Biological Features of IL-12 and IFN-Îó in the Pathogenesis of Systematic Lupus Erythematosus
Received: 26-Sep-2017 / Accepted Date: 12-Oct-2017 / Published Date: 16-Oct-2017 DOI: 10.4172/2161-0681.1000323
Abstract
Systemic lupus erythematosus is a chronic autoimmune disease characterized by abnormal immune response with overproduction of auto-antibodies, self-destructive complexes and hyperactivity of both T and B lymphocytes. Although the exact pathogenesis of SLE remains unclear, it has been found that the imbalance of Th1/Th2, subsequent cytokines and anti- or pro-inflammatory mediators could reflect the stage and phenotype of SLE. Among diverse cytokines involved in the disease progression, IL-12 and IFN-γ gain much attention for their biological functions in SLE. There existed many divergences on the expression levels of IL-12 while few conflicts were demonstrated on elevated levels of IFN-γ in SLE patients. By analysis of gene polymorphisms, it was suggested that positive correlations were observed between IL-12B rs3212227, IL-12B rs17860508 and susceptibility to and severity of SLE in Polish patients. In addition, the combination of the IL-12B rs17860508 GC allele and/or IL-12B rs3212227 C allele could increase the risk of SLE progression and/or severity of SLE parameters in Polish population. Furthermore, it was also found that the incorporation of IFN-γ R1 Met 14/Val 14 and IFN-γ R2 Gln 64/Gln 64 genotypes was a potential risk factor for SLE. Based on the precious researches, IL-12 and IFN-γ targeted gene therapy was developed and found to be effective in murine lupus. Intramuscular injection of IFN-γR/IgG1Fc fusion protein encoded cDNA and suppressive ODN was found to decrease the severity of disease and promote survival of lupus mice. On the other hand, intramuscular injection of IL-12 and IL-18 encoded plasmids alone or together was also observed to have therapeutic effects on murine lupus. All in all, this review mainly introduces the biological features and the potential therapeutic effects of IL-12 and IFN-γ in the pathogenesis of SLE.
Keywords: Systemic lupus erythmatosus; Interleukin-12; Interferongamma; Pathogenesis
Introduction
Introduction
Systemic lupus erythmatosus (SLE) is a representative multisystem autoimmune disease, characterized by abnormal immune response including overproduction of auto-antibodies, deposition of immune complexes and hyperactivity of both T and B lymphocytes, exerting severe damage to multiple organs and connective tissues in large population throughout the world [1]. Owing to genetic predisposition and environmental factors, SLE mainly occurs in child-bearing period female with gender distribution of 9:1 to male [2]. SLE is a heterogeneous autoimmune disorder with diverse clinical manifestations ranging from subtle skin lesion to severe multisystem dysfunctions including pericarditis, respiratory distress, osteoporosis and renal failure [3]. With its unpredictable manifestations and extensive organ injuries, investigators tried to figure out the paradigm of bio-markers fluctuation in SLE to evaluate susceptibility and progression of the disease, which could be used to make treatment decisions at the early stage of disease and decrease the damage to SLE patients [4].
Several decades of research into SLE blood markers has generated few widely accepted biomarkers including double-stranded DNA (ds- DNA), complement and auto-antibodies for prediction, diagnosis and treatment. Benefit from these traditional biomarkers, standards including SLE activity index (SLEDAI), systemic lupus activity measure (SLAM) and British Isles Lupus Assessment Group (BILAG) were developed to evaluate SLE while its extensive organ coverage brought inconvenience to routine clinical practice [5]. With the appearance of cell signaling profile in SLE, a series of non-traditional and emerging biomarkers including cytokines, growth factors, chemokines and acute phase reactants were found to have potential clinical effects in SLE. Diverse characteristics and introduction of traditional and non-traditional biomarkers for SLE were described elsewhere [6]. Cross communication among various cells, especially immune cells, play vital roles in the pathogenesis of SLE and cytokines gain much attention for their abilities to mediate adjacent and long-distance communications among immune cells. A great number of studies have addressed the expression profile of Th1 or Th2-related cytokines in SLE and tested the biological effects in animal lupus models following the knockdown or overexpression of these cytokines [7].
