is visible on LUS. The B-line was reduced after 16 hours, showing that the pulmonary edoema was becoming better. Improved oxygenation could be possible. Pulmonary ultrasonography allows for visual monitoring of prone ventilation. The patient was also given mechanical breathing, high-flow nasal oxygen, oseltamivir, lopinavir/ritonavir, abidol, and cefoperazone-sulbactam treatment at the same time.
Conclusion: The success of this case's therapy was largely due to LUS-guided care.
Keywords
Lung ultrasonography; Patients; Coronavirus; Pulmonary edoema; Computed tomography; Arrhythmia; Acute respiratory distress syndrome; Prone position ventilation
Introduction
Acute severe respiratory syndrome
The coronavirus type 2 (SARS-CoV-2) is one of the coronavirus subtypes. Since December 2019, this viral illness has been affecting a number of people in Wuhan City, Hubei Province, China [1]. The most recent kind is Omicron. Close contact with persons who are sick with this illness, including those who are asymptomatic, has been the source of infection in cases that have been reported thus far. The virus is mostly spread by infected droplets and direct contact with infected individuals. In general, the population is at risk. After infection, there are no unique clinical indications, and the majority of infected people exhibit symptoms like those of viral pneumonia, such as fever, coughing, painful muscles, etc. Results from the throat swab and imaging findings are mostly used to make the diagnosis of the illness. The primary characteristics of computed tomography imaging (CT) [2] include early many tiny patchy shadows and interstitial alterations, observable in the extrapulmonary zone, numerous ground glass infiltrations and infiltrates in both lungs, and pulmonary consolidation in severe instances. Pleural effusions don't happen often. There are four categories for clinical classification: mild, typical, severe, and critical. People who fit one of the following descriptions are thought to be critically ill: Illnesses needing intensive care unit (ICU) monitoring and care include shock, organ failure, and respiratory failure necessitating mechanical ventilation. Dyspnea and respiratory failure can develop fast in cases of severe and serious disease. LUS is useful, non-radioactive, and essentially unrestricted by environmental conditions, making it a frequent choice for usage in urgent and emergency situations. Pulmonary edoema, lung consolidation, and pleural effusion are often symptoms of acute respiratory distress syndrome (ARDS). The major symptoms are diffuse comet tail indications, which were first detected by ultrasonography before chest radiography. Pleural effusion can also be measured using LUS. Additionally, lung re-expansion or prone ventilation are frequently needed to treat lung consolidation brought on by ARDS. Lung ultrasonography (LUS) is crucial in the assessment of lung re-expansion and prone ventilation, helping to identify any difficulties through changes in the B-line, consolidation, and other symptoms. Coronavirus disease 2019 (COVID-19) causes respiratory failure and lung damage, which present as ARDS-like symptoms and may proceed to ARDS. As a result, pulmonary ultrasonography is crucial in the management of COVID-19.
Case presentation
On January 30, 2020, an 82-year-old man who had a worsening cough, expectoration, and a three-day fever of 38 °C was taken to a nearby hospital. He needed long-term oxygen treatment for his everyday activities because his symptoms had been steadily becoming worse after light exertion. He was given ambroxol and piperacillintazobactam in the neighbourhood hospital, but his symptoms did not get any better. The nucleic acid testing of SARS-CoV-2 from a throat swab returned positive results the next day. These findings, together with those from the CT scan, supported the coronavirus disease of 2019 (COVID-19) diagnosis. The patient was subsequently sent to the Affiliated Jinhua Hospital's Department of Intensive Care Medicine, Zhejiang University School of Medicine, for further care. All methods carried out for this study complied with the Declaration of Helsinki (as updated in 2013) and the ethical guidelines established by the institutional and/or national research committee(s). The patient's written informed consent was acquired before this case report and the associated pictures could be published. The editorial office of this journal has a copy of the written consent on file for examination.
Previous sickness: For more than 10 years, he had a history of asthma and chronic obstructive pulmonary disease (COPD), with his cough and expectoration always getting worse in the winter and spring.
Inspection of the body: The patient was aware but not in a good mood when examined. He had a barrel chest, pursed lips, and shortness of breath. His blood pressure was 170/84 mmHg, his temperature was 36.5 ºC, and his oxygen saturation (SpO2) was 78%. Proiosystole and arrhythmia were also seen. None of the lower limbs had edoema.
Laboratory assessments: The blood tests showed the following results: C-reactive protein (CRP) 20.1 mg/L; white blood cells 7.39×109/L; neutrophils (N) 0.926; absolute lymphocyte count 0.27×109/L; hemoglobin 155 g/L; and platelets 112×109/L.
Diagnosis: A chest CT revealed emphysema, bullae in the left upper lung, persistent right lower lung infection, and bronchitis. The following diagnoses were made: (I) pneumonia (critical illness) caused by a novel coronavirus (SARS-CoV-2); (II) respiratory failure; and (III) acute aggravation of COPD.
