Research Article Open Access
Benefit of Hearing Aids on Treatment Outcome in Neuro-Music Therapy for Chronic Tinnitus
Heike Argstatter* and Miriam Grapp
German Centre for Music Therapy Research, DZM e.V. Heidelberg, Germany
- *Corresponding Author:
- Heike A
German Centre for Music Therapy Research DZM e.V. Heidelberg, Germany
Tel: 06221-83 38 60
E-mail: Heike.Argstatter@dzmheidelberg.de
Recieved date: Jan 13, 2016; Accepted date: Feb 3, 2016; Published date: Feb 08, 2016
Citation: Argstatter H, Grapp M (2016) Benefit of Hearing Aids on Treatment Outcome in Neuro-Music Therapy for Chronic Tinnitus. J Biomusic Eng S1:005. doi:10.4172/2090- 2719.S1-005
Copyright: © 2016 Argstatter H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomusical Engineering
Abstract
Background: Epidemiological data indicate that many patients suffering from tinnitus also present some degree of hearing loss. Auditory stimulation procedures are promoted as important therapy option. One music-based intervention is the neuro-music therapy according to the Heidelberg model, a manualized short term intervention comprising nine 50-minutes sessions of individualized music therapy over a period of five consecutive days. The modules are based on the individual tinnitus pitch. We expected that patients presenting hearing loss would benefit from the use of hearing aids and achieve better results than patients not provided with suitable hearing aids.
Methods: A retrospective analysis of patients attending a neuro-music therapy was conducted. Patients were eligible if they suffered from chronic, tonal tinnitus. The target Group consisted of n=40 presenting hearing loss compensated for with suitable hearing loss (=Group A). A matched sample of n=40 patients presenting hearing loss but not provided with hearing loss (Group B) formed the comparison Group and a sample of n=40 patients with normal hearing thresholds (Group C) served as controls. All three samples were stratified according to the variables tinnitus handicap (i.e. initial TQ-score) and gender. For patients in Group A and B also hearing loss in the region of the tinnitus pitch was considered.
Results: There was a significant overall improvement in tinnitus questionnaire total scores (TQ) from baseline (admission) to end of treatment. However about 8 out of 10 patients in Groups A and C accomplished a reliable reduction in TQ scores, compared to about 3 out of 10 patients in Group B. Conclusions: Based on these results, we conclude that a hearing aid can compensate for a hearing loss and improve the chance for positive therapy outcome while if a hearing loss is not compensated for, success rate declines considerably.
Keywords
Music therapy; Tinnitus; Hearing loss; Hearing aids; Acoustic stimulation; Outcome assessment (Health care); Retrospective studies
Introduction
Background
Tinnitus, the enduring perception of ringing or buzzing sounds without an external source, is a complex, multifactorial disease. It affects about 1 in 10 adults and as such is one of the most common symptoms in ENT medicine [1,2]. It has considerable economical impact [3]. Tinnitus can significantly affect quality of life including insomnia, attention deficits and emotional problems such as anxiety, irritability or depression. Three factors leading to tinnitus perception are prominent in discussion [4]: 1. Changes in the sensory input due to damages of inner ear cells, 2. Psychological strains and their underlying neurophysiological network and 3. Changes in the neural activity in the brain.
The tinnitus pitch is associated the frequency spectrum of impaired hearing [5,6]. One reason for this phenomenon is a “central gain” mechanism [7]. The sensory loss of certain frequencies might increase neural activity in the central auditory system in order to compensate for the missing information. Normally, the limbic system may identify and inhibit an irrelevant sensory signal and thus prevent it from reaching consciousness. In case that this limbic regulation is compromised, the ‘noise-cancellation’ mechanism [8] breaks down and the tinnitus signal is relayed to the auditory cortex where it becomes part of conscious perception.
The impaired signal processing consequently downgrades speech intelligibility, deteriorates musical enjoyment and triggers communicative constraints.
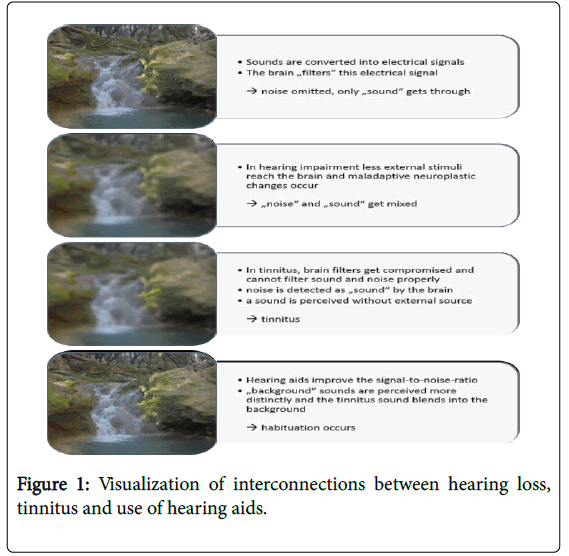
In order to counteract these problems, auditory stimulation procedures are promoted as important therapy options [9,10]. Hearing aids augment the volume of the external natural soundscape while sound generators use synthetic external sounds (broadband-noise, musical stimuli) [11]. If external sounds are amplified, they can be perceived more distinctly and the tinnitus blends into the ambient noise. This makes it more difficult to consciously perceive the tinnitus. Figure 1 depicts a diagram to visualize possible interconnections between hearing loss, tinnitus percept and use of hearing aids especially in tinnitus conditions.