According to previous reports, interferon-α (IFN-α), soluble interleukin-2 receptor(sIL-2R) and soluble tumor necrosis factor receptor (sTNFR) were thought to be potential biomarkers of SLE; IL-1Ra, IL-6, IL-10, IL-12p40, IL-13, IL-16, IL-18 and TNF were found out to have possible effects while IFN-γ, IL-12 and IL-15 were not promising to be biomarkers of SLE [8]. Though IL-12 and IFN-γ may not be proper biomarkers for clinical diagnosis, the results of researches on them triggered switching of Th1/Th2 mediated immune trend and their targeted therapies were proved to be effective in murine lupus [7,9]. Besides, abnormal expressive forms of IL-12 and IFN-γ were universally reported and contradictory data were collected among different ethnicities and regions. No consensus has been reached yet partly due to insufficient researches and there were few papers that systematically demonstrated the observation of IL-12 and IFN-γ though the two cytokines were closely related to each other in expression profile. Type I IFN-targeted therapy including monoclonal antibody of IFN-α and its receptor have completed phase II and III clinical trials while other IFNs, such as IFN-γ, and recently discovered type III IFNs were still tested in animal models [10]. Therefore, this review was conducted to demonstrate the present findings and divergences of IL-12 and IFN-γ in SLE and summarize novel targeted therapies of the two cytokines, which can be a rough knowledge basis for further researches on cytokines in SLE.
IFN-γ and its regulatory roles in SLE
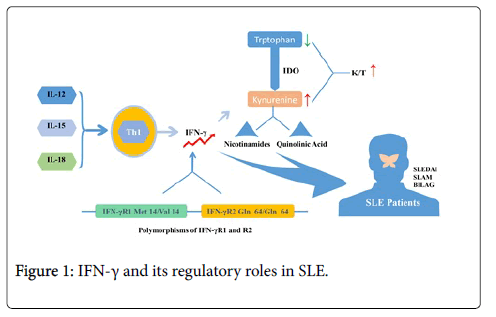
Interferon-gamma (IFN-γ), as one kind of water-soluble dimer cytokine is the only member of type II interferon, initially called macrophage activation factor. The biologically active IFN-γ is formed by two anti-parallel interlocking monomers, which is composed of six alpha helices consisting of one core and a fragment sequence extending at the C-terminal region. IFN-γ is a typical kind of cytokine secreted by Th1 cell and its expression profile is regulated by a series of IFN-inducing factors including IL-12, IL-15 and IL-18. It was reported that serum levels of IFN-γ in lupus patients were higher than in controls [11-14]. Min et al. investigated the associations between IFN-γ and lupus nephritis, and defective IFN-γ production was observed in 17 patients with lupus nephritis compared with 23 patients with un-nephritic lupus. A subtle correlation was found between SLEDAI scores and serum IL-4/IFN-γ ratios [15]. No distinctions in mitogen-stimulated and phytohemagglutinin-induced IFN-γ levels were observed between patients and healthy controls in some literatures. However, evident correlation of IFN-γ with SLAM scores was reported in other studies [16,17]. Apart from directly evaluating the IFN-γ levels in patients and controls, researches also detected some downstream products of IFN-γ. With the stimulation of IFN-γ, enzyme indoleamine dioxygenase (IDO), which can transform tryptophan into kynurenine, is further converted to final substances like nicotinamides and quinolinic acid. Widner et al. detected the levels of serum tryptophan, kynurenine and the kynurenine/tryptophan quotient (K/T) in 55 SLE patients in order to know the enzyme activity of IDO. Compared with healthy donors, there were lower tryptophan levels and higher kynurenine levels and K/T quotients in SLE patients. Besides, a slight correlation between K/T quotients and SLEDAI scores was established [18]. Polymorphisms of IFN-γR1 and R2 have been found in some lupus patients, and incorporation of IFN-γR1 Met14/Val14 and IFN-γR2 Gln64/Gln64 genotypes has been demonstrated to be a potential risk factor for SLE [19]. Nonetheless, with the mitogenic stimulation, higher expression levels of IFN-γ were detected from mononuclear cells in peripheral blood of lupus patients than controls [20]. It is very interesting to find that some patients would suffer from severe lupus-like disease, if they were treated with IFN-γ for unrelated autoimmune diseases [21,22].