Treatment: The patient received treatment with oseltamivir (75 mg, nasal feeding, twice daily), lopinavir/ritonavir (3 tablets, nasal feeding, twice daily), abidol (2 tables, nasal feeding, three times daily), and cefoperazone-sulbactam (2 g, intravenously, three times daily). The patient was also placed on high-flow nasal oxygen (flow of 60 L/ min and oxygen concentration of 100%) and cefoperazone-sulbactam (2 g, administered intravenously three times each day). The findings of his blood gas were as follows: Lactic acid (Lac) 1.8 mmol/L, PH (7.46 potential of hydrogen), PCO2 (32.5 mmHg), PO2 (61.7 mmHg), SO2 (91.8% oxygen saturation), and PO2/fraction of inspired oxygen (FiO2) (oxygen index) (62) are the values recorded. The patient was put on non-invasive mechanical ventilation the next morning, January 31, 2020. The patient started to challenge the device in the afternoon. In the evening, we intubated the patient using invasive mechanical ventilation after switching back to high-flow oxygen but finding that the patient was desaturating. The ventilator's settings were mode pressure control ventilation, pressure control 12 cmH2O, positive end expiratory pressure 6 cmH2O, respiratory rate times per minute, and oxygen concentration 60%. Immediately following intubation, a LUS was performed, which revealed the following: an enhanced pleural line echo at each scan point; a weak or nonexistent pleural sliding sign; no A-line; B-lines at each scanning point, which increased in amount with gravity distribution; the fragment sign at the upper, lower, and posterior blue points of the left lung; and the curtain sign at both diaphragmatic points. The posterior blue point on either side had an enormous number of B-lines, the LUS showed. His blood gases were examined after nine hours and showed that Lac was 2.5 mmol/L, PH was 7.37, PCO2 was 42.3 mmHg, PO2 was 50.5 mmHg, and SO2 was 84.4%. The LUS revealed that B-lines had grown at the posterolateral alveolar and/or pleural syndrome (PLAPS) point of each side, dropped at the left posterior blue point, and vanished at the right posterior blue point. He had blood gas readings of Lac 2.0 mmol/L, PH 7.40, PCO2 39.3 mmHg, PO2 70.4 mmHg, and SO2 92.7% after 16 hours. At the left and right posterior blue spots, the LUS revealed that the B-lines were no longer present. The left upper blue spots were where the fragment symbol could be observed. Back in the supine position, we noticed that the B-lines in the left and right lungs had grown at the upper blue point, lower blue point, and PLAPS point. Due to the minor side effects of lopinavir and Abidor and the absence of symptoms like nausea, vomiting, and diarrhoea, the treatment's nutritional delivery went without a hitch. Additionally, the antibiotics did not increase, and the inflammatory indicators remained steady.
Discussion
Bedside LUS is practical, affordable, non-intrusive, dynamic, reproducible, and real-time. According to Cortellaro et al. [3], bedside ultrasonography has a much greater sensitivity for diagnosing pneumonia than chest X-rays (CXR), which had sensitivity rates of 69% and 96%, respectively. For these approaches, respectively, Parlamento et al. [4] demonstrated sensitivities of 75% and 96.9% to diagnose pneumonia. As a result, ultrasonography is more useful than CXR in the diagnosis of pneumonia.
The patient whose state we are presenting here went from severe to critical quickly after arrival, then it progressed to ARDS [5]. Regardless of diagnosis, the examination of the lung state during this time was crucial for determining the impact of treatment and directing ongoing care. The health of the lungs is frequently evaluated using chest radiographs and CT scans. For patients with ARDS, however, who must be in an isolation unit and are extremely sick, these procedures are challenging to carry out and difficult to repeat. LUS is therefore the best option.
Although ultrasonography has been used as a diagnostic tool for lung problems since the 1960s [6], it has only lately become popular in intensive care settings. Gases and liquids both make up the lung. The liquid sinks while the gas rises. The normal lung tissue is totally reflected by ultrasound, resulting in artefacts like A-lines or a few B-lines. When ultrasound identifies varying levels of deaeration of lung tissue, the percentage of fluid varies and various occurrences happen. We have proof thanks to these artefacts, which include tissue-like indications, fragment signs, and more than a dozen additional signals [7,8]. The diagnosis of ARDS in patients with COVID-19 can thus be made using bedside LUS as a novel technique [9-11].