Research has proven that hearing aids might have a considerable impact on tinnitus perception: Up to two-thirds of the patients provided with hearing aids report some relief of their tinnitus when using it [10]. Henry, et al. even state, that “all patients with significant hearing loss need to have previously or simultaneously addressed their amplification requirements as part of maximizing the effectiveness of tinnitus intervention” [12]. Albeit, epidemiological data indicate that many patients suffering from tinnitus and sensorineural hearing loss are not provided with suitable hearing aids [13].
In recent years, a number of music-based interventions and a variety of commercially produced sound based devices such as Neuromonics® [14], tailor-made notched music training (TMNMT) [15], acoustic coordinated reset (CR) neuromodulation [16] were developed. Their underlying notion is the desynchronization of the pathologically increased neuronal synchrony leading to tonotopic changes in the auditory cortex by musical means. Common to these approaches is that the music is spectrally contoured to each individual’s audiometric and tinnitus profile. Recent research [17] certifies that sound therapies are promising to alleviate the perception of tinnitus, though it comes second which kind of sound device is used [18].
In line with these treatments, the neuro-music therapy according to the Heidelberg model [19] tailors the treatment to the individual tinnitus pitch of the patients. Main parts of the training depend on the patients’ ability to discriminate auditory stimuli. Since the neuro-music therapy tackles the tinnitus in an active way and relies on the use of music instruments (e.g. piano, tam-tam gong), the musical stimuli used in the neuro-music therapy are not artificially contoured or altered apart from adapting the volume of the intervention to the hearing thresholds of the patients. The perceived pitch of the tinnitus displays considerable variability in the course of the treatment [20]. Prior to each intervention, the individual tinnitus frequency is assessed by a pitch matching procedure in order to train the appropriate neural regions by the tone sequences. This procedure of individualized and continuously adapted training is unique in comparison to other treatments aiming at directed stimulation of the auditory system [21,22].
Seen from this perspective, we propose that therapy outcome depends on the hearing threshold in the frequency range of the individual tinnitus pitch.
The use of appropriate hearing aids should lift up the hearing threshold considerably enhancing the chance for positive treatment outcome.
Specifically we expected that patients presenting sensorineural would benefit from the use of hearing aids compensating for the hearing loss and achieve better therapy results than patients not provided with suitable hearing aids.
Material and Methods
Trials design
A retrospective analysis of patients attending a neuro-music therapy at the outpatient tinnitus department at the German Center of Music Therapy research during the period 01/01/2012 to 30/06/2015 was conducted.
Patients were eligible for this study if they suffered from chronic, tonal tinnitus and did not present any somatic or psychiatric diagnosis linked to the tinnitus (Table 1).
| Inclusion | Exclusion |
|---|---|
| Clinical diagnosis of chronic tinnitus persisting for a minimum of 6 months | Tinnitus related to anatomic lesions of the ear, toretrocochlear lesions or to cochlear implantation |
| Adults, aged 18 years or older | Tinnitus is concomitant symptom of a known systemic disease (such as Ménière’s disease, vestibular schwannoma, endolymphatic hydrops) |
| Patients are able to understand, read and speak German fluently | Tinnitus is noisiform (broadband or narrow-band noise; e.g. hissing, whooshing) or atonal (kricking, clacking, rumbling) or has different sound components or is pulsatile, intermittent or non-persistent |
| Constant tinnitus (no interruptions >1 hour 6 months before admission) | One or two-sided deafness |
| tinnitus with determinable center frequency à tinnitus is musically educible (e.g. sinus tone, beeping, whistling)” | Clinical diagnosis of severe mental disorder or psychiatric or neurological disease (psychosis, epilepsy, Parkinson’s disease, dementia, alcohol or drug abuse) |
| Inability to discontinue drugs known to be associated with tinnitus (high-dose aspirin, quinidine, aminoglycosides) or psychotropic medication before entry into the study |
Table 1: Inclusion and exclusion criteria.
For the current trial, especially the overall hearing threshold (classified according to WHO grades 0-3 [23]) and the hearing threshold in the region of the predominant tinnitus pitch were of importance. More information on the grades of hearing impairment can be found under http://www.who.int/pbd/deafness/ hearing_impairment_grades/en/.
In case of hearing levels resembling sensorineural (i.e. WHO grades 1-3), it was recorded whether the patients were supplied with sufficient hearing aids, ensuring a hearing level better than 60 dBHL, i.e. at most moderate hearing loss [23] in the tinnitus frequency region. An audiologist rechecked appropriate hearing thresholds prior to the treatment.
Patients fitted with such adequate hearing devices were allocated in Group A, patients diagnosed with hearing loss not compensated for were allocated in Group B. Patients in Group C presented normal hearing thresholds (hearing level better than 25 dB, WHO grade 0).
Examinations
All patients underwent a standardized initial audiological, psychological and otolaryngological examination as recommended by the Tinnitus Research Initiative [24].
Tinnitus pitch was estimated by a pitch-similarity rating. A detailed description of this procedure can be found in [20].
Psychological complaints were assessed using the German version of the tinnitus questionnaire (TQ) [25]. This well validated inventory comprises 52 items and records tinnitus related complaints. The range of global TQ-score values is between the minimum score of 0 and the maximum score of 84, whereas high values indicate high tinnitus related distress.