In recent years, a variety of techniques have been applied to measure the expression levels of cytokines in lymphoid organ and tissue in mouse models of SLE [23]. Elevated IFN-γ levels were detected in the MRL-Faslpr lupus mice especially at the late stage of disease [24-29]. Transgenic mice with over-expressed IFN-γ would develop T-cell dependent lupus-like syndrome and deposits of antinuclear auto-antibodies in kidney [6,30]. Jacob et al. firstly came up with the idea that IFN-γ played an important role in the pathogenesis of lupus [31]. In the study, they found that conditions were aggravated in (New Zealand Black (NZB) × New Zealand White (NZW)) F1 (B × W) lupus mice that were treated with IFN-γ or its inducers, while the onset of SLE was delayed in those receiving anti-IFN-γ antibody at an early stage. Following these findings, Ozmen et al. treated lupus mice with soluble IFN-γ or recombinant IFN-γ R, or with anti-IFN-γ antibody. They found longer overall survival and decreased histologic serologic parameters of lupus in B × W mice receiving sIFN-γ R or anti-IFN-γ. By contrast, those with the treatment of IFN-γ died earlier than controls. Moreover, only if all the mice were treated with anti-IFN-γ antibody or sIFN-γ R at the early stage of lupus disease were the treatments effective. The reason might be that the very high levels of the ligand formed late in the disease process could not be completely neutralized [32]. However, other studies indicated that application of monoclonal IFN-γ antibody exerted no influence on disease severity and mortality of MRL-Faslpr mice [33]. Transgenic mice that IFN-γ and IFN-γR genes were deleted were used to research the role of IFN-γ in lupus. In the study, it was found that survival and disease parameters significantly decreased. Besides, hypergammaglobulinemia with transition from IgG2a to IgG1 predominance was detected in IFN-γ- deleted transgenic lupus mice while the subclass of IgG1 anti-dsDNA auto-antibodies did not increase with the decreased levels of IgG2a anti-dsDNA auto-antibodies. Moreover, it was found that the progression of glomerulonephritis and rate of early death were suppressed even in heterozygous IFN-γ deleted lupus mice which were reduced by 50% in IFN-γ expression but not in auto-antibody and immune complexes in kidney [34,35]. In summary, all these findings suggest that IFN-γ may promote lupus progression in both humor-mediated and cell-mediated manners (Figure 1).
IL-12 and its regulatory roles in SLE
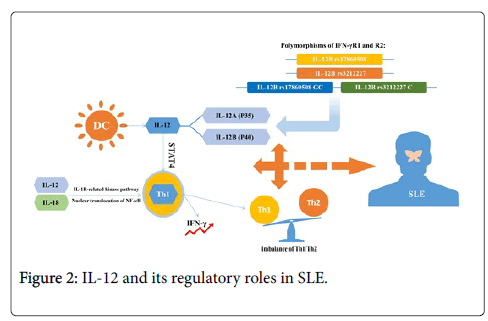
IL-12, a 70-kDa heterodimer secreted by activated dendritic cells and macrophages, stimulates early helper T cells to differentiate into Th1 cells and promotes the development and proliferation of Th1 cells via signal transducer and activator of transcription 4 (STAT4). IL-12 can induce IFN-γ production in T helper type 1 (Th1) cells via IL-1 receptor-related kinase pathway together with IL-18, resulting in nuclear translocation of the NF-κB complex [36]. Therefore, IL-12 promoted synthesis of IFN-γ and generation of Th1 cells while it suppressed progression of Th2 cells [37,38]. Past investigations have well documented the regulation of IL-12 in vitro, which positively correlated with elevated levels of IFN-γ and could be inhibited by IL-10 and IL-4 [39,40]. Serum IL-10 positively correlated with anti-ds DNA antibody, an significant marker presenting in 70% of SLE patients, while IL-12 was reported to negatively correlate with anti-ds DNA antibody [41]. It was suggested that the serum levels of IL-12 in active SLE patients were higher than in the controls in some studies while other investigators found defective IL-12 production in lupus patients [36,42]. IL-12 is composed of p35 (IL-12A) and p40 (IL-12B) and the two subunits are homologous to EBs and P28, two components of IL-27, which have both pro-inflammatory and anti-inflammatory effects on the pathology of autoimmune disease and play diverse roles in Th cell responses [43,44]. Since gene polymorphisms could modify the expression profile of cytokines, investigators began to study the association between polymorphisms of IL-12 and IL-27 and pro gression of SLE. In these studies, they found that there existed positive correlations between IL-12B rs17860508 and susceptibility to and severity of SLE, and IL-12B rs3212227 positively correlated with severity of SLE in Polish patients. Besides, the combination of the IL-12B rs17860508 GC allele and/or IL-12B rs3212227 C allele increased the risk of SLE and/or the severity of SLE parameters in Polish population [45]. Other studies have showed that C allele in IL-12B 3′ UTR connected with higher production of IL-12p70 while GC allele in IL-12B promoter binding with Sp1 increased gene transcription and cytokine activity [46-52]. All these alleles might increase the levels of IL-12 and affect the immune response via regulation of T cell activity, resulting in abnormalities in SLE. However, Sanchez, et al. investigated the IL-12B polymorphisms mentioned above in SLE patients and did not find the relationships between these SNPs and the predisposition to SLE or the progression of lupus nephritis in the Spanish population [53]. Hirankarn et al. demonstrated that the A allele and A/A genotype of IL-12 at 3′ untranslated region in SLE patients had few associations with proteinuria in Thais population [54]. Discrepancies among reports of IL-12 gene polymorphisms might be explained by the heterogeneity of different diseases, distinct ethnicities, limited sample size and diverse genotyping methods. Some reports revealed that serum levels of IL-12 were uncorrelated with disease activity while its p40 subunit was correlated with SLEDAI scores and immunosuppressive therapy could downregulate the levels of this subunit of IL-12 [55-57]. Min et al. investigated the associations between IL-12 and lupus nephritis, and they observed defective IL-12 production in 17 patients with lupus nephritis compared with 23 patients with un-nephritic lupus [15]. Except for defective IL-12 production, hyperproduction of IL-6 and IL-10 was detected via a series of studies on SLE [58,59]. On the other hand, as for these SLE patients with increased IL-12 and IFN-γ, they were also found to have higher Th1/Th2 ratios in the peripheral blood mononuclear cells. Predominance of Th1 cells in the blood and kidneys was particularly potent in patients with diffuse proliferative nephritis [60]. Apart from IFN-γ, elevated levels of IL-12 were also detected in the kidney-infiltrating mononuclear cells and tubular epithelial cells of MRL-Faslpr mice, a model of murine lupus (Figure 2) [61-63].
