Autism spectrum disorder and the promise of Artificial Intelligence
Received: 08-Dec-2021 / Manuscript No. JCALB-21-50116 / Editor assigned: 21-Dec-2021 / PreQC No. JCALB-21-50116 (PQ) / Reviewed: 05-Jan-2022 / QC No. JCALB-21-50116 / Revised: 10-Jan-2022 / Manuscript No. JCALB-21-50116 (R) / Published Date: 17-Jan-2022 DOI: 10.4172/2375-4494.1000428
Abstract
Since the U.S. Centers for Disease Control and Prevention began tracking the prevalence of autism spectrum disorder (ASD) over twenty years ago, rates have tripled, with an estimated one in 44 children now receiving a diagnosis [1]. Early ASD diagnosis and intervention during the critical neurodevelopmental window is recommended to enhance long-term outcomes [2-4]; yet many families experience diagnostic delays and challenges accessing services. Diagnostic barriers include long waits for specialist assessment, lengthy and fragmented evaluation processes, and limited primary care diagnostic capacity. Race, ethnicity, gender, geography, and socioeconomic status contribute to further delays for some populations [5-8]. Even after an ASD diagnosis is received, health services may struggle to fund and deliver targeted and timely interventions to the rapidly growing number of children requiring treatment. Data driven approaches to scale, streamline and enhance the quality of diagnostic and therapeutic ASD care available to families are urgently required. This narrative literature review considers the practice change potential of one such approach: Artificial Intelligence (AI) applied to the field of ASD. After providing a brief overview of AI in healthcare, we review a number of ASD specific AI-based approaches and consider their potential to augment current ASD diagnostic or treatment pathways. Key challenges associated with integrating AIbased technologies into clinical practice are also considered.
Keywords
Autism spectrum disorder; Artificial Intelligence; Healthcare; Algorithms; Human intelligence
An overview of AI in healthcare
Availability of massive and expanding quantities of digitized healthcare data, together with advances in computational and storage technologies and machine learning approaches, have opened up new possibilities for AI in healthcare [9]. A multidisciplinary branch of computer science, AI leverages computers to develop systems or algorithms capable of undertaking or partaking in tasks that would otherwise rely completely on human intelligence [10]. Within the healthcare sector strong economic investment in AI-powered technologies [11] has contributed to an exponential growth in topical research [12]. This focus has begun to filter into clinical practice with over 160 AI-powered devices having already been granted regulatory clearance, approval or marketing authorization by the Food and Drug Administration (FDA) [13].
With capabilities to extract clinically meaningful insights from rapidly increasing volumes of healthcare data that have exceeded human analytic capacity [14], AI offers multiple opportunities to augment healthcare practice. Machine learning, a subtype of AI where algorithms are applied to large datasets to look for patterns, can be used to create models that encapsulate those patterns to help predict outcomes [15,16]. Recent reviews have highlighted the promise of machine learning to enhance risk prediction, streamline some diagnostic practices, and support a more data-driven approach to clinical decisionmaking [17,18]. AI-enabled tools may improve accuracy and efficiency of diagnosis, leading to treatment or intervention, and scalability by quickly managing repetitive processes, storing and handling large amounts of data, and providing support for diagnostic or treatment decisions that may reduce the probability for mistakes [19,20]. Use of deep neural networks to augment interpretation of medical scans and other image based data, is one AI application that has received considerable attention [14,21]. A number of recent studies have also explored the therapeutic potential of AI-powered technologies, as well as its capacity to streamline administrative tasks [22]. For example, natural language processing based AI solutions are being leveraged to automate clinical documentation [23,24]. Such approaches show potential to improve workplace efficiency and increase the time clinicians can dedicate to patient care.
A number of promising ASD specific AI-based approaches have been described in the recent literature. The following section of this review highlights key challenges within the ASD diagnostic and therapeutic space and considers the potential of some of these technologies to augment care pathways.
