Bioavailability Study of Gingerol and Ginger Extract Capsule in Healthy, Adult, Human Subjects under Fasting Conditions
Received: 03-Apr-2024 / Manuscript No. WJPT-24-128784 / Editor assigned: 08-Apr-2024 / PreQC No. WJPT-24-128784 (PQ) / Reviewed: 23-Apr-2024 / QC No. WJPT-24-128784 / Revised: 10-Mar-2025 / Manuscript No. WJPT-24-128784 (R) / Published Date: 17-Mar-2025
Abstract
A comparative bioavailability study was conducted to assess gingerol and ginger extract capsule in healthy adult human subjects under fasting conditions. The study employed an open-label, balanced, randomized, single-dose, two-treatment, two-period, two-sequence, two-way crossover design. The genus Zingiber, encompassing ginger (Zingiber officinale Roscoe), was the focus due to its pharmacological properties, particularly its antiemetic effects attributed to its constituents, including gingerols. Thirty normal healthy adult male subjects were recruited, receiving oral doses of ginger extracts standardized to 5% gingerols, ranging from 100 mg to 2.0 g. Rapid absorption was observed with Tmax values ranging from 55 to 65.6 minutes and elimination half-lives ranging from 75 to 120 minutes at the highest dose. No serious adverse events were reported during active medication periods. The study indicated a statistically significant effect of gingerol in the initial treatment period, with no significant difference observed overall.
Keywords
Gingerol; Ginger extract capsule; Oral dose; Concentration of drug, Human subjects; Male subjects
Introduction
Bioavailability and Bioequivalence studies play a major role in developing new drug products and their generic equivalents and thus attract considerable attention globally. Bioequivalence is a strategy to introduce generic equivalents of brand name drugs to lower the cost of medication through proper assessment as directed by the international regulatory authorities.
Therefore, a thorough understanding is required for bioavailability and bioequivalence concepts and basic regulatory considerations for conducting bioavailability and bioequivalence studies [1]. The original manufacturer of the medication has made minor modifications to the manufacturing process or reformulated the drug product in ways that can be persuasively argued to have no bearing on the bioavailability [2]. It is advised to conduct a BE study under fed conditions in addition to a BE study under fasting conditions when administering oral immediate-release drug products [3]. Details of the general approach are provided in the following sections [4]. A larger acceptance range may be acceptable in exceptional cases if justified clinically [5]. Moreover, Central Drugs Standard Control Organization (CDSCO) mentioned in the guidelines that bioavailability and bioequivalence data is therefore required to be furnished with applications for new drugs, as required under schedule Y, depending on the type of application being submitted [6]. In some cases, “bioinequivalence” is observed because of insufficient numbers of subjects entered into the BE study [7]. Healthcare professionals, the pharmaceutical industry, consumers and government officials are all concerned about generic substitution. Numerous scholarly articles have raised concerns about the approval standards for generic products, which may not always guarantee therapeutic equivalency. [8]. Unfavorable physiochemical properties, e.g. low solubility, metastable modifications, instability etc. Documented evidence for bioavailability problems [9]. The feeling that one is about to vomit is called nausea, which usually proceeds, but does not always lead to vomiting. Healthy adult human male volunteers participated in the study. The screening procedure includes demographic data, laboratory investigations and physical examination. The inclusion criteria for the study, their age should be within 18-45 years of age and Body Mass Index (BMI) should be within, 18.5 kg/m2-24.99 kg/m2. The volunteers should have no history of using tobacco, known history of alcoholism or drug abuse, past and present status of drug addiction or history of smoking or alcohol intake. Study participants should have no history of blood donation or history of participation in a drug research study for 90 days prior to participating in the study. Medical history, including any past and present illness, any chronic illness, persistent cough and any family history of diseases and history of any allergies (food/drug/any other) data was obtained from the volunteers. Physical and medical examination was performed for each subject, which included checking of vital signs (Blood Pressure (BP), pulse, temperature and respiration). Recording of ECG was performed through 12-lead electrodes for checking heart rate, rhythm and specific findings (if any). Chest X-ray (PA view) and systemic examinations like cardiovascular, respiratory, abdomen, nervous and musculoskeletal were done. The heart and aorta should be within normal range, lung fields should be clean, the bony cage should appear normal and the diaphragm should be clear. The subjects should be free from liver disease and abstained from taking any drug for two weeks prior to and during the study period. Drinking alcoholic beverages, coffee and tea was not allowed at least one month prior to and during the entire period of the study. One method of examining laboratory parameters is a Complete Blood Count (CBC). Within 60 hours, metabolites from an oral dosage of 50 mg/kg of 6-gingerol were eliminated as bile (48%) and urine (16%). Zingerone at 100 mg/kg was shown to have comparable elimination patterns to 6-gingerol, with 40% excreted in urine and 50% in feces over the course of 24 hours. Emesis occurring at two times median tmax or earlier.