Outcome and statistical analysis
Primary outcome measure was the change in Tinnitus Questionnaire Total Scores TQ [25] from baseline (admission) (T0) to end of treatment (T1) (immediately after the last session). An investigator different to the therapists administered the TQ.
Statistical analysis was performed using the program IBM SPSS Statistics 22. Level of significance was determined to a p-value <0.05.
Possible baseline differences in demographic or tinnitus related variables were assessed by Pearson’s chi-square test for ordinal data (gender; tinnitus localization) and analysis of variance (if appropriate with Scheffé post-hoc tests) or paired t-test for related samples if variables were parametric (age; initial TQ, tinnitus pitch at admission, tinnitus masking level).
For the outcome criteria “change in TQ” at different assessment times, a repeated measures analysis of variance (two assessment times: baseline (admission), T0 and end of treatment, T1, for three independent samples (Groups A, B, C) was conducted.
Apart from statistical significance, individual changes were assessed according to the concept of clinical significance [26]. This method allows for calculating a reliable change index, i.e. a criterion level above which changes are unlikely to be due to simple measurement unreliability. Patients with a reliable change in TQ-scores are called “responders”. The formula for criterion level RC is 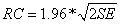 (with
(with 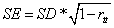 =standard error of the TQ, SD=standard deviation of the norming sample=17.6 and rtt=0.94). For an RC ≥ 1.96, the difference is statistically significant at the 0.05 significance level. In order to compute the RC for the global TQ score, standard deviation and reliability of the TQ manual [25] were used. This yields a critical difference of 6.1 points.
=standard error of the TQ, SD=standard deviation of the norming sample=17.6 and rtt=0.94). For an RC ≥ 1.96, the difference is statistically significant at the 0.05 significance level. In order to compute the RC for the global TQ score, standard deviation and reliability of the TQ manual [25] were used. This yields a critical difference of 6.1 points.
In order to predict parameters possibly influencing therapy outcome, a logistic regression model was calculated using “therapy success” (reliable change in the TQ yes/no) as binary dependent variable and the following variables as independent variables: demographic factors (gender, age), tinnitus related factors (initial tinnitus severity in the TQ, tinnitus frequency, time since onset of tinnitus, lateralization) and audiometric factors (hearing loss WHO grading, minimal masking level).
Intervention: the Heidelberg model of music therapy
The "Heidelberg model of music therapy" is a manualized short term music therapeutic intervention.
The therapy course is completed in five consecutive days with two therapy sessions per day. Each morning and each afternoon session lasts for 50 minutes, thereof 25 minutes of active music therapy and 25 minutes of receptive music therapy. The therapy is carried out by two trained music therapists. One therapist performs the active modules, the other the receptive modules. The interventions are structured into distinct modules (Table 2 depicts a treatment schedule)
| Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|
| Morning Session | •TPE | •TPE | •TPE | •TPE | •TPE |
| •Counselling | •RT (Control) | •RT •NACP |
•RT •NACP |
•RT (transfer to daily life application); •NACP |
|
| •RT (Introduction) | •RelaxT (Consolidation) | •HT | •SM | •SM: Real-life-simulation | |
| Interval (morning-afternoon) | •RT hourly for 3 minutes | •RT hourly for 3 minutes •RelaxT hourly for 5 minutes |
•RT hourly for 3 minutes •RelaxT hourly for 5 minutes |
•RT hourly for 3 minutes •RelaxT once |
|
| Afternoon Session | •TPE | •TPE | •TPE | •TPE | |
| •RT (check of correct execution) | •RT •NACP |
•RT •NACP |
•RT •NACP |
||
| •RelaxT (Introduction) | •RelaxT •HT |
•SM | •SM | ||
| Interval (overnight) | •RT hourly for 3 minutes •Creation of a mental “place of retreat” |
•Tinnitus-Map •RT hourly for 3 minutes •RelaxT hourly for 5 minutes |
•RT hourly for 3 minutes •RelaxT bi-hourly for 5 minutes |
•RT hourly for 3 minutes •RelaxT once |
Table 2: Treatment schedule.
In the beginning of the therapy, all patients receive a comprehensive individualized personal instruction. This counselling lasts for 50 minutes and is delivered by a trained psychologist. A cognitive model of tinnitus based on neuroscientific principles should be established.
The active music therapy modules consist of a “Resonance Training” and a “Neuroauditive Cortex Training”. Prior to these active modules, the individual tinnitus pitch is measured since the modules are based on the individual tinnitus pitch. The “Resonance Training” is a purely vocal exercise and intended to stimulate the auditory pathway by means of somatosensory innervations. The patients have to practice the Resonance Training for three minutes every hour during the interval between the sessions.
The “Neuroauditive Cortex Training” contains a detailed “intonation-training”. A trained therapist performs tone sequences on a piano, which the patients are required to sing along. The tone sequences are composed of unfamiliar, atonal combinations of subsequent single tones in ad lib performance based on the individual tinnitus pitch. By a systematic and targeted training of inaccurately intonated musical the patients increased their ability to actively filter out irrelevant information and to concentrate on relevant acoustic stimuli. In addition to an increased auditory attention control this training aimed at a neuronal reorganization of the auditory cortex.
