Biodiesel Production from the Seeds of Mimusops elengi Using Potassium Aluminium Silicate as Novel Catalyst
Received: 04-Jul-2017 / Accepted Date: 17-Jul-2017 / Published Date: 24-Jul-2017 DOI: 10.4172/2576-1463.1000165
Abstract
Biodiesel is considered as most promising alternative for fossil fuels because of its robust combustion characteristics and self-sustainability. Identifying a suitable feed stock with good lipid content, optimized reaction parameters will help standardizing this fuel globally. This paper deals with production of biodiesel from the oil of Mimusops elengi by heterogeneous catalyzed transesterification reaction. Methanol in stoichiometric molar ratio was used as the solvent for reaction whereas potassium aluminium silicate was used heterogeneous catalyst with a weight corresponding to weight of oil. Maximum oil content present in the seed was found to be 13%, which was determined using Soxhlet’s extraction and the dominant acids found in the extracted oil were stearic acid and Palmitic acids. The molar ratio was found out to be 1:9, with catalyst concentration of 2% weight of oil, for a temperature of 60oC. A maximum yield of 73.4% was obtained using this novel catalyst with stearate as dominant methyl ester. The novel catalyst can be reused again for the reaction without compromising the conversion yield.
Keywords: Biodiesel; Mimusops elengi seeds; Potassium aluminium silicate; Soxhlet’s extraction methanol; GC-MS spectrophotometer; Transesterification
23090Introduction
The increase in the trend for employing bio diesel to reduce emissions and zero sulphur contents, in combustion applications [1] has attracted researchers to works on various bio oils extracted from various non-edible seeds [2]. Mimusops elengi is a non- edible seed obtained from the family of Sapotaceae . The various parts of this plant possess their unique medicinal property, hence making it an important flora in the country. These seeds are not having any medicinal properties, but the kernel of the seeds have been proved to possess high percentage of oil content. So this oil was found to be a feedstock source for bio diesel which has unique property of biodegradability, non-toxicity and reusability [3]. The extraction of lipids from these solid seed samples was satisfactory when using Soxhlet’s extraction than compared to other possibilities [4]. Various researches has been carried out in developing biodiesel with high heating value (39000 kJ/kg) in order to range anywhere between the HHVs of petrol (42,000 kJ/kg) and coal (32000 kJ/kg) [5], even though this idea was initiated by Novak and Kraus in early 1970’s [6]. These lipids are nothing but 3 fatty acid esters grouped with 1 glycerol molecule which splits up during Trans-esterification reaction [7]. The transesterification process involving the reaction of bio lipids with Methanol in presence of catalyst is the most preferred method for yielding Fatty Acid Methyl Ester (Bio diesel) [8], which are then subjected for engine testing for evaluating its performance, emission characteristics [9]. The yield of bio diesel mainly depends upon the quantity of lipids present, amount of suitable catalyst along with reaction parameters itself. The catalyst involved in the reaction not only speeds up the rate of reaction but also enhances the bio diesel yield [10], which makes the decision of the suitable catalyst a critical one. The use of heterogeneous catalyst has made a recent trend in biodiesel production. These lipids and Fatty Acid Methyl Esters (FAME) are usually hydrophobic which makes them insoluble in water. Various sources of bio-lipids includes Vegetable oil from edible products, waste vegetable oil, varieties of animal lipids derived from yellow, tallow and tard grease and nonedible oils from seeds of Neem, Jatropha, castor etc.
Materials And Methodology
Sample collection
The Mimusops elengi being a most common tree in local, these seeds can be easily collected from various nurseries and seed collectors. Also these seeds are available in Ayurvedic centers that use these plants for medicinal purposes. The seeds are cleared and separated from the other impurities present along with it. Then, the outer shells of the seeds are removed to get the oil content kernels. After removing them, they are cleansed with water to remove any impurities and dust present in it. The cleansed seeds are then dried up in hot air oven for 5 h duration over 110°C. This removes the moisture content and also reduces the chances of seed contamination. The baked seeds are the crushed into coarse powder and is then subjected for Soxhlet extraction. A total of 500 g of M. elengi seeds were used for in this process.
Extraction of lipids using Soxhlet’s apparatus
The coarse powder is packed in highly porous packets such that each weighs about 25 g. These are then packed In 250 ml capacity Soxhlet tube at the bottom. The n-Hexane used as solvent in this method is taken in 500 ml round bottom flask. The flask and Soxhlet tube are connected in such a way that the round bottom flask is at the bottom and at the top it is provided with a water condensing jacket for the vaporized hexane to get condensed. The setup is the mounted on a heater and the temperature is maintained at 60°C. On heating, the hexane get convert in to vapor and this vaporized hexane passes through the tube and gets condensed and comes in contact with the seed powder, which then extracts oil from it and then again it comes back to round bottomed flask due to flow continuity. This makes the oil to get collected in the flask and the hexane to re vaporize and re condense to extract more oil from seeds. This cycle repeats until the oil is completely extracted from the seeds. The duration of the process and the number of cycles depends upon the amount of fatty acid content in the seed. The end of process is indicated change in colour of the in the flask and disappearance of turbidity in Soxhlet tube. Then the extracted oil is subjected to fractional distillation where the n-Hexane solvent is isolated from the oil because of variance in their boiling point, on heating.
