Atypical Manifestations of Posterior Reversible Encephalopathy Syndrome (PRES) Complicating Cytotoxic Chemotherapy during the Treatment of Acute Lymphoblastic Leukemia in a Child
Received: 10-Feb-2019 / Accepted Date: 01-Mar-2019 / Published Date: 09-Mar-2019 DOI: 10.4172/2572-4983.1000181
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a rare cause of acute encephalopathy but can be observed in children under chemotherapy. We herein report a case of PRES in a 13-year-old boy with an acute lymphoblastic leukemia following treatment with intrathecal and intravenous chemotherapy. On Magnetic Resonance Imaging (MRI) the thalamic nuclei and cortex involvement accompanied the classical subcortical localization in parietal and occipital lobes. In addition, cytotoxic edema, instead of the vasogenic edema classically occurring in PRES, was observed. Cortical atrophy was seen after regression of other MRI findings. This case shows that PRES with an atypical appearance on MRI should be considered in children in case of acute encephalopathy occurring during chemotherapy treatment.
Keywords: PRES; Restricted diffusion; Chemotherapy
Introduction
Posterior reversible encephalopathy syndrome (PRES) is clinically characterized by headache, confusion, seizure, altered consciousness and visual disturbances with a typical Magnetic Resonance Imaging (MRI) appearance [1]. Diagnosis of PRES is based on both clinical presentation and imaging. On MRI, usual presentation consists of vasogenic edema in the subcortical white matter of the parietooccipital lobes with relative sparing of the cortex [2,3]. The global incidence of PRES is unknown. According to retrospective studies of cases seen between 1988 and 2008, PRES has been reported in patients aged 4 to 90 years with a significant female predominance [4]. Many patients have comorbidities including severe conditions such as bone marrow or solid organ transplantation, chronic renal failure, and chronic hypertension [4]. We present a case of a 13-year-old boy with PRES due to chemotherapy administration and with atypical involvement of thalamic nuclei and cortex and with presence of cytotoxic edema.
Case Report
A 13-year-old boy with T cell lymphoblastic leukemia was treated with a combination of intrathecal (methotrexate, cytarabine) and intravenous (vincristine, L-asparaginase, daunorubicine, cyclophosphamide) chemotherapy as well as a high dose of corticosteroids. A few hours after the first intrathecal chemotherapy administration, the patient developed abrupt headaches and status epilepticus. The blood pressure was normal. He was treated for his status epilepticus and for his post-dural-puncture headache with intravenous caffeine administration.
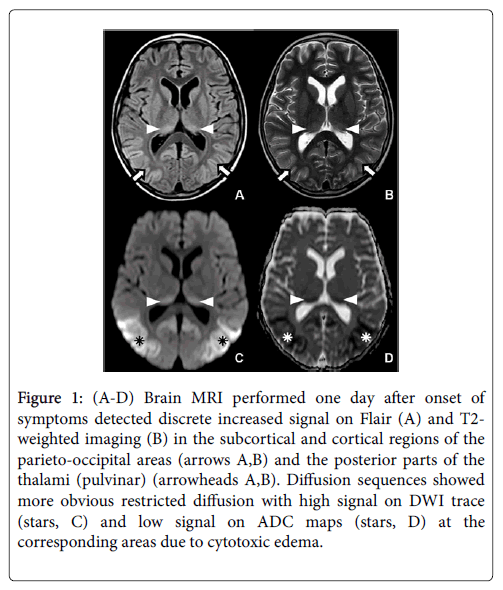
MRI demonstrated symmetric and bilateral abnormal high signal intensity on FLAIR (fluid-attenuated inversion recovery) and T2- weighted imaging sequences, located in the parietal and occipital cortex, involving subcortical white matter such as the pulvinar nuclei and associated with slight gyral swelling and sulcal effacement (Figure 1A and 1B). Diffusion weighted imaging (DWI) showed more obvious high signal on DWI b1000 with low signal on apparent diffusion coefficient (ADC) images typical for diffusion restriction in these locations (Figure 1C and 1D). There was no pathological enhancement after gadolinium injection.
Figure 1: (A-D) Brain MRI performed one day after onset of symptoms detected discrete increased signal on Flair (A) and T2- weighted imaging (B) in the subcortical and cortical regions of the parieto-occipital areas (arrows A,B) and the posterior parts of the thalami (pulvinar) (arrowheads A,B). Diffusion sequences showed more obvious restricted diffusion with high signal on DWI trace (stars, C) and low signal on ADC maps (stars, D) at the corresponding areas due to cytotoxic edema.