“Tinnitus Reconditioning” has distinct features which combines musical distractors with psychophysiological relaxation and imagination of mental well-being. This module increases the auditory filtering and supports a modified emotional processing of the tinnitus sound. Patients listen to prerecorded relaxation music and imagine a positive autobiographic episode (e.g. reminiscence of a holiday experience) which serves as anchor stimulus. During the relaxation exercise, the tinnitus sound is integrated intermittently into the background music. Subsequently, the patients have to set up compilation of situations aggravating or intensifying the tinnitus (‘tinnitus map’). The patients visualize these aversive situations mentally during the relaxation and thus learn to decouple tinnitus and aversive associations. The volume of the music is adapted to the individual hearing level compensating for a potential hearing deficit. For this purpose, before the training session started, the patients have to set the background music to a convenient level so they could easily listen to the music while still being able to follow verbal instructions from the therapist.
The treatment modules have been described in more detail by Argstatter, et al. [19].
Results
Patients
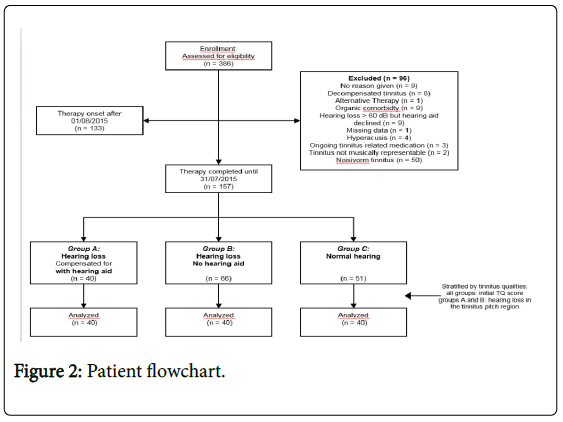
Overall n=386 were enrolled in an initial intake examination. N=96 patients had to be excluded because they met exclusion criteria, another n=133 patient did not terminate their therapy by 30/06/2015. N=157 patients met the inclusion criteria and completed their music therapy by 31/08/2013. Among them, n=106 patients presented sensorineural requiring hearing aids but only n=40 were provided with suitable hearing aids. These patients represented the target Group and were allocated to Group A. The remaining n=66 patients served as comparison Group and were allocated to Group B. N=51 patients had normal hearing capacities (WHO grade 0) and formed the control Group C.
In order to avoid a distortion of results due to influencing variables such as initial tinnitus strain, hearing loss in the region of the tinnitus pitch and gender, a sample of n=40 patients from the subgroup B and C was taken and analysed. All three samples were stratified according to the two variables “initial TQ-score” and “gender”. Since the patients in Group C had no hearing loss, only for patients in Groups A and B hearing loss in the region of the tinnitus pitch had to be considered. Figure 2 depicts the patient flow.
Baseline data
Due to the stratification, there were no significant differences between the patients groups presenting hearing loss (Groups A and B) in the variables age (t-test: t(78)=-1.13, p=0.262), hearing loss in the range of the tinnitus frequency (t-test: t(88)=-0.77, p=0.442) and initial tinnitus strain (TQ-score; t-test: t(78)=-1.49, p=0.140). Patients in need for wearing hearing aids (Groups A and B) were older than normal hearing patients (Group C) (ANOVA F(2,117)=11.02, p<0.001) (Table 3).
| Group A (Hearing Loss+Hearing Aid) | Group B (Hearing Loss+no Hearing Aid) | Group C (normal Hearing) | Statistics | |
|---|---|---|---|---|
| Age | 60.0 ± 12.4 (18/78) | 57.0 ± 11.7 (30/82) | 48.3 ± 10.4 (20/67) | ANOVA: p<0.00 A-B: p=0.501; A-C: p=0.000; B-C: p=0.005 |
| Gender [âÂ?Â?/âÂ?Â?] | 27/13 | 26/14 | 22/18 | c2: p =0.474 |
| Tinnitus frequency [Hz] [M± SD. Min/Max] | 4904 ± 2397 (1004/11.504) | 5878 ± 2423 (442/11.947) | 6173 ± 3300 (300/11.177) | ANOVA: p=0.100 |
| Duration of tinnitus [yrs] [M± SD. Min/Max] | 10.8 ± 9.5(1/32) | 9.0 ± 8.9 (1/38) |
5.3 ± 6.5(1/25) | ANOVA: p=0.016 A-B: p=0.631; A-C: p=0.018; B-C: p=0.168 |
| Localisation Tinnitus [right/left/both sides] | 13/8/19 | 4/7/2029 | 6/9/2025 | c2: p=0.086 |
| Mean hearing level1 right ear[dB HL][M± SD. Min/Max] | 38.9 ± 14.3 (18/69) | 26.9 ± 12.5 (6/69) | 11.4 ± 4.8(1/24) | ANOVA: p<0.001 A-B/A-C/B-C: p<0.000 |
| Mean hearing level 1 left ear [dB HL][M± SD.Min/Max] | 35.0 ± 13.1 (15/64) | 25.4 ± 8.3 (13/46) | 10.8 ± 4.0(4/21) | ANOVA: p<0.001 A-B/A-C/B-C: p<0.000 |
| Hearing loss WHO grading right ear [Grade 0/1/2/3/4] | 9/14/14/3/0 | 25/10/4/1/0 | 40/0/0/0/0 | A-B χ2: p=0.002 |
| Hearing loss WHO grading left ear [Grade 0/1/2/3/4] | 11/16/11/2 | 23/14/3/0/0 | 40/0/0/0/0 | A-B χ2: p=0.012 |
| Mean hearing level tinnitus frequency right ear2 [dB HL] [M± SD. Min/Max] | 58.1 ± 13.2 (40/95) | 54.0 ± 16.1 (30/100) | 14.2 ± 6.2(5/25) | ANOVA: p<0.001 A-B: p=0.420A-C/B-C:p<0.000 |
| Mean hearing level tinnitus frequency left ear3 [dB HL] [M± SD. Min/Max] | 52.1 ± 12.8 (30/75) | 52.1 ± 14.5 (30/85) | 14.9 ± 5.7(5/23) | ANOVA: p<0.001A-B: p=1.000A-C/B-C: p<0.000 |
| Hearing loss WHO grading right ear tinnitus frequency2 [Grade 0/1/2/3/4] | 0/3/17/11/1 | 0/9/16/6/2 | 31/0/0/0/0 | A-B χ2: p=0.185 |
| Hearing loss WHO grading left ear tinnitus frequency3 [Grade 0/1/2/3/4] | 0/7/16/4/0 | 0/10/19/6/1 | 34/0/0/0/0 | A-B χ2: p=0.659 |
| Tinnitus loudness[dB HL] [M± SD.Min/Max] | 56.0±11.7 (33/78) | 53.7±14.9 (31/100) | 14.9±5.7(5/25) | ANOVA: p<0.001 A-B: p=0.664 A-C/B-C: p<0.000 |
Table 3: Patients and symptoms at baseline.