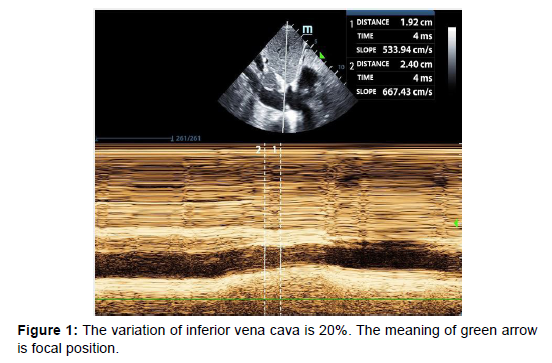
The occurrence, intensity, or timing of ARDS in patients with ARDS is not a standalone risk factor for short-term demise. But ARDS does play a major role in 2-year mortality [12]. This implies that the patient may pass away from other main illnesses even if they do not pass away from ARDS itself. Therefore, doctors should work to enhance treatment plans for the underlying condition that produced ARDS during the acute phase of the condition. Many years prior to his present hospitalisation, the patient's COPD and hypertension were both diagnosed. After tracheal intubation, circulatory failure set in, necessitating the administration of norepinephrine to keep blood pressure stable. At this point, the cause of the shock had to be discovered right away. For the purpose of identifying shock causes, which are categorised by hemodynamic factors, the fluid administration limited by lung sonography (FALLS) protocol [13] integrates cardiac ultrasonography based on the blue or blue-plus protocol as described by Lichtenstein. The patient's severe weight reduction left him with a barrel-shaped chest, making it difficult to access the cardiac regions. The ejection fraction (EF) was between 30 and 50 percent, the heartbeat was normal, and there was no serious cardiogenic pulmonary edoema, according to the available subxiphoid 4-chamber cardiac section and inferior vena cava section. The inferior vena cava allowed for the measurement of respiratory variation, and its value was 20% (Figure 1). Given that the inferior vena cava's respiratory fluctuation during mechanical ventilation was larger than 18%, we assumed that the patient was fluid sensitive. Due to the thorough evaluation of the size ratio of the 4 cardiac chambers and the fluctuation of the inferior vena cava, we temporarily ruled out obstructive shock. Other areas of the heart could not be evaluated, and the isolation wards did not have access to invasive hemodynamic monitoring devices like the Swan- Ganz catheter and pulse index continuous cardiac output (PiCCO). As a result, it was impossible to determine the cardiac output precisely and septic shock could not be totally ruled out. In conclusion, we thought the patient's heart activity was satisfactory and that there was volume responsiveness, however there were B-lines in various lung regions, as shown by LUS. We employed colloidal fluids to prevent the likelihood of fluid delivery worsening pulmonary edoema. We later learned that blood pressure improved and norepinephrine treatment was progressively reduced until the volume of colloid had reached around 600 mL. Colloidal fluids were maintained for resuscitation after the blood pressure had stabilised. The use of diuretics was intended to help the pulmonary edoema, however this strategy was not successful. It was challenging to determine if ARDS was the primary factor that caused the pulmonary edoema. By depending just on the B-lines, it is challenging to identify ARDS from acute cardiogenic pulmonary edoema [14]. By this time, the FALLS procedure had been used to determine the shock's causes in this case, and the appropriate treatment had been administered. The ultrasound findings were as expected for ARDS, including consolidation of the sub pleural anterior wall, weakened or lost sliding pleural sign, normal lung tissue, irregular thickening of the pleural line, and uneven distribution of B-lines.
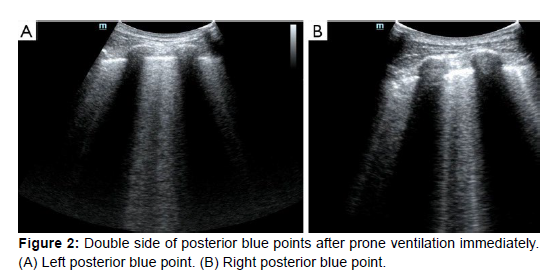
These results demonstrated that the pathogenesis of ARDS resulted in diverse lung alterations. The next action was to undertake prone position ventilation in light of the low SpO2 and the enhanced circulation. According to Guérin et al. [15], early use of prone position ventilation can dramatically lower mortality at 28 and 90 days in patients with severe ARDS. LUS was carried out as soon as the patient was in the prone position [16]. B-lines could be seen at both the left and right posterior blue sites, with the left side being more pronounced and heterogeneous than the right (Figure 2). We repeated the LUS after nine hours. On the left posterior blue point, the B-lines were still visible, although they were less prominent than before. B-lines weren't present on the right posterior blue point, but they were more numerous than before on both sides of the PLAPS points. The SpO2 was kept between 88% and 92% at this point, and the oxygenation status had increased from around 60% to 100%, indicating that the lung tissue in the gravity-dependent portions was involved. After 16 hours, we repeated the LUS in the prone position. Both posterior blue points were devoid of B-lines, and the left posterior blue point displayed a mild fragment indication [17].