IL-12 and IFN-γ targeted therapy for SLE
Systemic lupus erythmatosus is an auto-immune disease with a malfunction of self-tolerance that causes a variety of severe system disorders. Past investigations have clearly addressed the complexity of cellular and humoral abnormalities under the influence of environment, predisposing gene and sex hormone in both animal models and patients. In clinical practice, immunosuppressant (e.g., cyclophosphamide, tacrolimus and prednisolone) and NSAIDs (nonsteroidal anti-inflammatory drugs) were widely used and proved to be effective in both treatment and prevention. However, severe adverse effects including infertility, infection and amenorrhea frequently occurred and exerted immense sufferings to SLE patients. Therefore, based on the profile of cytokine imbalance, a series of novel therapies including B lymphocyte inhibition, costimulation supression and cytokine antagonists were developed [64,65]. The damages of diverse organ and tissue are caused by different immune responses in SLE. The hormonal factors closely related to capillary damage in glomeruli, lungs, dermal tissues while damage for interstitium of kidneys, lacrimal, and salivary glands were caused by cell-mediated immunity [66,67]. Therefore, investigators thought single cytokine-targeted therapy might not be adequate because of complex cytokine network but a certain cytokine targeted therapy had potent effects with cascade reaction of immune response. Here we mainly introduce IL-12 and IFN-γ targeted therapy for SLE. Although the results of IL-12 and IFN-γ targeted therapy are proved to be effective in animal models, whether its therapeutic effects remain the same in patients still needs further clinical studies to certify.
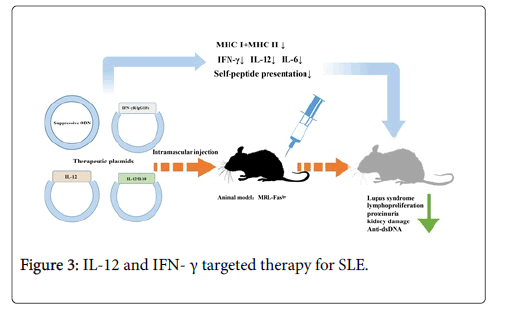
IFN-γ targeted therapy: Since studies of diverse levels showed the therapeutic effects of IFN-γ suppressing methods in murine lupus, intramuscular injection of a non-viral vector with IFN-γR/IgG1Fc fusion protein encoded cDNA has been regarded by investigators as a novel method to interfere IFN-γ expression [68]. Different from truncated IFN-γ receptor, fusion compound was a kind of disulfide-linked homodimer possessing longer T1/2 and higher avidity [69]. By treatment with IFN-gR/Fc vector before symptoms appeared, serum levels of IFN-γ and parameters in lupus would effectively decrease in MRL-Faslpr mice. Moreover, even at the early stage of disease, this kind of treatment could still make a difference. It was infrequent to find that suppression of single target had significant effects on this chronic immune disease with various pathogenic factors [46,70]. Until now, the mechanism how the blockade of IFN-γ reduces the deterioration of murine lupus has not been fully understood. One possible explanation was that suppression of IFN-γ lead to reduction of MHC class I and II molecules in renal tubular epithelial and mononuclear cells, destroying self-peptide presentations and responses [47]. Besides, a series of pro-inflammatory factors like monocyte chemattractant protein-1 and intercellular adhesion molecule 1 have also been detected to be suppressed following the down regulation of IFN-γ [48]. Compared with viral vectors, it was simple to finish intramuscular injection of plasmid vector of IFN-γR/IgG1Fc with higher safety and lower toxicity [49]. Moreover, this gene targeted therapy permits long-term production of fusion protein and migration of injected DNA to other organs, which have advantages over the usage of sIFN-gR.