Outcomes and estimation
Main outcome criterion: TQ change: At baseline, all patients presented nearly identical TQ values (Group A mean=38.7 points, Group B mean=34.4 points, Group C mean=34.5 points; p=0.218).
Overall, there was a significant improvement in TQ scores (Table 4) (repeated ANOVA, main effect time F(117,1)=162.4, p<0.001). Although, the groups performed unequal (interaction Group X Time F (117,2)=15.9, p<0.001): patients in Group A and Group C achieved a higher reduction by median 12.0 points (Group A) and 14.5 points (Group C) compared to Group B with a reduction by median 3.0 points. There was no statistically significant difference between Group A and C (p=0.676) but Groups A and C outperformed Group B in statistically equal measure (p<0.001 respectively).
| Group A (Hearing loss + Hearing Aid) | Group B (Hearing loss + no hearing aid) | Group C (normal hearing) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TQ-Pre | 38.7 ± 11.6 | 34.4 ± 13.8 | 34.5 ± 11.7 | |||||||
| TQ-Post | 26.3 ± 10.7 | 30.6 ± 13.8 | 20.3 ± 11.0 | |||||||
| TQ-Pre-Post absolute | 12.4 ± 9.1 | 3.9 ± 7.6 | 14.1 ± 9.2 | |||||||
| TQ-Pre-Post relative | 32% ± 24% | 9% ± 24% | 42% ± 25% | |||||||
| Repeated ANOVA for TQ scores pre-post | ||||||||||
| ANOVA | Scheffé post-hoc | |||||||||
| F-value | df | p | ||||||||
| Main effect time (Pre vs. Post) |
162.4 | 132.1 | <0.001 | |||||||
| Main effect group (Group A, B, C) |
2.6 | 132.2 | 0.075 | |||||||
| Interaction group x time | 15.9 | 132.2 | <0.000 | Group A | Group B <0.001 | |||||
| Group C 0.676 | ||||||||||
| Group B | Group C <0.001 | |||||||||
Table 4: TQ scores and statistical results.
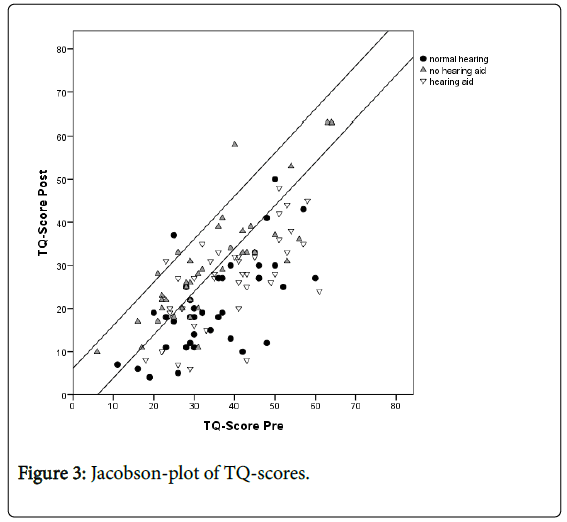
According to the concept of “clinical significance”, about 8 out of 10 patients in Groups A (hearing loss with hearing aid) and C (normal hearing) accomplished a reliable reduction in TQ scores (Table 5) (“positive responder”), compared to about 3 out of 10 patients in Group B (hearing loss without hearing aid). There was only one negative responder in Groups A and C respectively, while there were three negative responders in Group B. A chi-square test backed up these group differences (χ2(4)=28.4, p<0.001). Figure 3 depicts a Jacobson-plot of TQ-scores indicating the proportions of patients with reliably changed scores, responders and non-responders.
| Pre-Post | |||
|---|---|---|---|
| Group A | Group B | Group C | |
| ”Positive Responder“ | (Hearing Loss+Hearing Aid) | (Hearing loss+no hearing aid) | (normal hearing) |
| 77.5% | 32.5% | 85.0% | |
| =reliable improvement (more than-6.1 TQ-points) | (n=31) | (n=13) | (n=34) |
| “Non-Responder“ | 20.0% | 60.0% | 12.5% |
| =no change | (n=8) | (n=24) | (n=5) |
| (less than ± 6.1 TQ-points | |||
| “Negative Responder” | 2.50% | 7.50% | 2.50% |
| =reliable deterioration (more than+6.1 TQ-points) | (n=1) | (n=3) | (n=1) |
Table 5: Individual changes in Tinnitus-Questionnaire Scores according to the concept of “clinical significance”.