As was already noted, ARDS's lung damage and exudative alterations are gravity-dependent. The amount of B-lines in the lung tissue close to the anterior chest wall dramatically increased after 16 hours of breathing in the prone position. Ventilation changes in the prone position indicated that the wounded lung tissue was well able to expand. We thus implemented a ventilation strategy for this patient with modest tidal volume breathing and occasional lung retention after returning to the supine position. We administered a tidal amount of around 6 mL/kg, and we checked lung retention every 30 minutes. The B-line at the top and lower blue spots had drastically shrunk by around 2 hours. The main goal of various treatment plans for ARDS patients is to increase oxygenation while reducing additional lung damage [18]. However, individuals with ARDS frequently experience multiple organ failure and shock together. Along with fixing the oxygenation, we also need to address issues with circulation and volume status. To determine the reason for the hemodynamic instability and to establish the groundwork for more prone position ventilation, we performed the FALLS protocol in conjunction with pulmonary and cardiac ultrasonography. The bedside lung ultrasound in emergency (BLUE) protocol more accurately indicated the recoverability of the lung before and after prone position breathing by comparing the characteristics of oxygenation and circulation. In conclusion, more precise scheduling of the use of extracorporeal membrane oxygenation (ECMO) and highfrequency oscillating ventilation is required.
Conclusion
The main goal of various treatment plans for ARDS patients is to increase oxygenation while reducing additional lung damage. However, individuals with ARDS frequently experience multiple organ failure and shock together. Along with fixing the oxygenation, we also need to address issues with circulation and volume status. To determine the reason for the hemodynamic instability and to establish the groundwork for more prone position ventilation, we performed the FALLS protocol in conjunction with pulmonary and cardiac ultrasonography. The BLUE procedure more accurately represented the recoverability of the lung by comparing the oxygenation and circulation parameters before and after prone position breathing.
Acknowledgement
Not applicable.
Conflict of Interest
Author declares no conflict of interest.
References
- Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S (2020) Novel Wuhan (2019-nCoV) Coronavirus. Am J Respir Crit Care Med 201:7-8.
- Lei J, Li J, Li X, Qi X (2020) CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 295:18.
- Cortellaro F, Colombo S, Coen D, Duca PG (2012) Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J 29:19-23.
- Parlamento S, Copetti R, Di Bartolomeo S (2009) Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med 7:379-384.
- Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA (2021) CT of Post-Acute Lung Complications of COVID-19. Radiology 301:383-395.
- Weil MH, Shubin H (1971) Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 23:13-23.
- Lichtenstein D (2014) Lung ultrasound in the critically ill. Curr Opin Crit Care 20:315-322.
- Pesenti A, Musch G, Lichtenstein D, Mojoli F, Amato MB, et al. (2016) Imaging in acute respiratory distress syndrome. Intensive Care Med 42:686-698.
- Kumar A, Weng Y, Graglia S, Chung S, Duanmu Y, et al. (2021) Interobserver Agreement of Lung Ultrasound Findings of COVID-19. J Ultrasound Med 40:2369-2376.
- Peixoto AO, Costa RM, Uzun R, Fraga ADM, Ribeiro JD, et al. (2021) Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: A systematic review. Pulmonology 27:529-62.
- Villén Villegas T (2021) Lung ultrasound in COVID-19: What has it contributedand what can it contribute? Emergencias 33:331-2.
- Fuchs L, Feng M, Novack V, Lee J, Taylor J, et al. (2019) The Effect of ARDS on Survival: Do Patients Die From ARDS or With ARDS? J Intensive Care Med 34:374-382.
- Lichtenstein DA (2015) BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 147:1659-1670.
- Seiler C, Klingberg C, Hårdstedt M (2021) Lung Ultrasound for Identification of Patients Requiring Invasive Mechanical Ventilation in COVID-19. J Ultrasound Med 40:339-351.
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, et al. (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:159-168.
- González-Seguel F, Pinto-Concha JJ, Aranis N, Leppe J (2021) Adverse Events of Prone Positioning in Mechanically Ventilated Adults With ARDS. Respir Care 66:1898-911.
- Fan E, Brodie D, Slutsky AS (2018) Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 319:698-710.
- Fan H, Tong H, Chen K (2022) Lung ultrasound-guided treatment for acute respiratory distress syndrome in a critically ill patient with severe COVID-19: a case report. Ann Palliat Med 11:3794-3803.
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Indexed atGoogle Scholar, Crossref
Citation: Ziane S (2023) Application of LUS to Treat Acute Respiratory DistressSyndrome (ARDS) in a Critically Ill Patient with Severe COVID-19. J Palliat CareMed 13: 530. DOI: 10.4172/2165-7386.1000530
Copyright: © 2023 Ziane S. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2374
- [From(publication date): 0-2023 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 2006
- PDF downloads: 368