Past studies have revealed that synthetic oligodeoxynucletides (ODN) with TTAGGG motif, which highly accumulated in telemeric region of mammalian chromosomes, could be suppressed to treat autoimmune disease [50-52]. Sano et al. reported that ODN without CpG motifs functioned as an adjuvant to induce Th2 differentiation and Dong et al. reported that glomerulonephritis and survival of lupus mice model were improved by synthetic ODN with significant reduction of anti-ds DNA auto-antibody, IL-12, IFN-γ and IL-6. Besides, they also tested whether ODN had negative effects on animal well-being, which was measured by body weight and general activity, and made a conclusion that no obvious impairments were observed. Therefore, suppressive ODN may be an ideal method to treat autoimmune disease like SLE. However, the mechanism how ODN suppression influences inflammatory immune responses still needs further investigation. It was reported that ODN suppression blocked the signal pathway which could regulate the production of IL-12 and IFN-γ, causing the balance of Th1/Th2 changed [71-73].
IL-12 targeted therapy: Hagiwara et al. explored the therapeutic effects of IL-12-encoding plasmid on lupus according to the former concept that lupus was a Th2 related disease for shifting polarized immune response from Th2 to Th1. Murine lupus model was established and all mice were treated with IL-12-encoding plasmid DNA via intramuscular injection every 4 weeks at age of 4 weeks. Results demonstrated that accumulation of CD4 (−) CD8 (−) T cells was greatly inhibited accompanying with reduced splenomegaly and lymphadenopathy. Besides, it was also found that severity of glomerulonephritis and proteinuria weakened and serum IgG anti- DNA auto-antibody titers reduced while serum levels of IFN-γ increased. Therefore, the balance of cytokines tended to Th1 but the mechanism of this intervention was not totally depended on an IFN-γ- mediated pathway [74,75].
It has been reported that injection of recombinant IL-12 or IL-18 proteins might aggravate the lupus-like symptoms in lupus mice, and elevated serum levels of IL-12 and IL-18 were observed in SLE patients [14,76-79]. Neumann et al. conducted intramuscular injection of IL-12 and IL-18 encoded plasmids alone or together on specific mouse models of SLE over expressing both TNF-γ and IFN-γ. Through the study, they observed decreased serum levels of TNF-γ and weakened productive ability of lymphocytes to produce IFN-γ in vitro. Synergistical injection of two plasmids not only attenuated the severity of lupus syndromes, lymphoproliferation in secondary lymphoid organs but also relieved proteinuria, kidney damage, and pneumonitis with decreased serum TNF-α concentration. Cells from lymph node secreted fewer levels of IFN-γ in IL-12/IL-18-treated mice while anti-dsDNA IgG levels were not affected (Figure 3) [80].
Summary
The imbalance of cytokines plays an important role in the pathogenesis of systemic lupus erythematosus with elevated levels of auto-antibody, deposits of complement and activation of immune responses [81,82]. Among various cytokines overexpressed or suppressed in SLE, abnormal production of IL-12 and IFN-γ gained much attention in old researches. Nevertheless, conflicts on IL-12 expression profile in SLE patients have been arising. Some studies demonstrated that IL-12 was upregulated in serum of patients at the active stage of SLE, while others reported defective IL-12 production in lupus patients [36,42]. As for IFN-γ, divergences were not so obvious and past literatures tend to suggest that serum levels of IFN-γ were higher in lupus patients than in controls [10-15]. In addition, IFN-γ was also associated with symptomatology of SLE, in which a subtle correlation was found between SLEDAI scores and serum IL-4/IFN-γ ratios and evident correlation of IFN-γ with SLAM scores was also documented [16,17]. Based on clinical discoveries, murine lupus model was applied to explore the internal mechanism and potential therapeutic effects. Treatment of IFN-γ or its inducers accelerated the lupus progression while IFN-γ antibody, recombinant IFN-γR presented longer overall survival and decreased histologic serologic parameters of lupus and significantly delayed lupus onset in B × W lupus mice [10].