Materials and Methods
Laboratory report for volunteers
In our study, we screened a total of 58 volunteers. Out of 58 volunteers, 30 volunteers passed and 28 volunteers failed because of below mentioned reasons (Table 1).
| Total number of volunteers screened for the study | 58 |
| Number of volunteers passed during screening | 30 |
| Number of volunteers failed during screening | 28 |
Table 1: No. of volunteers.
The present study we screened total 60 volunteers. Out of 58 volunteers, 30 volunteers passed and 28 volunteers failed because some variable changes in laboratory parameters like high plasma glucose, serum albumin was more than normal range, high LDL cholesterol, high alkaline phosphate, high blood pressure, low haemoglobin, RBC count was low, detail of screen failure summarized in Table 2.
|
Volunteers |
Reason of failure |
|
8 volunteers |
High plasma glucose |
|
4 volunteers |
Serum albumin was more than normal range |
|
3 volunteers |
High LDL cholesterol |
|
2 volunteers |
High alkaline phosphate |
|
5 volunteers |
High blood pressure |
|
3 volunteers |
Low hemoglobin |
|
3 volunteers |
RBC count was low |
Table 2: Evaluation of screen failure.
The mean age, height, weight and BMI ± SD values for normal healthy human subjects recruited in the study and those analyzed were 28.77 ± 5.207 years, 166.915 ± 5.408 Kg, 22,075 ± 3.089 Kg/m2 respectively. The demographic data was summarized in Table 3.
| Age (year) | Height (cm) | Weight (kg) | BMI (kg/m2) | |
| Number | 32 | 32 | 32 | 32 |
| Median | 28 | 167 | 62 | 22.55 |
| Mean | 28.774 | 166.915 | 61.521 | 22.075 |
| Standard deviation | 5.207 | 5.408 | 8.488 | 3.089 |
| Minimum | 41 | 179 | 75 | 25.96 |
| Maximum | 20 | 154 | 16 | 1.51 |
Table 3: Evaluation of demographic and other baseline characteristics.
Body temperature for period I analysed: 98.03333 ± 0.152753, period II: 98.13333 ± 0.305505. Respiratory rate for period I analysed: 22.66667 ± 1.154701, period II: 55.33333 ± 1.154. Pulse rate for period I analysed: 76 ± 2, period II: 78 ± 2. Summarized in Table 4.
|
Group |
Body temperature (F0) |
Respiratory rate (/min) |
Pulse rate (/min) |
|||
|
Average |
Average |
Average |
||||
|
Period I |
Period II |
Period I |
Period II |
Period I |
Period II |
|
|
21-25 years |
97.9 |
97.8 |
22 |
24 |
78 |
80 |
|
26-30 years |
98 |
98.2 |
24 |
26 |
76 |
78 |
|
31-35 years |
98.2 |
98.4 |
22 |
26 |
74 |
76 |
|
36-40 years |
98 |
98.4 |
20 |
24 |
72 |
74 |
Table 4: Evaluation of vital signs.