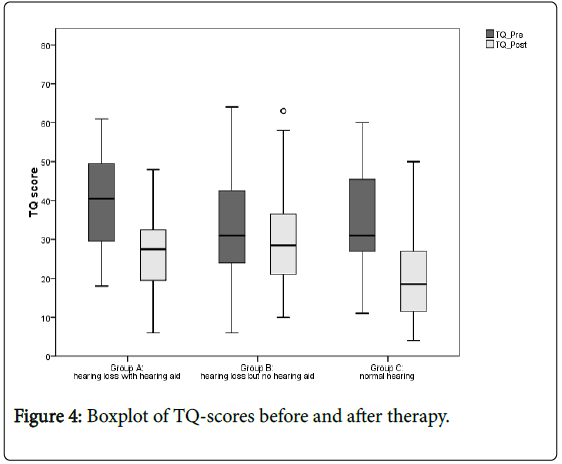
In a further analysis, the chance for positive vs. negative therapy outcome was estimated using odds ratio calculation. Positive outcome was expressed as reliable improvement according to the concept of clinical significance (i.e. more than 6.1 points decline in TQ scores), negative outcome was both no-change or reliable deterioration (less than 6.1 points decline in TQ scores) (Figure 4). Compared to patients in Group B, patients from both Group A and C had an 3-times higher chance for positive therapy outcome (OR: 95% CI=1.6-5.5; χ2(1)=16.4, p<0.001) and compared to Group B patients from Group A had a 2.6- times higher chance for positive outcome (OR: 95% CI=1.6-4.2; χ2 (1)=22.7, p<0.001).
There was no statistical difference between patients from Groups A and C (OR 95%-CI=0.9-1.4; χ2(1)=0.39, p=0.390.
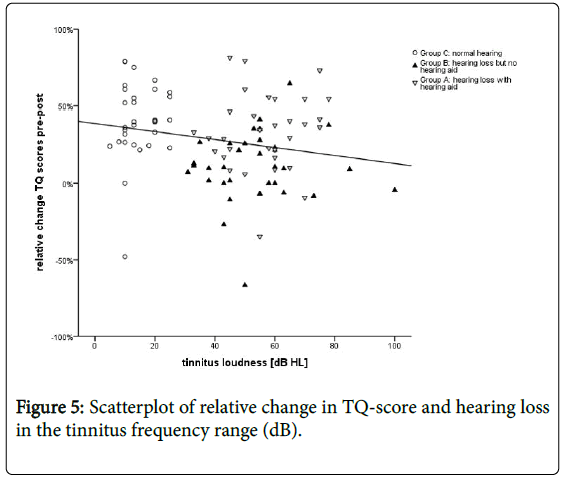
There was a negative correlation between change in TQ-scores and hearing loss in the region of the tinnitus pitch (Spearmen-rho=-0.327, p<0.001) (Figure 5).
Discussion
Music therapy is a short term intervention with considerable impact on the subjective tinnitus percept. Mean overall reduction in TQscores was estimated to about 10.1 ± 9.7 points (or 27 ± 28%). About two thirds of all patients in our sample were positive responders in the sense of clinical significance. These numbers are in line with results from previous trials [19,27].
The main question of the current trial was to find out, if and to the extent to which hearing aids have an impact on therapy outcome.
The analysis of the three groups under consideration revealed distinct differences. Patients in Groups A (hearing loss with hearing aid) and Group C (normal hearing) did not perform differently and achieved a nearly equal reduction in TQ-scores and similar proportions of responders. Accordingly, the chance for positive therapy outcome expressed as odds ratio was equal for patients from Group A compared to group C (OR of about 1.0). In contrast, patients in Group B (hearing loss without hearing aid) were significantly worse off than the patients in Groups A and C in terms of overall TQ-score reduction and proportion of responders. In Group B, three patients experienced an aggravation of their symptoms as revealed by clinically significant higher TQ scores after therapy (negative responders) compared to one patient in Groups A and C respectively. This poor result was reflected by the Odds ratio calculation that revealed an about three times higher chance for positive therapy outcome for patients from Groups A (2.6) and C (3.3) contrasted with patients from Group B.
A further correlation analysis backed up this notion since there was a significant negative correlation between hearing threshold in the region of the tinnitus pitch and relative change in TQ-scores-the higher the masking level, the lower the decline in TQ scores. This linear relationship may be discussed as evidence of the dependency of the therapy success on hearing capacity in the range of the tinnitus frequency.
Based on these results, we conclude that a hearing aid can compensate for a hearing loss and improve the chance for positive therapy outcome while if a hearing loss is not compensated for, success rate declines considerably.