Apart from clinical and animal studies, researches of gene polymorphisms were also performed and positive relationships between IL-12B rs3212227, IL-12B rs17860508 and susceptibility to and severity of SLE in Polish patients were observed. In addition, the combination of the IL-12B rs17860508 GC allele and/or IL-12B rs3212227 C allele increased the risk of SLE progression and/or severity of SLE parameters in Polish population [45]. Moreover, incorporation of IFN-γR1 Met14/Val14 and IFN-γR2 Gln64/Gln64 genotypes has been explored to be a potential risk factor for SLE. Based on the biological features of IL-12 and IFN-γ, scientists have developed the gene therapies aiming to correct IFN-γ and IL-12 expressive profile and have found their effects were promising in murine lupus. Intramuscular injection of IFN-γR/IgG1Fc fusion protein encoded cDNA and suppressive ODN were found to attenuate the severity of disease and promote survival of lupus mice. On the other hand, it was also found that intramuscular injection of IL-12 and IL-18 encoded plasmids alone or together had therapeutic effects on murine lupus. In general, abnormal expression of IL-12 activate Th1 cells and trigger upregulation of IFN-γ from Th1 cells towards Th1-predonminant immunity, causing subsequent production of autoantibodies and complements in SLE. On the other hand, gene polymorphisms of IL-12 and IFN-γ may partly describe the abnormal production of IL-12 and IFN-γ. Considering the severe adverse effects of classic immunosuppressant, IL-12 and IFN-γ targeted gene therapies were established and proved to be effective in murine lupus while multi-center clinical trials still need further progression.
Acknowledgements
This study was supported by Grants National Natural Science Foundation of China (No.81573217), and Scientific Research of BSKY from Anhui Medical University (No. XJ201301).
Conflict of Interest
The authors declare no conflict of interest.
References
- Arriens C, Mohan C (2013) Systemic lupus erythematosus diagnostics in the ‘omics’ era. Int J Clin Rheumatol 8: 671-687.
- Tikly M, Navarra SV (2008) Lupus in the developing world-is it any different? Best Pract Res Clin Rheumatol 22: 643-655.
- D’Cruz DP, Khamashta MA and Hughes GR (2007) Systemic lupus erythematosus: the lancet. Lancet 369: 587-596.
- Stypi?ska B, Paradowska-Gorycka A (2015) Cytokines and MicroRNAs as Candidate Biomarkers for Systemic Lupus Erythematosus. Int J Mol Sci 16: 24194-24218.
- Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, et al. (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64: 2677-2686.
- Arriens C, Wren JD, Munroe ME (2017) Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology (Oxford) 56: i32-i45.
- Dolff S, Bijl M, Huitema MG, Limburg PC, Kallenberg CGM, et al. (2011) Disturbed Th1, Th2, Th17 and Treg balance in patients with systemic lupus erythematosus. Clin Immunol 141: 197–204.
- Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, et al. (2016) Dysregulation of innate and adaptive serum mediators precede systemic lupus erythematosus classification and improve prognostic accuracy of autoantibodies. J Autoimmun 74: 182–193.
- Hayashi T (2010) Therapeutic Strategies for SLE Involving Cytokines: Mechanism-Oriented Therapies Especially IFN-γ Targeting Gene Therapy. J Biomed Biotechnol.
- Mathian A, Hie M, Cohen-Aubart F (2015) Targeting interferons in systemic lupus erythematosus: current and future prospects. Drugs 75: 835-846.
- Wong CK, Ho CY, Li EK, Lam CW (2000) Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9: 589–593.
- Guimarães PM, Scavuzzi BM, Stadtlober NP, Franchi Santos LFDR, Lozovoy MAB, et al. (2017) Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol 95: 824-831.
- Chiba A, Tamura N, Yoshikiyo K, Murayama G, Kitagaichi M, et al. (2017) Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res Ther 19: 58.
- Qian-Ling Ye, Run-Nan Guo Liu, Hui Qin, Yuan-Yuan Shen, Bin Wang, et al. (2017) Elevated plasma levels of IL-12 and IFN-γin systemic lupus erythematosus. J Clin Exp Pathol 10: 3286-3291.
- Min DJ, Cho ML, Cho CS, Min SY, Kim WU, et al. (2001) Decreased production of interleukin-12 and interferon-gamma is associated with renal involvement in systemic lupus erythematosus. Scand J Rheumatol 30: 159–163.
- Viallard JF, Pellegrin JL, Ranchin V, Schaeverbeke T, Dehais J, et al. (1999) Th1 (IL-2, interferon-γ [IFN-γ]) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin Exp Immunol 115: 189–195.
- Barcellini W, Rizzardi GP, Borghi MO, Nicoletti F, Fain C, et al. (1996) In vitro type-1 and type-2 cytokine production in systemic lupus erythematosus: lack of relationship with clinical disease activity. Lupus 5: 139–145.
- Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, et al. (2000) Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 201: 621–630.
- Yao X, Chen ZQ, Gong JQ, Chen M, Li AS, et al. (2007) The interferon-gamma receptor gene polymorphisms (Val14Met and Gln64Arg) are not associated with systemic lupus erythematosus in Chinese patients. Arch Dermatol Res 299: 367-371.