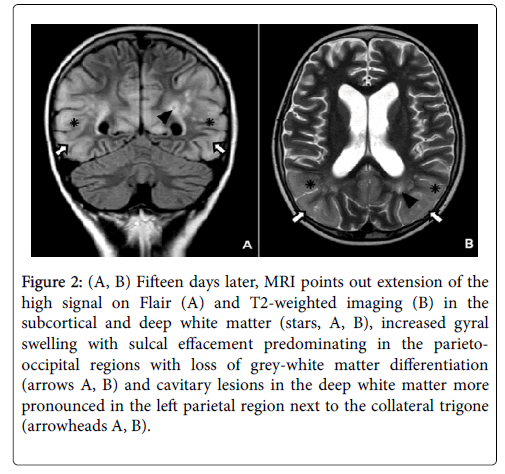
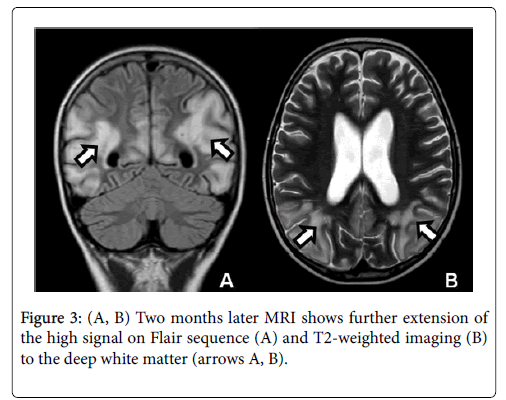
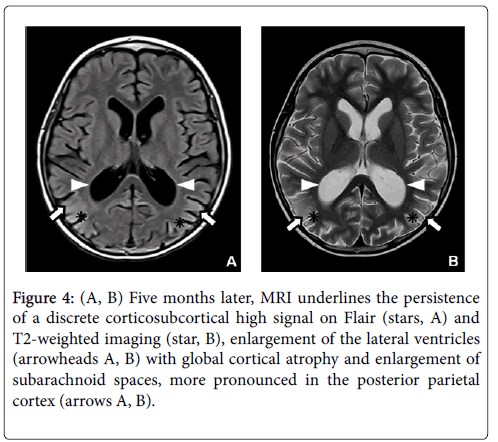
The diagnosis of early PRES was made on the basis of symptoms, clinical history (intrathecal and intravenous chemotherapy) and abnormal findings on MRI. Two weeks later, the follow-up MRI showed loss of grey-white matter differentiation with extension of signal changes into the subcortical and deep white matter and increased gyral swelling and sulcal effacement predominating in the parieto-occipital lobes. Furthermore, some cavitary lesions appeared in the deep white matter, especially in the left parietal region next to the collateral trigone (Figure 2). A follow-up MRI was performed two months later because of the persistence of delirium with transient visual disturbances. The control MRI showed even more extensive signal abnormalities in the parieto-occipital lobes with decreased cortical thickness compared to the acute stage presumably due to a combination of diffuse cortical atrophy with some remaining cortical swelling (Figure 3). The five months follow-up MRI revealed an important regression of signal abnormalities. However, the cortical atrophy was very evident, with a slight increase in the size of lateral ventricles and subarachnoid space (Figure 4).
Figure 2: (A, B) Fifteen days later, MRI points out extension of the high signal on Flair (A) and T2-weighted imaging (B) in the subcortical and deep white matter (stars, A, B), increased gyral swelling with sulcal effacement predominating in the parietooccipital regions with loss of grey-white matter differentiation (arrows A, B) and cavitary lesions in the deep white matter more pronounced in the left parietal region next to the collateral trigone (arrowheads A, B).
Figure 4: (A, B) Five months later, MRI underlines the persistence of a discrete corticosubcortical high signal on Flair (stars, A) and T2-weighted imaging (star, B), enlargement of the lateral ventricles (arrowheads A, B) with global cortical atrophy and enlargement of subarachnoid spaces, more pronounced in the posterior parietal cortex (arrows A, B).
Discussion
Posterior reversible encephalopathy syndrome (PRES) is a rare cause of acute encephalopathy in children under chemotherapy including methotrexate administration [5,6]. This condition was first described in adults by Hinckey in 1996, as a syndrome consisting of headache, confusion, seizure and visual disturbance associated with lesions predominating around posterior lobes [1,7]. Hypertension including toxemia of pregnancy is the main condition favoring the appearance of PRES.
Other causes could be post transplantation conditions, immunosuppression status, autoimmune diseases like systemic lupus erythematosus, infection, sepsis, shock, metabolic disorders, tumor lysis syndrome and cancer chemotherapy such as cytotoxic agents (cytarabine, vincristine, L-asparaginase and methotrexate), antiangiogenic agents and immunomodulatory cytokines [2,4,8]. Pathogenesis of PRES is until now not well understood. In children as in adults, neurotoxicity of chemotherapy cytotoxic agents could be related to endothelial damage with release of endothelin, prostacyclin, and thromboxane A [9,10]. The cytokine release results in a sudden increase in systemic blood pressure, which is counterbalanced by sympathetic autoregulatory vasoconstriction. Owing to physiologically lower sympathetic innervation, this process is less efficient in the vertebrobasilar circulation than in the carotid arterial tree, which could explain the posterior brain involvement [9]. Autoregulation breakdown in cerebral circulation leads to cerebral hyperperfusion resulting in disruption of the blood-brain barrier, fluid extravasation into the interstitium and eventually, vasogenic edema [9]. Upon imaging, PRES usually reveals itself as a vasogenic edema involving subcortical white matter of the parieto-occipital lobes bilaterally [2,10].