Yield calculation
The extracted oil is heated up to 70°C for 30 min in a water bath, to remove the residual solvent and volatile impurities present in it. Then the amount of oil is measured using a measuring cylinder and the oil quantity is noted down. The yield percentage of the M. elengi oil is calculated using the following formula:
oil content (%) = Weight of the oil/weight of the sample×100
Fatty acid analysis
GC-MS chromatography was used to perform the qualitative and quantitative analysis of the extracted bio oil and biodiesel. The technical specifications of the gas chromatography and mass spectrometry have been listed below in Tables 1 and 2 [11,12].
| Equipment | Agilent 6890 chromatograph |
| Injector Liner | direct/2 mm |
| Column | 15 m all tech ec-5 (25 u id, 0.25 u thickness) |
| Split Ratio | 10:01 |
| Oven Temperature | 35°C/2 min |
| Ramp | 20°C/min @ 300°C for 5 min |
| Helium Carrier Gas | 2 ml/min (constant flow mode) |
Table 1: The technical specification of gas chromatograph equipment.
| Equipment | JOEL GC mate II bench top |
| Type | Double focusing magnetic sector MS |
| Operation mode | Electron Ionization (EI) mode |
| Software | TSS-2001 |
| Resolving power | 1000 (20% height definition) |
| Scanning feature | 25 m/z to 700 m/z @ 0.3 s/scan |
| Inter scan delay | 0.2 s |
Table 2: The technical specification of mass spectrophotometer equipment.
Trans-esterification reaction
The GC-MS analysis report has revealed the amount of fatty acid present in the extracted oil. Using the empirical formula, the molecular weight of the oil is calculated. Considering the weight of the oil and methanol, the molar ratio for the process is calculated and found out to be in a ratio of 1:9. The reaction is carried out by reacting one part of oil with nine part of methanol in presence of 2% weight of heterogeneous catalyst under regulated temperature of 60°C at 600 rpm. Then the product is made to get settled into two separate layers under the influence of gravity separation, for 2-4 h depending upon the settling rate. The top layer is usually the fatty acid methyl ester whereas the settlement at the bottom is glycerol.
Results and Discussion
The gas chromatography result reported the presence of 13% fatty acid content in the extracted oil. The molecular weight of the Mimusops elengi oil was determined with the help of equation [13].
M (TG)=92.09-3+3(M (HA)-17
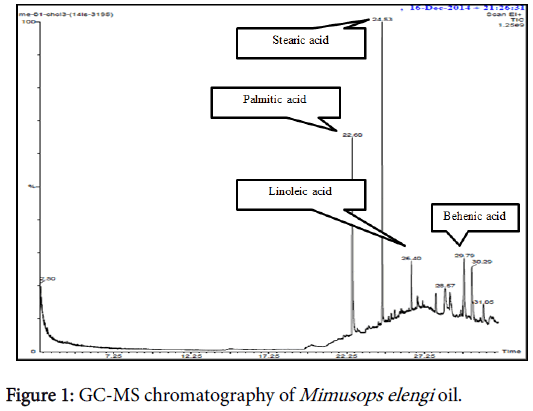
The characterization of the bio oil extracted form M. elengi seeds were done with the means of GC-MS Spectrophotometer. Confirmation of various fatty acids such as stearic acid, palmitic acid, Behenic acid and linoleic acid was done based on GC-MS spectral data and comparison was made with standard GC-MS NIST Library search. Figure 1 represents the GC-MS spectra of M. elengi oil which shows the above mentioned fatty acids present in it. The presence of these fatty acids holds the responsibility for the biodiesel production during the transesterification process. The dominant fatty acids present in M. elengi oil are tabulated below in Table 3 based upon the library search for the corresponding spectra.
| S. No. | Fatty Acid | Molecular Formula | Carbon Number | Retention Time |
|---|---|---|---|---|
| 1 | Palmitic acid | C16H32O2 | C22:0 | 22.60 |
| 2 | Stearic acid | C18H36O2 | C18:0 | 24.63 |
| 3 | linoleic acid | C18H32O2 | C18:2 | 26.40 |
| 4 | Behenic acid | C22H44O2 | C21:0 | 29.79 |
Table 3: The fatty acid present in Elengi oil.
The GC-MS results helped in obtaining the average molecular weight of the elengi bio oil and meanwhile strong molecular ion peaks [M+] were exhibited in mass spectra data. Based upon the GC and MS analysis report, there was a sufficient amount of desired fatty acids present in the Elengi oil that is important for synthesis of biodiesel.
By using the above equation, the molecular weight of the M. elengi bio oil was determined and had a molecular weight of 826.46 g/mole. The synthesis of bio diesel using Trans esterification was carried out in various molar ratio combinations from 1:3-1:12 by varying the proportion of methanol by 3. The most efficient and high yield bio diesel was achieved at the molar ratio of 1:9, thereby standardizing this ratio for throughout this batch of bio diesel production. The most consistent, high quality yield was obtained for catalyst amounting 2% weight of oil.