AI in the ASD diagnostic landscape
Current diagnostic challenges
While ASD can reliably be diagnosed as young as 18 months of age, diagnostic challenges and workforce capacity issues in the U.S. are leading to prolonged wait times and delayed initiation of ASD specific treatments. The current average age of ASD diagnosis remains high at over four years [1,25], with roughly 27% of children still undiagnosed at age 8 [8]. While equity of access to diagnostic assessments is improving [1], certain groups such as girls, children who are non-white, of lower socio-economic status, or rural residing, have been noted in the literature to be more often un-diagnosed, mis-diagnosed, or receive a delayed diagnosis [7,26]. ASD evaluation is based on behavioral observations, highlighting the need for more objective methods for ASD assessment with the potential to better understand heterogeneity and identify potential phenotypes that could guide treatment. In response, calls have been made to develop a more data-driven and equitable approach to ASD diagnosis.
ASD has traditionally been diagnosed in specialist settings in the U.S. The dramatic rise in the number of children requiring ASD evaluations has exceeded specialist capacity, however, and resulted in prolonged waits for specialist evaluations. To help facilitate access to early intervention services, the recently updated 2020 American Academy of Pediatrics (AAP) clinical report encourages general pediatricians to make an initial diagnosis of ASD within the medical home for those not requiring specialist referrals [27]. Increasing primary care diagnostic capacity could reduce some of the pressure placed on specialist services and potentially streamline diagnosis and treatment initiation. Unfortunately, current ASD diagnostic tools can be difficult to use in primary care settings as they are time intensive and often require specialist training to administer [28]. They have also only been clinically validated for in-person use, presenting an additional challenge in the context of the ongoing COVID-19 pandemic [29]. Novel approaches that could be administered remotely or within the primary care setting, could potentially decrease some of these diagnostic challenges and workforce capacity issues.
Emerging AI-based innovations
There are a growing number of studies exploring the potential utility of AI in ASD screening and diagnosis. Within the brain magnetic resonance imaging study space, a recent systematic review and meta-analysis identified 43 studies investigating machine learning for ASD diagnosis [30]. Diagnostic accuracy appeared highest for the structural magnetic resonance imaging sub-group of studies, however, multiple methodological limitations were noted across studies. Further robustly designed follow-up trials are needed to clarify the utility of these approaches.
AI is also being used to mine electronic medical records and uncover ASD comorbidity patterns which could enhance screening practices. One novel ASD prediction approach [31] developed digital bio-markers based on the medical histories of patients aged 6 years and under. Data from a commercial claims and encounters database along with data from de-identified diagnostic records from a separate large medical center database was used to train and validate the model. For children over two years of age, the study investigators were able to leverage this model to identify ASD high risk with an area under the receiver operating characteristic above 80%. The study authors note that the autism comorbid risk score (ACoR) they were able to estimate from this work, displays a superior predictive performance to commonly used questionnaire-based screeners and is potentially less biased across demographic groups. An ACoR is an estimate of the likelihood of later ASD diagnosis based on the comorbidity history. An earlier topical study [32] similarly drew on electronic medical records (20K+ patients) and listed medical comorbidities to develop algorithms to detect clinically unique ASD subgroups.
Computer vision AI technology is also being applied in the ASD screening space. Recently, researchers have used deep learning facial image analysis to propose a more objective ASD screening solution [33]. This work draws on noted phenotypic facial differences between typically developing children and children with ASD [34]. The resulting model had 95% classification accuracy. The authors highlight the potential of such an approach to address the subjectivity of current screening practices. Further model training on race-specific datasets could potentially also address some of the racial biases [26] apparent in current practice. A mobile device app designed to capture and distinguish between the eye-gaze patterns of typically developing toddlers and toddlers with ASD has also recently been developed [35]. Designed for use in pediatric primary care settings, the app uses differences detected in the gaze patterns of children with ASD, including poorer coordination of gaze with speech, and reduced gaze response to social stimulus. Application of AI to kinematic features has also shown some potential within the screening and diagnosis space (for a review see [36]). One topical study [37] used a supervised machine learning approach to differentiate between typically developing children and children with severe levels of functional impairment related to ASD based on seven upper limb movement features. Analysis of speech prosody with machine learning techniques has also been explored with some success in ASD screening research [38].