Haemoglobin range for period I: 15.05667 ± 0.628039 (g/dl) and period II: 13.975 ± 0.5245(g/dl)
Total RBC count for period I: 5.78125 ± 0.157766 (μl) and period II: 4.66425 ± 0.720262 (μl)
Total WBC counts for period I: 7.9275 ± 0.670358 (μl) and period II: 7.91 ± 0.654037 (μl) summarized in Table 4
Platelet count for period I: 2.822 ± 0.185035 and period II: 2.70325 ± 0.144368 (lakhs/μl). Summarized in Table 5.
| Age | 21-25 years | 26-30 years | 31-35 years | 36-40 years | ||||
| P I | P II | P I | P II | P I | P II | P I | P II | |
| Haematology | ||||||||
| Hemoglobin (g/dL) | 14.74 | 13.72 | 14.7 7 | 13.87 | 15.78 | 13.57 | 14.65 | 14.74 |
| Total RBC count (μL) | 5.836 | 4.076 | 5.546 | 5.078 | 5.876 | 4.036 | 5.867 | 5.467 |
| Total leucocyte count (μL) | 7.745 | 7.477 | 8.846 | 7.745 | 7.243 | 8.876 | 7.876 | 7.542 |
| Differential count | ||||||||
| Platelet count (lakhs/μL) | 2.985 | 2.87 | 2.867 | 2.638 | 2.556 | 2.765 | 2.88 | 2.54 |
Table 5: Determination and evaluation of haematology of study subject.
Glucose for period I: 91.28013 ± 0.943309 (mg/dl) and period II: 88.55825 ± 0.94327 (mg/dl)
Urea for period I: 20.53475 ± 0.442382 (mg/dl) and period II: 19.4635 ± 0.485118 (mg/dl).
Creatinine for period I: 0.913 ± 0.092679 (mg/dl) and period II: 0.83375 ± 0.057314 (mg/dl)
Cholesterol for period I: 179.304 ± 13.8745 (mg/dl) and period II: 168.745 ± 12.78742 (mg/dl)
Bilirubin total for period I: 0.82825 ± 0.074289 (u/l) and period II: 0.821 ± 0.04535 (u/l).
Bilirubin direct for period I: <0.04 (u/l) and period II: <0.04 (u/l).
SGOT (AST) for period I: 26.3985 ± 1.047195 (u/l) and period II: 26.579 ± 0.819054 (u/l)
SGPT (ALT) for period I: 30.22625 ± 0.679424 (u/l) and period II: 30.98525 ± 0.77207(u/l). Summarized in Table 6.
| Age | 21-25 years | 26-30 years | 31-35 years | 36-40 years | ||||
| P I | P II | P I | P II | P I | P II | P I | P II | |
| Glucose (mg/dL) | 92.536 | 88.456 | 90.562 | 89.654 | 90.5465 | 87.367 | 91.476 | 88.756 |
| Urea (mg/dL) | 20.385 | 19.281 | 21.183 | 20.181 | 20.386 | 19.282 | 20.185 | 19.11 |
| Creatinine (mg/dL) | 0.891 | 0.788 | 0.989 | 0.891 | 0.791 | 0.781 | 0.981 | 0.875 |
| Cholesterol (mg/dL) | 163.787 | 153.877 | 196.327 | 183.557 | 183.337 | 173.778 | 173.765 | 163.768 |
| Bilirubin total (U/L) | 0.89 | 0.783 | 0.768 | 0.784 | 0.895 | 0.842 | 0.76 | 0.875 |
| Bilirubin direct (U/L) | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 |
| SGOT (U/L) | 25.487 | 26.579 | 27.649 | 26.645 | 25.484 | 26.534 | 26.876 | 25.654 |
| SGPT (U/L) | 30.532 | 31.756 | 29.372 | 30.375 | 30.576 | 31.566 | 30.475 | 30.384 |
Table 6: Evaluation of bio-chemical markers.
The colour of urine was straw yellow and its appearance is clear for both the periods.
The pH and specific gravity period I was found to be 5.594 ± 0.14977 and 1.013667 ± 0.002082 normal.
The pH and specific gravity period II was found to be 5.804 ± 0.149663 and 1.015333 ± 0.002309 normal.
Protein, urobilinogen, ketone, bile pigments and nitrite values in urine routine analysis were found to be negative. Summarized in Table 7.
| Age | 21-25 years | 26-30 years | 31-35 years | 36-40 years | ||||
| P I | P II | P I | P II | P I | P II | P I | P II | |
| Urine routine analysis | ||||||||
| Colour | Straw yellow | Straw yellow | Straw yellow | Straw yellow | Straw yellow | Straw yellow | Straw yellow | Straw yellow |
| Appearance | Clear | Clear | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.829 | 5.936 | 5.518 | 5.657 | 5.725 | 5.839 | 5.448 | 5.648 |
| Specific gravity | 1.015 | 1.017 | 1.013 | 1.012 | 1.014 | 1.013 | 1.015 | 1.014 |
| Protein mg/dl | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Glucose mg/dl | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Urobilinogen mg/dL | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Ketone mg/dL | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Bile pigments mg/dL | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Blood | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Nitrite | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
Table 7: Determination of urine markers in study subjects.