Generally it seems essential to account for the individual hearing loss in acoustic training. Sound based interventions contour their musical stimuli to the patients audiometric and tinnitus profile in order to ensure that the training stimuli reach the auditory cortex in an appropriate way.
In literature exists sparse data only concerning the impact of hearing loss and/or compensation of hearing loss. The only controlled trial investigating a so called “music therapy” [28] revealed contrary results: The stimuli used in that trial were spectrally contoured musical files either compensating for hearing loss or even overcompensating hearing loss. While stimuli compensating for the hearing loss were not beneficial in suppressing tinnitus, overcompensating hearing loss actually worsened tinnitus, both clinically and electrophysiologically.
From a music therapeutic point of view however, passive sounding is not a music therapy as circumscribed by the world federation of music therapy (WFMT) [29]. The WFMT defines music therapy as “the use of music and/or its musical elements (sound, rhythm, melody and harmony) by a qualified music therapist, with a client or group, in a process designed to facilitate and promote communication, relationships, learning, mobilisation, expression, organisation and other relevant therapeutic objectives in order to meet physical, emotional, mental, social and cognitive needs.”
Accordingly, the Neuro-Music Therapy concept presented here pursues another approach. One key issue of the neuro-music therapy is to ensure that the regime is intrinsically motivating. Main parts of the therapy consist of active music therapy modules-and active music therapy guarantees the lasting co-operation of the patients as reflected in the very low dropout rates during therapy. As regards content of the therapy, the patients tackle their individual tinnitus sounds in an active way. The music therapy makes use of specific tonal material, matched to the individual tinnitus frequency as suggested by animal models [30] and research on tinnitus treatments [31]. It offers a training at both normal hearing frequencies and in the region of hearing loss or tinnitus frequency. Hence there is no need and no purpose for spectrally changing the music used [14,32], which considerably facilitates the application of the treatment. However, as the results indicate, the hearing capacity heavily influences therapy outcome: patients have to be able to process the stimuli used-and the better the hearing the greater the chance for positive therapy outcome.
Generalizability, limitations and future directions
The patients presenting hearing loss in our trial are representative for the “hearing loss patient” compared to other trials [33]. In our sample, patients presenting normal hearing thresholds (Group C) were about 10 years younger than patients with hearing loss (Groups A and B). This result is not surprising since hearing loss usually is age related and in a retrospective analysis, it is difficult to find age-matched normal hearing controls. Even though results from previous studies [19,34], did not reveal an influence of age on therapy outcome, in a future trial, patients should be stratified according to age controlling for this intervening variable.
Patients supplied with hearing aids (Group A) reported more monaural tinnitus than the two other groups. Possibly this factor has to be taken into consideration in future since unilateral tinnitus might have different causes than binaural tinnitus and might affect the patients in a different way, influencing therapy outcome.
Another pending issue is the way patients deal with their hearing impairment. Different coping styles have been proven to correlate with neuronal activities in chronic tinnitus [35]. Adaptive coping strategies might enhance both the acceptance of the hearing impairment and the necessity of hearing aids. Those patients possibly present higher internal motivation to actively engage in a therapy for the accompanying tinnitus. Maladaptive coping strategies on the other hand foster passive and avoidance strategies e.g. simply not acknowledging the hearing loss. Those patients might neglect the use of hearing aids and expect a more medicinal treatment outline-thus diminishing the personal responsibility and engagement of the patients necessary for a treatment such as the music therapy. A detailed assessment of coping styles might be fruitful to further explore these assumptions. Currently, a prospective controlled trial addressing this question is under way.
From previous studies it is known, that the majority of patients attending a music therapy treatment does not undergo any further treatment-apart from hearing aid fitting [34]. Therefore it would be of interest, to evaluate long term effects of this therapy (one to two years from the last session) with regard to hearing aid use. Probably some of the patients not provided with hearing aids at the time of the music therapy might reconsider fitting hearing aids. Probably, a continued lack of amplification will have prolonged negative impact on long term outcomes.
The impact of the neuro-music therapy has been evaluated by objective measurement, such as cortical evoked potentials [36] and brain imaging data [37] proving a positive effect on both objective hearing level and neuronal auditory structures. The assessment of auditory profiles incorporating audiometry and electroencephalography in order to evaluate a possible influence of hearing threshold is scheduled for a future trial.
References
- Heller AJ (2003) Classification and epidemiology of tinnitus. Otolaryngol. Clin North Am 36: 239-248.
- Hoffmann HJ, Reed G (2004) Epidemiology of tinnitus. Theory and management. Sales and distribution, BC Decker, Hamilton, Ont, Lewiston, NY, USA.
- Maes IHL, Cima RFF, Vlaeyen JW, Anteunis LJC, Joore MA (2013) Tinnitus: A Cost Study. Ear Hear 34:508-514.
- Langguth B, Kreuzer PM, Kleinjung T, Ridder D de (2013) Tinnitus: causes and clinical management. The Lancet Neurology 12: 920-930.
- Goebel G, Hiller W (2001) STI Strukturiertes Tinnitus-Interview. Hogrefe, Göttingen, Germany.
- Koelsch S (2009) A neuroscientific perspective on music therapy. Ann N YAcad Sci 1169: 374-384.
- Noreña AJ (2011) An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev 35:1089-1109.
- Rauschecker JP, Leaver AM, Mühlau M (2010) Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66: 819-826.