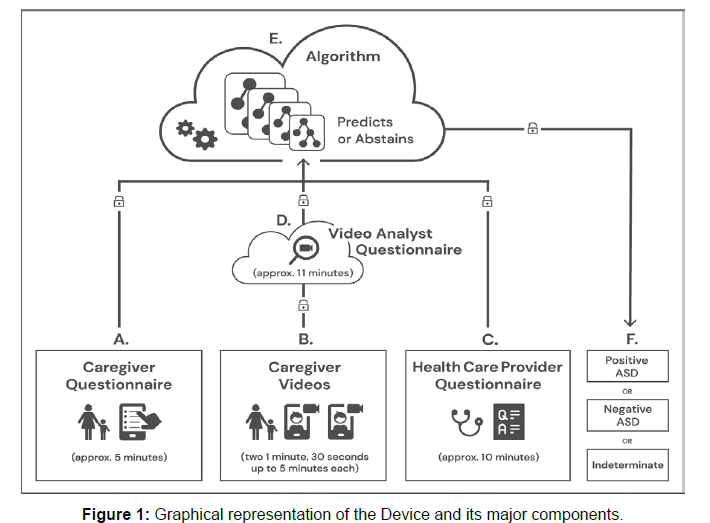
A prescription AI-based Software as a Medical Device [39], prospectively validated, has been developed to aid in the diagnosis of ASD in primary care settings. Following strong clinical trial results, the Device was granted FDA marketing authorization in 2021 [40]. The Device is a diagnosis aid, rather than a standalone diagnostic tool. It leverages a machine learning algorithm developed using patient record data from thousands of children with diverse conditions, presentations, and comorbidities who were either diagnosed with ASD or confirmed not to have ASD based on standardized diagnostic tools and representing both genders across the supported age range [41-45]. Questionnaires combining data and clinical experience were developed to identify the maximally predictive behavioral features of ASD based on the categories of social communication, verbal communication, facial expression, and repetitive behaviors as outlined by DSM-5 criteria. The Device combines 3 independent inputs, all of which can be completed remotely (see figure 1). Uploaded information is evaluated based on predictive features that are most indicative of ASD and one of 3 outputs are provided for the primary care provider to use in conjunction with their clinical judgment: ASD positive, ASD negative, or indeterminate/no result. The latter output acts as a risk control measure [46,47] when information is insufficiently granular to make a diagnostic recommendation with confidence (Figure 1).
Figure legend: A. Caregiver uses smartphone to answer a brief questionnaire, B. Caregiver uploads two short (1 minute, 30 seconds up to 5 minutes) home videos of their child to be scored by trained video analysts, and C. their primary care physician (or other qualified health care provider) independently answers a short clinical question set in approximately 10 minutes. These inputs are securely transmitted to the D. trained analysts where video features are extracted. E. The caregiver, primary care physician, and video analyst inputs are combined into a mathematical vector for machine learning analysis and classification. F. The Device provides a result of ‘ASD positive’ or ‘ASD negative’ or an indeterminate output (no result).
Approaches such as those highlighted above illustrate the breadth of novel AI-based work being undertaken in the ASD screening and diagnostic space. Next, we briefly review key challenges and some potential AI-based solutions within the ASD intervention and therapeutics space.
AI in the ASD treatment landscape
Current treatment challenges
Tailored interventions during the critical neurodevelopmental window can enhance long term outcomes, including gains in cognitive [48,49] and adaptive functioning [4,50], receptive and expressive language use [51], and social skills [3,52]. Despite recognition of the value of early intervention, only one in three children with ASD in the U.S. is thought to be receiving standard of care treatment [53]. Reasons for these gaps include inconsistent access to care providers, especially across regional and remote areas [54], an insufficient number of trained care providers to meet the growing need for ASD intervention, and high out-of-pocket treatment costs, in some cases up to $80,000 per year [55]. Difficulties accessing in-person care providers due to the ongoing Covid-19 pandemic has further curtailed treatment access for some families.