The serology for HIV, HBsAg, anti-HCV, RPR/VDRL were found to be non-reactive. Summarized in Table 8.
| Age | 21-25 years | 26-30 years | 31-35 years | 36-40 years | ||||
| P I | P II | P I | P II | P I | P II | P I | P II | |
| HIV | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive |
| HBsAg | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Anti-HCV | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive |
| RPR/VDRL | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive | Non-reactive |
Table 8: Determination of serology.
The study subjects electrocardiogram and chest X-ray were found normal. The clinical pathology of the study subjects was 100% within the normal limit. The serology report of the subjects who participated were found to be 100% non-reactive.
Urine analysis for drug of abuse of the study subjects was found to be 100% negative. Summarized in Table 9.
| Urine analysis | Period I | Period II |
| Amphetamine | Negative | Negative |
| Benzodiazepines | Negative | Negative |
| Barbiturates | Negative | Negative |
| Cocaine | Negative | Negative |
| Morphine | Negative | Negative |
| THC | Negative | Negative |
Table 9: Urine analysis for drug of abuse.
Period I and period II, the check-in process was completed approximately in 1 hours. In period I and period II, check-out process were completed within 30 minutes (Table 10).
| Volunteers | Period I | Period II | ||
| Check-in Day 1 | Check-out Day 2 | Check-in Day 1 | Check-out Day 2 | |
| 6 volunteers | 4.20 pm | 8.15 am | 4.25 pm | 8.00 am |
| 7 volunteers | 5.10 pm | 8.30 am | 4.40 pm | 8.25 am |
| 4 volunteers | 5.15 pm | 8.40 am | 5.00 pm | 8.30 am |
| 5 volunteers | 6.25 pm | 8.45 am | 5.55 pm | 8.40 am |
| 8 volunteers | 7.00 pm | 9.00 am | 6.45 pm | 9.00 am |
Table 10: Check-in and check-out timings of the study.
Results
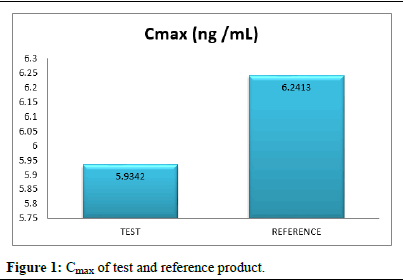
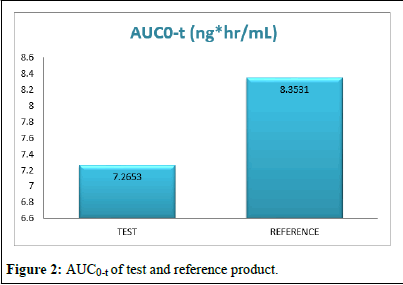
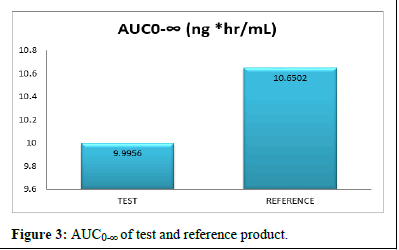
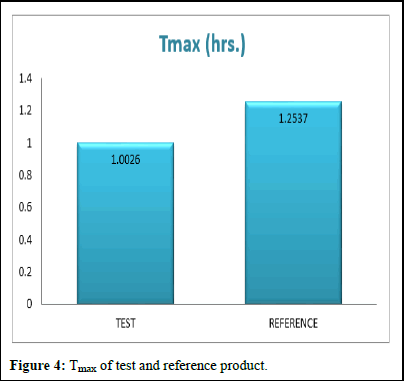
Pharmacokinetic parameters like test product and reference product Cmax, AUC0-t, AUC0-∞, and Tmax concentration range of standard deviation presented in the (Figures 1-4) (Table 11).