According a study of McKinney about 76 patients, the incidence of regions of involvement was parieto-occipital, 98.7%; posterior frontal, 78.9%; temporal, 68.4%; thalamus, 30.3%; cerebellum, 34.2%; brainstem, 18.4%; and basal ganglia, 11.8%. The incidence of atypical manifestations was enhancement, 37.7%; restricted diffusion, 17.3%; hemorrhage, 17.1%; and unilateral variant, 2.6% [11]. In case of chemotherapy including methotrexate administration, areas of restricted diffusion and decreased apparent diffusion coefficient diffusion can be detected indicating cytotoxic edema due to direct cellular derangement. Cytotoxic edema could be explained by the hyperperfusion with vasogenic edema causing mass effect with compression of local microcirculation [12]. Another theory suggests that the cytotoxic edema results in vasoconstriction leading to cytotoxicity [12].
Treatment of leukemia with a combination of chemotherapy cytotoxic agents is reported in the literature as a rare condition at risk of PRES due to neurotoxicity [2,6]. Several conditions may be similar to PRES in children; the ambiguous clinical manifestations and an atypical radiological appearance can confuse PRES with other diagnosis such as myelinolysis, encephalitis and acute disseminated encephalomyelitis (ADEM). In these cases, clinical history and imaging follow-up are used if necessary. However, the final diagnosis in our case was atypical PRES due to treatment by intrathecal and intravenous chemotherapy agents. In our patient, cytotoxic edema with restricted diffusion could be explained by the direct toxic effect on brain parenchyma due to metabolic derangement [13,14]. In PRES, just as in other conditions of brain parenchyma edema, areas with normal or increased diffusion (vasogenic edema) completely reverse, while areas with reduced diffusion (cytotoxic edema) are usually associated with permanent injury such as in our case and may result in cortical atrophy [5,15].
Conclusion
PRES following combination chemotherapy during the treatment of acute lymphoblastic leukemia has previously been reported in few cases. In our case an atypical involvement of thalamic nuclei and cortex with restricted diffusion due to a cytotoxic edema was observed. This case highlights that PRES should be considered in children in cases of extensive and very acute encephalopathy due to neurotoxicity clearly related to chemotherapy arising in children during chemotherapy treatment even when MRI presentation is atypical.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, et al. (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334: 494-500.
- Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am J Neuroradiol 29: 1036-1042.
- Bartynski WS, Boardman JF (2007) Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 28: 1320-1327.
- Legriel S, Pico F, Azoulay E (2011) Understanding Posterior Reversible Encephalopathy Syndrome. Annual update in intensive care and emergency medicine. Springer, Germany.
- Dicuonzo F, Salvati A, Palma M, Lefons V, Lasalandra G, et al. (2009) Posterior reversible encephalopathy syndrome associated with methotrexate neurotoxicity: conventional magnetic resonance and diffusion-weighted imaging findings. J Child Neurol 24: 1013-1018.
- Ziereisen F, Dan B, Azzi N, Ferster A, Damry N, et al. (2006) Reversible acute methotrexate leukoencephalopathy: atypical brain MR imaging features. Pediatr Radiol 36: 205-212.
- Shin RK, Stern JW, Janss AJ, Hunter JV, Liu GT (2001) Reversible posterior leukoencephalopathy during the treatment of acute lymphoblastic leukemia. Neurology 56: 388-391.
- Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 29: 1043-1049.
- Tortori-Donati P, Rossi A (2005) Pediatric Neuroradiology: Brain, Neck and Spine. Springer, Berlin, Germany; p462.
- Fugate JE, Rabinstein AA (2015) Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 14: 914-925.
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, et al. (2007) Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 189: 904-912.
- Rykken JB, McKinney AM (2014) Posterior reversible encephalopathy syndrome. Semin Ultrasound CT MR 35: 118-135.
- Fisher MJ, Khademian ZP, Simon EM, Zimmerman RA, Bilaniuk LT (2005) Diffusion-weighted MR imaging of early methotrexate-related neurotoxicity in children. AJNR Am J Neuroradiol 26: 1686-1689.
- Baehring JM, Fulbright RK (2008) Delayed leukoencephalopathy with stroke-like presentation in chemotherapy recipients. J Neurol Neurosurg Psychiatry 79: 535-539.
- Barkovich AJ, Raybaud C (2012) Pediatric Neuroimaging. (5thedn), Williams & Wilkins, Philadelphia, USA.
Citation: Kadi R, Malasi S, Ziereisen F, Christophe C (2019) Atypical Manifestations of Posterior Reversible Encephalopathy Syndrome (PRES) Complicating Cytotoxic Chemotherapy during the Treatment of Acute Lymphoblastic Leukemia in a Child. Neonat Pediatr Med 5: 181. DOI: 10.4172/2572-4983.1000181
Copyright: © 2019 Kadi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3575
- [From(publication date): 0-2019 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 2779
- PDF downloads: 796