Emerging AI-based innovations
A number of promising AI-based ASD treatment innovations are explored in the literature, including the use of robot assisted therapy [56]. Studies have variously utilized autonomous, partially autonomous, and non-autonomous robot models [57]. Social robottherapy studies have reported improvements in eye contact, emotional recognition and expression, imitation, shared attention to a common object, turn-taking, motor skills and learning behaviors [57-60]. Much of this research is still experimental, however, and large clinical validation studies are needed to quantify the real world potential of such technologies in ASD treatment [61].
Preventing meltdowns using AI technology is another interesting area of ASD intervention research. Behavioral precedents to challenging behavior in children with ASD were identified in one study using deep learning techniques [62]. Researchers then developed a caregiver alert mechanism. This mechanism was designed to provide caregivers with timely warnings so they could potentially intervene prior to behavior escalation. A more recent study [63] similarly sought to develop meltdown prevention signals via AI computer-vision techniques which facilitated real-time facial expression monitoring in children with ASD.
In other research, neural networks have been leveraged to predict how children with ASD will respond to behavioral therapy [64]. This approach was used to explore the relationship between therapy, supervision intensity, age, and gender, on mastery of learning outcomes. Machine learning models have also been built to provide predictive recommendations for the most suitable types of technological treatment interventions for children with ASD [65]. Treatment recommendations were made based on singular or combination symptom patterns. Recent research [66] has also explored the use of an AI-augmented learning and applied behavior analytics platform to personalize ASD intervention. Study authors highlight the potential of such approaches to enhance data-driven clinical decisionmaking, improve intervention efficacy and streamline care delivery.
Clinical adoption challenges
Despite advances in utilizing AI in healthcare, widespread clinical adoption has been limited. Barriers to broader adoption include difficulties integrating such technologies with existing electronic medical record systems, and ethical and legal concerns [67-69]. Regulatory frameworks that account for the differences between AIbased devices and other types of medical devices [70] also require further development. In addition, many AI models require large datasets to train on. However, combining multiple datasets presents technical challenges, and concerns over data privacy and ownership have been raised [71]. The quality of data supplied to a machine learning algorithm is also of critical importance; data bias or imbalance can limit model generalizability and perpetuate pre-existing inequities if not accounted for [22,72]. Questions about a lack of transparency in certain types of AI algorithms and the implications of this within the context of clinical decision-making, have also been raised [14]. Several different approaches to algorithmic explainability and trustworthiness have been proposed in response to this concern [14,73]. Clinician and patient focused AI education is also required prior to broad deployment of these technologies within care pathways. Currently, however, AI education in medical training is patchwork and insufficient [74-77]. Some patients have also expressed concern over data-security and safety standards for AI-based health technologies [78].
Conclusion
Rapidly rising demand for ASD evaluations and treatment [1] has strained workforce capacity and led to suboptimal care for some children. Understaffing, long specialist wait lists, lengthy and complex assessment processes, demographic biases, and limited primary care evaluation capacity have all contributed to delays in diagnosis and treatment initiation [79]. Reduced in-person care options during the ongoing Covid-19 pandemic has further exacerbated access challenges. AI-based innovation in the ASD treatment and diagnostic space shows potential to help address some of these practice challenges. However, additional research is needed to comprehensively clarify the utility and efficacy of many of these approaches. Challenges relating to regulation, data selection and integration, algorithmic transparency and patient and clinician preparedness will all need to be addressed prior to widespread clinical adoption. While much of this research is still in its infancy, the promise of AI in the field of ASD has become clear. As sophistication of machine learning approaches increase, and the volume of digitized medical information and computational power and storage capabilities continue to expand, the impact of intelligent technology on the healthcare landscape will likely accelerate at a rapid pace [10,80].
Conflicts of interest
Dr Shannon, Mr. Abbas, Dr Chettiath, Dr. Salomon and Dr. Taraman are employees of Cognoa and have Cognoa stock options. Dr. Taraman additionally receives consulting fees for Cognito Therapeutics, volunteers as a board member of the AAP - OC chapter and AAP – California, is a paid advisor for MI10 LLC, and owns stock for NTX, Inc., and HandzIn.