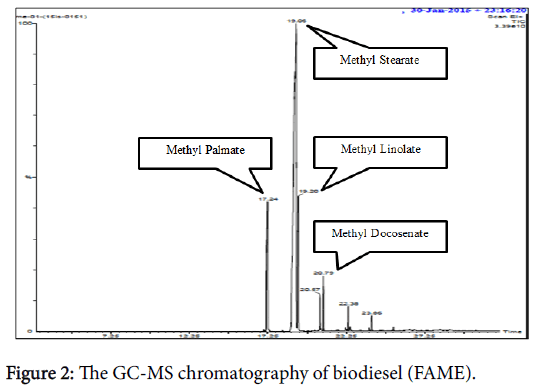
The base catalyzed Trans esterification reaction was followed for conversion of Elengi oil into bio diesel under regulated conditions. The GC-MS spectrophotometer analysis was done on the synthesized biodiesel and Figure 2 represents the spectra of M. elengi oil ester post conversion. The spectral data of GC-MS spectrophotometer confirmed the yield of Fatty Acid Methyl Ester (FAME) and the esters converted from the oil are tabulated in Table 4. Overall transesterification of fatty acid into FAME was found to 73.4%.
| S. No. | Fatty Acid | FAME | Retention Time |
|---|---|---|---|
| 1 | Stearic Acid | Methyl 9,10-methylene-octadecanoate | 19.06 |
| 2 | Behenic Acid | Methyl 11-docosenoate | 20.79 |
| 3 | Palmitic acid | Methyl 14-methylhexadecanoate | 17.34 |
| 4 | Linoleic acid | Methyl linolate | 19.2 |
Table 4: The fatty acid methyl ester present in Elengi oil.
Conclusion
The maximum yield of bio-diesel from Mimusops elengi oil was achieved by regulating various parameters like molar ratio, amount of KAlSiO3 catalyst used, time period of the reaction, methodology involved and temperature conditions. The bio-diesel synthesis was carried out using trans-esterification process between Elengi seed oil and methanol with potassium aluminium silicate as a heterogeneous catalyst under monitored temperature of 60°C. The resultant product was analyzed using GC-MS spectrophotometric and confirmed the presence of fatty acid methyl ester, which proves the bio-diesel formation. A 73.4% of yield was achieved from this process using a novel catalyst and various physiochemical properties and performance based analysis would be done for characterizing and improvising the bio-diesel for further future works.
References
- Antolin G, Tinaut FV, Briceno Y, Castano V, Perez C, et al. (2002) Optimisation of biodiesel production by sunflower oil transesterification. Bioresour Technol 83: 111-114.
- Demirbas A (2008) New liquid biofuels from vegetable oils via catalytic pyrolysis. Energy, Education, Science and Technology 21: 1-59.
- Manirakiza P, Covaci A, Schepens P (2001) Comparative study on total lipid determination using soxhlet, roese-gottlieb, bligh & dyer, and modified bligh & dyer extraction methods. J Food Compost Anal 14: 93-100.
- Demirbas A, Demirbas I (2007) Importance of rural bioenergy for developing countries. Energy Convers Manage 48: 2386-2398.
- Demirbas A (2008) Biodiesel: a realistic fuel alternative for Diesel engines. London: Springer.
- Novak JT, Kraus DL (1973) Degradation of long chain fatty acids by activated sludge. Water Res 7: 843-851.
- Sonntag N (1979) Reactions of fats and fatty acids. Bailey’s industrial oil and fat products (4th edn.).
- Gunstone FD, Hamilton RJ (2001) Oleochemicals manufacture and applications. Sheffield Chemistry and Technology of Oils and Fats
- Singh PJ, Khurma J, Singh A (2010) Preparation, characterisation, engine performance and emission characteristics of coconut oil based hybrid fuels. Renew Energy 35: 2065-2070.
- Leng TY, Mohamed AR, Bhatia S (1999) Catalytic conversion of palm oil to fuels and chemicals. Can J Chem Eng 77: 156-162.
- https://www.agilent.com/cs/library/specifications/Public/5989-3290EN.pdf
- http://www.medwow.com/med/mass-spectrometer/jeol/jms-gcmate/1924.model-spec
- Komers K, Stloukal R, Machek J, Skopal F (2001) Biodiesel from rapeseed oil, methanol and KOH. 3. Analysis of composition of actual reaction mixture. Eur J Lipid Sci Technol 103: 363-371.
Citation: Srinivasan GR, Palani S, Ranjitha J (2017) Biodiesel Production from the Seeds of Mimusops elengi Using Potassium Aluminium Silicate as Novel Catalyst. Innov Ener Res 6: 165. DOI: 10.4172/2576-1463.1000165
Copyright: ©2017 Srinivasan GR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3146
- [From(publication date): 0-2017 - Aug 16, 2025]
- Breakdown by view type
- HTML page views: 2283
- PDF downloads: 863