|
Pharmacokinetic parameters |
N |
Test product |
N |
Reference product |
||
|
Mean |
S.D |
Mean |
S.D |
|||
|
Cmax (ng /mL) |
30 |
5.9342 |
2.7851 |
30 |
6.2413 |
4.6521 |
|
AUC0-t (ng*hr/mL) |
30 |
7.2653 |
4.02864 |
30 |
8.3531 |
4.2356 |
|
AUC0-∞ (ng*hr/mL) |
30 |
9.9956 |
5.2456 |
30 |
10.6502 |
5.62 |
|
Tmax (hrs.) |
30 |
1.0026 |
0.3865 |
30 |
1.2537 |
0.5742 |
Table 11: The ln-transformed mean pharmacokinetic parameters for both the reference and test formulations.
The comparison of test and reference product maximum concentration is more bioavailability in reference product (Figure 1).
Figure 1: Cmax of test and reference product.
The comparison of test and reference product AUC0-t in more bioavailability in reference product (Figure 2).
Figure 2: AUC0-t of test and reference product.
The comparison of test and reference product AUC0-∞ in more bioavailability in reference product (Figure 3).
Figure 3: AUC0-∞of Test and reference Product
The comparison of test and reference product Tmax is more bioavailability in reference product (Figure 4).
Figure 4: Tmax of test and reference product.
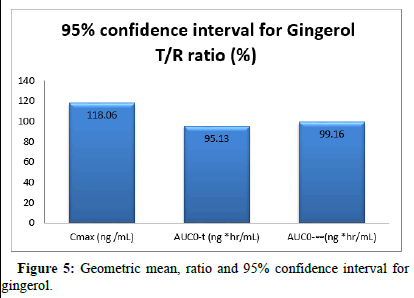
Pharmacokinetic parameters like test product and reference product Cmax, AUC0-t, AUC0-∞ and Tmax Concentration range comparison between the study of more bioavailability present in the reference product and better significant effect present in the test product. The comparative study to get a 95% confidential interval of Gingerol (Table 12).
|
Geometric mean, ratio and 95% confidence interval for Gingerol |
||
|
Pharmacokinetic parameters |
T/R ratio (%) |
95% CI |
|
Cmax (ng /mL) |
118.06 |
96.54-114.23 |
|
AUC0-t (ng *hr/mL) |
95.13 |
75.62-101.20 |
|
AUC0-∞ (ng *hr/mL) |
99.16 |
81.54-112.08 |
Table 12: The 95% confidence intervals of ln-transformed parameters for Gingerol.
95% Confidence Interval (CI): This is a statistical measure used to express the uncertainty around an estimated parameter. In pharmacokinetics, it's often used to determine the precision of estimated parameters such as Cmax and AUC.
T/R ratio: This likely refers to the ratio of test (T) to reference (R) formulations in bioequivalence studies. These studies compare different formulations of a drug to ensure they produce similar concentrations in the body (Figure 5).
Cmax: Represents peak concentration. Shows significant difference.
AUC0-t: Indicates overall exposure, with a lower impact.
AUC0-∞: Represents comprehensive exposure, with a moderate impact.
Figure 5: Geometric mean, ratio and 95% confidence interval for gingerol.
Discussion
Acceptable range of bioequivalence is generally 0.8%-1.25% for the test or reference ratio of average values, when the parameters are logarithmically transformed. The acceptable range is generally ± 0.2 for the relative difference in vivo parameters between reference and test products, when the raw data are used. Two different medicinal products is said to be therapeutically equivalent when their active constituent is same, meeting same therapeutic moiety and show same clinical efficacy and safety. Pharmaceutical equivalence does not essentially imply therapeutic equivalence, as differences in the excipients or the manufacturing procedure and some other variables can lead to differences in product performance.
Both bioavailability and bioequivalence study focus the rate and extent to which the active pharmaceutical ingredient or therapeutic moiety was absorbed from a pharmaceutical drug product available at the systemic circulation. In vivo performance, in term of BA/BE, study was considered to be one aspect of product quality that provides a link to the performance of the safety and efficacy. For this reason, similar approaches to measure bioavailability in an NDA should generally be followed in demonstrating bioequivalence for an NDA for present study we screened total 58 volunteers. Out of 58 volunteers 30 volunteers passed and 28 volunteers failed because some variable changes in laboratory parameters like high plasma glucose, serum albumin was more than normal range, high LDL cholesterol, high alkaline phosphate, high blood pressure, low haemoglobin, RBC count is low.