References
- Maenner MJ, Shaw KA, Bakian AV (2021) Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR 70:1-16.
- Estes A, Munson J, Rogers SJ, Greenson J, Winter, J et al. (2015) Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. J Am Acad Child Adolesc. Psychiatry 54(7):580-587.
- MacDonald R, Parry-Cruwys D, Dupere S, Ahearn W (2014) Assessing progress and outcome of early intensive behavioral intervention for toddlers with autism. Res Dev Disabil 35:3632-3644.
- Smith T, Klorman R and Mruzek DW (2015) Predicting Outcome of Community-Based Early Intensive Behavioral Intervention for Children with Autism. J Abnorm Child Psychol 43:1271–1282.
- Delobel-Ayoub M, Ehlinger V, Klapouszczak D, Maffre T, Raynaud J-P, et al. (2015) Socioeconomic disparities and prevalence of autism spectrum disorders and intellectual disability. PloS one 10:e0141964.
- Oswald DP, Haworth SM, Mackenzie BK, Willis JH (2017) Parental report of the diagnostic process and outcome: ASD compared with other developmental disabilities. Focus on Autism and Other Developmental Disabilities. SAGE Publications 32(2):152–160.
- Wiggins LD, Durkin M, Esler A, Lee L-C, Zahorodny W, et al. Disparities in documented diagnoses of autism spectrum disorder based on demographic, individual, and service factors. Autism Res 13:464-473.
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, et al. (2009) Timing of Identification among Children with an Autism Spectrum Disorder: Findings from a Population-Based Surveillance Study. J Am Acad Child Adoles Psychiatry 48:474-483.
- Shu L-Q, Sun Y-K, Tan L-H, Shu Q, Chang AC (2019) Application of artificial intelligence in pediatrics: past, present and future. World J Pediatr 15:105-108.
- Chang AC. Intelligence-based medicine: artificial intelligence and human cognition in clinical medicine and healthcare. London, United Kingdom San Diego, CA, United States: Academic Press is an imprint of Elsevier; 2020. ISBN:978-0-12-823337-5
- Buch VH, Ahmed I, Maruthappu M (2018) Artificial intelligence in medicine: current trends and future possibilities. Br J Gen Pract 68:143-144.
- Kokol P, Završnik J, Vošner HB (2018) Artificial intelligence and pediatrics: A synthetic mini review. Pediatr Dimens 2:1-5.
- Katznelson G, Gerke S (2021) The need for health AI ethics in medical school education. Adv Health Sci Educ Theory Pract 26:1447-1458.
- Topol EJ (2019) High-performance medicine: the convergence of human and artificial intelligence. Nat Med 25:44-56.
- Nichols JA, Herbert Chan HW, Baker MAB (2019) Machine learning: applications of artificial intelligence to imaging and diagnosis. Biophysical Reviews 11: p. 111-118.
- Shah NH, Milstein A, Bagley P, Steven C (2019) Making Machine Learning Models Clinically Useful. JAMA 322:1351-1352.
- Chahal D, Byrne MF (2020) A primer on artificial intelligence and its application to endoscopy. Gastrointest Endosc 92: 813-820.
- Greenhill AT, Edmunds BR (2020) A primer of artificial intelligence in medicine. TIGE 22: 85-89.
- Ahuja AS (2019) The impact of artificial intelligence in medicine on the future role of the physician. PeerJ 7: e7702.
- Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, et al (2018) Scalable and accurate deep learning with electronic health records. NPJ Digital Medicine 1:18.
- Bressem KK, Adams LC, Gaudin RA, Tröltzsch D, Hamm B, et al (2020) Highly accurate classification of chest radiographic reports using a deep learning natural language model pre-trained on 3.8 million text reports. Bioinformatics 36:5255-5261.
- Greenfield D (2019) Artificial Intelligence in Medicine: Applications, implications, and limitations.
- Coiera E, Kocaballi B, Halamka J, Laranjo L (2018) The digital scribe. NPJ digital medicine Nature Publishing Group 1:1-5.