- Gerez L, Shkolnik T, Hirschmann O, Lorber M, Arad G, et al. (1997) Hyperinducible expression of the interferon-gamma (IFNgamma) gene and its suppression in systemic lupus erythematosus (SLE). Clin Exp Immunol 109: 296–303.
- Machold KP, Smolen JS (1990) Interferon-gamma induced exacerbation of systemic lupus erythematosus. J Rheumatol 17: 831–832.
- Vedove CD, Del Giglio M, Schena D (2009) Drug-induced lupus erythematosus. Arch Dermatol Res 301: 99-105.
- Robak E, Robak T, Wozniacka A, Zak-Prelich M, Sysa-Jedrzejowska A, et al. (2002) Proinflammatory interferon-γ-inducing monokines (interleukin-12, interleukin-18, interleukin-15): serum profile in patients with systemic lupus erythematosus. Eur Cytokine Netw 13: 364–368.
- Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, et al. (2008) Inborn errors of interferon (IFN)-mediated immunity in humans: in-sights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev 226: 29–40.
- Shirai A, Conover J, Klinman DM (1995) Increased activation and altered ratio of interferon-gamma: interleukin-4 secreting cells in MRL-lpr/lpr mice. Autoimmunity 21: 107–116.
- Murray LJ, Lee R, Martens C (1990) In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol 20: 163–170.
- Umland S, Lee R, Howard M, Martens C (1989) Expression of lymphokine genes in splenic lymphocytes of autoimmune mice. Mol Immunol 26: 649–656.
- Manolios N, Schrieber L, Nelson M, Geczy CL (1989) Enhanced interferon-gamma (IFN) production by lymph node cells from autoimmune (MRL/1, MRL/n) mice. Clin Exp Immunol 76: 301–306.
- Prud’homme GJ, Kono DH, Theofilopoulos AN (1995) Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1 beta, interleukin-1 and interferongammamRNA in the lymph nodes of lupus-prone mice. Mol Immunol 32: 495–503.
- Seery JP, Carroll JM, Cattell V, Watt FM (1997) Antinuclear autoantibodies and lupus nephritis in transgenic mice expressing interferon gamma in the epidermis. J Exp Med 186: 1451–1459.
- Jacob CO, van der Meide PH, McDevitt HO (1987) In vivo treatment of (NZB×NZW) F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med 166: 798–803.
- Ozmen L, Roman D, Fountoulakis M, Schmid G, Ryffel B, et al. (1995) Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferongamma receptor inhibits the onset of glomerulonephritis. Eur J Immunol 25: 6–12.
- Nicoletti F, Meroni P, DiMarco R, Barcellini W, Borghi MO, et al. (1992) In vivo treatment with a monoclonal antivody to interferon-gamma neither affects the survival nor the incidence of lupus-nephritis in the MRL/lpr-lpr mouse. Immunopharmacol 24: 11–16.
- Peng SL, Moslehi J, Craft J (1997) Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest 99: 1936–1946.
- Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F (2008) Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol 154: 247–254.
- Lit LCW, Wong CK, Li EKM, Tam LS, Lam CWK, et al. (2007) Elevated gene expression of Th1/Th2 associated transcription factors is correlated with disease activity in patients with systemic lupus erythematosus. J Rheumatol 34: 89–96.
- Gui L, Zeng Q, Xu Z, Zhang H, Qin S, et al. (2016) IL-2, IL-4, IFN-? or TNF-a enhances BAFF-stimulated cell viability and survival by activating Erk1/2 and S6K1 pathways in neoplastic B-lymphoid cells. J Cytokine 84: 37-46.
- Faraji F, Rastin M, Arab FL (2016) Effects of 1,25-dihydroxyvitamin D3 on IL-17/IL-23 axis, IFN-? and IL-4 expression in systemic lupus erythematosus induced mice model. Iran J Basic Med Sci 19: 374-380.
- Liu TF (1998) Impaired production of IL-12 in system lupus erythematosus. II: IL-12 production in vitro is correlated negatively with serum IL-10, positively with serum IFN-gamma and negatively with disease activity in SLE. Cytokine 10: 148-153.
- Li CS, Zhang Q, Lee KJ, Cho SW, Lee KM, et al. (2009) Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol 24: 1692–1696.
- Serra M1, Forcales SV, Pereira-Lopes S, Lloberas J, Celada A (2011) Characterization of Trex1 induction by IFN-γ in murine macrophages. J Immunol 186: 2299-2308.
- Shen H, Xia L, Xiao W, Lu J (2011) Increased levels of interleukin-27 in patients with rheumatoid arthritis. Arthritis Rheum 63: 860-861.
- Pflanz SJ, Timans J, Cheung R, Rosales R, Kanzler H, et al. (2002) IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4 + T cells. Immunity 16: 779-790.