The body temperature, respiratory rate, pulse rate for the subjects recruited in the study in period I, II were analysed according to the age of 21-40 years were within normal limit.
The heamotology values for the subjects recruited in the study in period I, II were analysed according to the age of 21-40 years were within normal limit.
The differential count values for the subjects recruited in the study in period I, II were analysed according to the age of 21-40 years were within normal limit.
Bio-chemistry values for the subjects recruited in the study in period I, II were analysed according to the age of 20-40 years were within normal limit. In this screening procedure can be used to predict the subject health conditions in other words determination of fit or unfit in a particular study dosing before conducted in screening procedure. The screening procedure passed subjects are involved in the study.
The clinical pathology for both periods I and period II were found to be within normal limit and compared to be same.
Period I and period II, the check-in process was completed approximately in 1 hours. In period I and period II, check-out process were completed within 30 minutes.
Conclusion
In a study comparing gingerol and ginger extract capsules for antiemetic activity, Gingerol is a chemical constituents obtained by ginger. Ginger is a neutraceutical product and ginger extract capsule is a standard drug and there was no significant variation in the overall analysis. However, during the first treatment period before crossover, gingerol showed a better effect compared to ginger extract capsules, as demonstrated by statistical methods. No serious adverse events were reported during the active medication periods. Overall, a statistically significant effect of gingerol was observed only in the initial treatment period, not throughout the entire study.
Ethical Considerations
This protocol and the corresponding informed consent documents used to obtain informed consent of study subjects were reviewed by the Independent Ethics Committee Re-Registration Certificate no. ECRl83/lndtlTN/2013/RR-19. Study procedures were commencing only after receipt of letter of approval is received from the committee, for the same.
Conflict of Interest Statement
The authors report no conflicts of interest in this work.
Acknowledgements
I would like to express my sincere appreciation to Sankaralingam Bhuvaneswari College of pharmacy for their invaluable guidance and support during the preparation of this Research article. Their expertise and insights have greatly enriched the content.
Funding
Nill.
References
- Masten CL, Morelli SA, Eisenberger NI (2011) An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. Neuroimage 55: 381-388.
[Crossref] [Google Scholar] [PubMed]
- Food and Drug Administration (2003) Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products-general considerations. Food and Drug Administration.
- Choi SO, Jung SH, Um SY, Jung SJ, Kim JI, et al. (2004) Guideline for bioequivalence studies of generic products for topical use. J Pharm Investig 34: 333-340.
- Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE, et al. (2001) Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol Cell 8: 1105-1115.
[Crossref] [Google Scholar] [PubMed]
- Patel BA, Wunderlich RE (2010) Dynamic pressure patterns in the hands of olive baboons (Papio anubis) during terrestrial locomotion: Implications for cercopithecoid primate hand morphology. Anat Rec (Hoboken) 293: 710-718.
[Crossref] [Google Scholar] [PubMed]
- Usman MA, Garba M, Odunola MT, Sule MI, Ahmadu AA, et al. (2007) Comparative bioequivalence studies of three brands of paracetamol with panadol in healthy human volunteers. Niger J Pharm Sci 6.
- Fujita M, Charney DS, Innis RB (2000) Imaging serotonergic neurotransmission in depression: Hippocampal pathophysiology may mirror global brain alterations. Biol Psychiatry 48: 801-812.
[Crossref] [Google Scholar] [PubMed]
- Agarwal SP, Chairman DTAB (2014) Good clinical practices for clinical research in India.
- Guideline IHT (1996) Guideline for good clinical practice E6 (R1). ICH Harmon Tripart Guidel, 1996.
Citation: Sheely JJ (2025) Bioavailability Study of Gingerol and Ginger Extract Capsule in Healthy, Adult, Human Subjects under Fasting Conditions. World J Pharmacol Toxicol 8: 297.
Copyright: © 2025 Sheely JJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 361
- [From(publication date): 0-0 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 268
- PDF downloads: 93