- Kreimeyer K, Foster M, Pandey A, Arya N, Halford G, et al. (2017) Natural language processing systems for capturing and standardizing unstructured clinical information: a systematic review. J Biomed Inform 73:14-29.
- Van’t Hof M, Tisseur C, van Berckelear-Onnes I, van Nieuwenhuyzen A, Daniels AM, et al. (2019) Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism SAGE Publications 25: 862-873.
- Angell AM, Empey A, Zuckerman KE (2018) A review of diagnosis and service disparities among children with autism from racial and ethnic minority groups in the United States. Int Rev Res Dev Disabil 55:145-180.
- Hyman SL, Levy SE, Myers SM (2019) Identification, Evaluation, and Management of Children with Autism Spectrum Disorder Pediatrics 145: e20193447.
- Kaufman NK. (2020) Rethinking “gold standards” and “best practices” in the assessment of autism. Appl Neuropsychol Child 27:1-12.
- Dahiya AV, DeLucia E, McDonnell CG, Scarpa A (2021) A systematic review of technological approaches for autism spectrum disorder assessment in children: Implications for the COVID-19 pandemic. Res Dev Disabil 109:103852.
- Moon SJ, Hwang J, Kana R, Torous J, Kim JW (2019) Accuracy of machine learning algorithms for the diagnosis of autism spectrum disorder: Systematic review and meta-analysis of brain magnetic resonance imaging studies. JMIR ment health 6:e14108.
- Onishchenko Dmytro, Huang Yi, van Horne James, Smith Peter J, Msall Michael E, et al. (2021) Reduced false positives in autism screening via digital biomarkers inferred from deep comorbidity patterns. Sci Adv 7:eabf0354.
- Lingren T, Chen P, Bochenek J, Doshi-Velez F, Manning-Courtney P, et al. (2016) Electronic health record based algorithm to identify patients with autism spectrum disorder. PloS one 11:e0159621.
- Lu A, Perkowski M (2021) Deep Learning Approach for Screening Autism Spectrum Disorder in Children with Facial Images and Analysis of Ethnoracial Factors in Model Development and Application. Brain Sci 11:1446.
- Obafemi-Ajayi T, Miles JH, Takahashi TN, Qi W, Aldridge K, et al. Facial structure analysis separates autism spectrum disorders into meaningful clinical subgroups. J Autism Dev Disord 45:1302–1317.
- Chang Z, Di Martino JM, Aiello R, Baker J, Carpenter K, et al. Computational Methods to Measure Patterns of Gaze in Toddlers With Autism Spectrum Disorder. JAMA pediatr 175: 827-836.
- Ghosh T, Al Banna MH, Rahman MS, Kaiser MS, Mahmud M, et al. Artificial intelligence and internet of things in screening and management of autism spectrum disorder. Sustainable Cities and Society 74:103189.
- Crippa A, Salvatore C, Perego P, Forti S, Nobile M, et al (2015) Use of machine learning to identify children with autism and their motor abnormalities. J Autism Dev Disord 45:2146-2156.
- Nakai Y, Takiguchi T, Matsui G, Yamaoka N, Takada S (2017) Detecting abnormal voice prosody through single-word utterances in children with autism spectrum disorders: machine-learning-based voice analysis versus speech therapists. Percept Mot Skills 124:961-973.
- FDA. Software as a Medical Device (SaMD) FDA.
- Cognoa. (2020) Cognoa Receives FDA Marketing Authorization for First-of-its-kind Autism Diagnosis Aid.
- Abbas H, Garberson F, Liu-Mayo S, Glover E (2020) Multi-modular AI approach to streamline autism diagnosis in young children. Sci Rep 10:1–8.
- Abbas H, Garberson F, Glover E, Wall DP (2018) Machine learning approach for early detection of autism by combining questionnaire and home video screening. J Am Med Inform Assoc 25:1000–1007.
- Duda M, Haber N, Daniels J, Wall DP (2017) Crowdsourced validation of a machine-learning classification system for autism and ADHD. Transl Psychiatry 7:1133–1133.