- Deutsche Gesellschaftfür Hals-Nasen-Ohren-Heilkunde (2015) Leitlinie "Tinnitus”.
- Shekhawat GS, Searchfield GD, Stinear CM (2013) Role of hearing aids in tinnitus intervention: a scoping review. J Am Acad Audiol 24: 747-762.
- Hobson J, Chisholm E, RefaieEA (2012) Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev 11: CD006371.
- Henry JA, Zaugg TL, Myers PJ, Schechter MA (2008) Using Therapeutic Sound With Progressive Audiologic Tinnitus Management. Trends Amplif 12: 188-209.
- Zarenoe R, Ledin T (2013) A cohort study of patients with tinnitus and sensorineural hearing loss in a Swedish population. AurisNasus Larynx 40: 41-45.
- Hanley PJ, Davis PB (2008) Treatment of tinnitus with a customized, dynamic acoustic neural stimulus: underlying principles and clinical efficacy. Trends Amplif 12: 210-222.
- Okamoto H, Stracke H, Stoll W, Pantev C (2010) Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci U.S.A. 107: 1207-1210.
- Tass PA, Adamchic I, Freund H, Stackelberg T von, Hauptmann C (2012) Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restor Neurol Neurosci 30: 137-159.
- Hoare DJ, Adjamian P, Sereda M, Hall DA (2013) Recent technological advances in sound-based approaches to tinnitus treatment: a review of efficacy considered against putative physiological mechanisms. Noise Health 15: 107-116.
- Newman CW, Sandridge SA (2012) A comparison of benefit and economic value between two sound therapy tinnitus management options. J Am Acad Audiol 23: 126-138.
- Argstatter H, Grapp M, Hutter E, Plinkert PK, Bolay HV (2015) The effectiveness of Neuro-Music Therapy according to the Heidelberg model compared to a single session of educational counselling as treatment for tinnitus: a controlled trial. J Psychosom Res78: 285-292.
- Hutter E, Grapp M, Argstatter H, Bolay HV (2014) Music therapy for chronic tinnitus: variability of tinnitus pitch in the course of therapy. J Am Acad Audiol 25: 335-342.
- Herraiz C, Diges I, Cobo P, Aparicio JM, Toledano A (2010) Auditory discrimination training for tinnitus treatment: the effect of different paradigms. Eur Arch Otorhinolaryngol 267: 1067-1074.
- Teismann H, Okamoto H, Pantev C (2011) Short and intense tailor-made notched music training against tinnitus: the tinnitus frequency matters. PLoS ONE 6: e24685.
- World Health Organization (2014) Grades of hearing impairment.
- Langguth B, Goodey R, Azevedo A, Bjorne A, Cacace A, et al. (2007) Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog Brain Res 166: 525-536.
- Goebel G, Hiller W (1998) Tinnitus-Fragebogen (TF); ein Instrument zurErfassung von Belastung und Schweregradbei Tinnitus; Handanweisung. HogrefeVerl. fürPsychologie, Göttingen.
- Jacobson NS, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 59: 12-19.
- Argstatter H, Grapp M, Plinkert PK, Bolay HV (2012) Heidelberg Neuro-Music Therapy for chronic-tonal tinnitus-treatment outline and psychometric evaluation. Int Tinnitus J 17: 31-41.
- Vanneste S, van Dongen M, Vree B, Hiseni S, van der Velden E, et al. (2013) Does enriched acoustic environment in humans abolish chronic tinnitus clinically and electrophysiologically? A double blind placebo controlled study. Hear Res 296: 141-148.
- World Federation of Music Therapy (2011) Definition of music therapy.
- Noreña AJ, Eggermont JJ (2005) Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci 25: 699-705.
- Pineda JA, Moore FR, Viirre E (2008) Tinnitus treatment with customized sounds. Int Tinnitus J 14: 17-25.
- Sweetow RW, Sabes JH (2010) Effects of acoustical stimuli delivered through hearing aids on tinnitus. J Am Acad Audiol 21: 461-473.
- Martines F, Bentivegna D, Martines E, Sciacca V, Martinciglio G (2010) Assessing audiological, pathophysiological and psychological variables in tinnitus patients with or without hearing loss. Eur Arch Otorhinolaryngol 267: 1685-1693.
- Argstatter H, Grapp M, Hutter E, Plinkert P, Bolay HV (2012) Long-term effects of the "Heidelberg Model of Music Therapy" in patients with chronic tinnitus. Int J Clin Exp Med 5: 273-288.
- Vanneste S, Joos K, Langguth B, To WT, Ridder D de (2014) Neuronal correlates of maladaptive coping: an EEG-study in tinnitus patients. PLoS ONE 9: e88253.
- Low YF, Argstatter H, Bolay HV, Strauss DJ (2008) Evaluation of a compact tinnitus therapy by electrophysiological tinnitus decompensation measures. Conf Proc IEEE Eng Med Biol Soc 2008: 5132-5135.
- Krick CM, Grapp M, Daneshvar-Talebi J, Reith W, Plinkert PK, et al. (2015) Cortical reorganization in recent-onset tinnitus patients by the Heidelberg Model of Music Therapy. Front Neurosci (Frontiers in neuroscience) 9: 49.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11375
- [From(publication date):
specialissue-2016 - Aug 04, 2025] - Breakdown by view type
- HTML page views : 10412
- PDF downloads : 963