- Kosmicki JA, Sochat V, Duda M, Wall DP (2015) Searching for a minimal set of behaviors for autism detection through feature selection-based machine learning. Transl Psychiatry 5:514–514.
- Levy S, Duda M, Haber N, Wall DP (2017) Sparsifying machine learning models identify stable subsets of predictive features for behavioral detection of autism. Mol Autism 8:1–17.
- Cortes C, DeSalvo G, Gentile C, Mohri M (2016) Online learning with abstention. Int Conf Mach Learn 9:1059–1067.
- Kompa B, Snoek J, Beam AL (2021) Second opinion needed: communicating uncertainty in medical machine learning. NPJ Digit Med 4:1–6.
- Itzchak E, Zachor DA (2011) Who benefits from early intervention in autism spectrum disorders? Res Autism Spectr Disord 5:345–350.
- Flanagan HE, Perry A, Freeman NL (2012) Effectiveness of large-scale community-based intensive behavioral intervention: A waitlist comparison study exploring outcomes and predictors. Res Autism Spectr Disord 6:673–682.
- Dawson G, Rogers S, Munson J, Smith M (2010) Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Modell. Pediatrics 125:17-19.
- Vivanti G, Dissanayake C (2016) Outcome for children receiving the Early Start Denver Model before and after 48 months. J Autism Dev Disord 46:2441-2449. Indexed at Google Scholar Crossref
- Mazurek MO, Kanne SM, Miles JH (2012) Predicting improvement in social–communication symptoms of autism spectrum disorders using retrospective treatment data. Research in Autism Spectrum Disorders 6:535-545.
- Penev Y, Dunlap K, Husic A, Hou C, Washington P, et al. (2021) A Mobile Game Platform for Improving Social Communication in Children with Autism: A Feasibility Study. Appl Clin Inform 12:1030-1040.
- Ning M, Daniels J, Schwartz J, Dunlap K, Washington P, et al. (2019) Identification and quantification of gaps in access to autism resources in the United States: an infodemiological study. J Med Internet Res 21:e13094.
- Cidav Z, Munson J, Estes A, Dawson G, Rogers S, et al. (2017) Cost offset associated with Early Start Denver Model for children with autism. J Am Acad Child Adolesc Psychiatry 56:777-783.
- Palestra G, De Carolis B, Esposito F (2017) Artificial Intelligence for Robot-Assisted Treatment of Autism17-24.
- Saleh MA, Hanapiah FA, Hashim H (2021) Robot applications for autism: A comprehensive review. Disability and Rehabilitation: Assistive TechnoXlogy 16:580-602.
- Admoni H, Scassellati B (2017) Social eye gaze in human-robot interaction: a review. J hum robot interact 6:25-63.
- Palestra G, Varni G, Chetouani M, Esposito F (2016) A multimodal and multilevel system for robotics treatment of autism in children. InProceedings of the International Workshop on Social Learning and Multimodal Interaction for Designing Artificial Agents 1-6.
- Chevalier P, Martin JC, Isableu B, Bazile C, Iacob DO, et al. (2016) Joint attention using human-robot interaction: Impact of sensory preferences of children with autism. In 2016 25th IEEE International Symposium on Robot and Human Interactive Communication (RO-MAN) 849-854.
- Jaliaawala MS, Khan RA (2020) Can autism be catered with artificial intelligence-assisted intervention technology? A comprehensive survey. Artificial Intelligence Review 53:1039-1069.
- Patnam VS, George FT, George K, Verma A (2017) Deep learning based recognition of meltdown in autistic kids. In2017 IEEE International Conference on Healthcare Informatics (ICHI) 391-396.
- Silva JL, Oliveira I, Topolniak Z, Alvarez AB (2021) A CNN Approach Implemented to Emotional Facial Expression Recognition for the Prevention of Autistic Meltdowns. In2021 2nd Sustainable Cities Latin America Conference (SCLA) 1-6.
- Linstead E, German R, Dixon D, Granpeesheh D, Novack M, et al. (2015) An application of neural networks to predicting mastery of learning outcomes in the treatment of autism spectrum disorder. In2015 IEEE 14th International Conference on Machine Learning and Applications (ICMLA) 414-418.
- Akhtar N, Feeney M (2020) Predictive analytics using a Machine Learning Model to recommend the most suitable Intervention Technology for Autism related deficits. In2020 31st Irish Signals and Systems Conference (ISSC) 1-6.
- Ghafghazi S, Carnett A, Neely L, Das A, Rad P (2021) AI-Augmented Behavior Analysis for Children With Developmental Disabilities: Building Toward Precision Treatment. IEEE Systems, Man, and Cybernetics Magazine 7:4-12.
- Fiske A, Henningsen P, Buyx A (2019) Your robot therapist will see you now: ethical implications of embodied artificial intelligence in psychiatry, psychology, and psychotherapy. J Med Internet Res 21:e13216.
- Davenport T, Kalakota R (2019) The potential for artificial intelligence in healthcare. Future Hosp J 6:94.
- Floridi L, Cowls J, Beltrametti M, Chatila R, Chazerand P, et al. (2021) An Ethical Framework for a Good AI Society: Opportunities, Risks, Principles, and Recommendations. In Ethics, Governance, and Policies in Artificial Intelligence 19-39.
- Kagiyama N, Shrestha S, Farjo PD, Sengupta PP (2019) Artificial intelligence: practical primer for clinical research in cardiovascular disease. J Am Heart Assoc 8:e012788.
- He J, Baxter SL, Xu J, Xu J, Zhou X, et al. (2019) The practical implementation of artificial intelligence technologies in medicine. Nat Med.Nature Publishing Group 25: 30–36.
- Zhang H, Lu AX, Abdalla M, McDermott M, Ghassemi M (2020) Hurtful words: quantifying biases in clinical contextual word embeddings. Proceedings of the ACM Conference on Health, Inference, and Learning: 110–120.
- Ghassemi M, Oakden-Rayner L, Beam AL (2021) The false hope of current approaches to explainable artificial intelligence in health care. The Lancet Digital Health 3: e745-e750.
- Paranjape K, Schinkel M, Panday RN, Car J, Nanayakkara P. (2019) Introducing artificial intelligence training in medical education. JMIR medical education JMIR Publications Inc 5: e16048.
- Banerjee M, Chiew D, Patel KT, Johns I, Chappell D, et al. (2021) The impact of artificial intelligence on clinical education: perceptions of postgraduate trainee doctors in London (UK) and recommendations for trainers. BMC med educ Bio Med Central 21: 1–10.
- Dos Santos DP, Giese D, Brodehl S, Chon SH, Staab W, et al. (2019) Medical students’ attitude towards artificial intelligence: a multicentre survey. European Radiology 29: 1640–1646.
- Sit C, Srinivasan R, Amlani A, Muthuswamy K, Azam A, et al. (2020) Attitudes and perceptions of UK medical students towards artificial intelligence and radiology: a multicentre survey. Insights into imaging Springer 11: 1–6.
- Richardson JP, Smith C, Curtis S, Watson S, Zhu X, et al. (2021) Patient apprehensions about the use of artificial intelligence in healthcare. npj Digit Med 4: 1-6.
- Gordon-Lipkin E, Foster J, Peacock G. (2016) Whittling down the wait time: exploring models to minimize the delay from initial concern to diagnosis and treatment of autism spectrum disorder. Pediatr Clin North Am 63: 851–859.
- Wartman SA, Combs CD. (2018) Medical education must move from the information age to the age of artificial intelligence. Academic Medicine LWW 93: 1107-1109.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Cross Ref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Shannon J, Salomon C, Chettiath T, Abbas H, Taraman S (2022) Autism spectrum disorder and the promise of Artificial Intelligence. J Child Adolesc Behav 10: 428. DOI: 10.4172/2375-4494.1000428
Copyright: © 2022 Shannon J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4356
- [From(publication date): 0-2022 - Dec 12, 2025]
- Breakdown by view type
- HTML page views: 3699
- PDF downloads: 657