- Illei GG1, Tackey E, Lapteva L, Lipsky PE (2004) Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum 50: 2048-2065.
- Gottschalk T, Tsantikos E, Hibbs M (2015) Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol 6: 550.
- Balomenos D, Rumold R, Theofilopoulos AN (1998) Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest 101: 364-371.
- Kyttaris VC, Sfikakis PP, Juang YT, Tsokos GC (2005) Gene therapy in systemic lupus erythematosus. Curr Gene Ther 5: 677-684.
- Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, et al. (2003) Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol 171: 1393–1400.
- Dong L, Ito S, Ishii KJ, Klinman DM (2005) Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum 52: 651–658
- Zeuner RA, Verthelyi D, Gursel M, Ishii KJ, Klinman DM (2003) Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum 48: 1701-1707.
- Sano K, Shirota H, Terui T, Hattori T, Tamura G (2003) Oligodeoxynucleotides without CpG motifs work as adjuvant for the induction of Th2 differentiation in a sequence-independent manner. J Immunol 170: 2367–2373.
- Sánchez E, Morales S, Paco L, López-Nevot MA, Hidalgo C, et al. (2005) Interleukin 12 (IL12B), interleukin 12 receptor (IL12RB1) and interleukin 23 (IL23A) gene polymorphism in systemic lupus erythematosus. Rheumatology (Oxford) 44: 1136-1139.
- Hirankarn N, Tangwattanachuleeporn M, Wongpiyabovorn J, Wongchinsri J, Avihingsanon Y (2009) Association of IL-18 gene polymorphism (-137C) with arthritis manifestations in SLE: combined effect with IFN gamma gene polymorphism (+874A). Clin Rheumatol 28: 219-223.
- Amerio P, Frezzolini A, Abeni D, Teofoli P, Girardelli CR, et al. (2002) Increased IL-18 in patients with systemic lupus erythematosus: relations with Th-1, Th-2, pro-inflammatory cytokines and disease activity: IL-18 is a marker of disease activity but does not correlate with pro-inflammatory cytokines. Clin Exp Rheumatol 20: 535–538.
- Wong CK, Li EK, Ho CY, Lam CW (2000) Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 39: 1078–1081.
- Lauwerys BR, Van Snick J, Houssiau FA (2002) Serum IL-12 in systemic lupus erythematosus: absence of p70 heterodimers but presence of p40 monomers correlating with disease activity. Lupus 11: 384–387.
- Yu S-L, Kuan W-P, Wong C-K, Li EK, Tam L-S (2012) Immunopathological Roles of Cytokines, Chemokines, Signaling Molecules, and Pattern-Recognition Receptors in Systemic Lupus Erythematosus. Clin Dev Immunol.
- Lit LC, Wong CK, Li EK, Tam LS, Lam CW, et al. (2007) Elevated gene expression of Th1/Th2 associated transcription factors is correlated with disease activity in patients with systemic lupus erythematosus. J Rheumatology 34: 89–96.
- Chan RW, Lai FM, Li EK, Tam LS, Chow KM, et al. (2007) Intrarenal cytokine gene expression in lupus nephritis. Ann Rheum Dis 66: 886-892.
- Akahoshi M, Nakashima H, Tanaka Y, Kohsaka T, Nagano S, et al. (1999) Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum 42: 1644–1648.
- Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, et al. (1999) IL-12 drives IFN-gamma-dependent autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol 163: 6884–6891.
- Fan X, Oertli B, Wüthrich RP (1997) Up-regulation of tubular epithelial interleukin-12 in autoimmune MRL-Fas (lpr) mice with renal injury. Kidney Int 51: 79-86.
- Van Raalte DH1, Ouwens DM, Diamant M (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39: 81–93.
- Prud'homme GJ (2000) Gene therapy of autoimmune diseases with vectors encoding regulatory cytokines or inflammatory cytokine inhibitors. J Gene Med 2: 222–232.
- Bossù P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen, et al. (2003) IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci U S A 100: 14181–14186.
- Guimarães PM, Scavuzzi BM, Stadtlober NP, Franchi Santos LFDR, Lozovoy MAB, et al. (2017) Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol 95: 824-831.
- Lawson BR, Prud'homme GJ, Chang Y, Gardner HA, Kuan J, et al. (2000) Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest 106: 207-215.
- Gottenberg JE, Chiocchia G (2007) Dendritic cells and interferon-mediated autoimmunity. Biochimie 89: 856–871.
- Welcher AA, Boedigheimer M, Kivitz AJ, Amoura Z, Buyon J, et al. (2015) Blockade of interferon-gamma normalizes interferon-regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol 67: 2713–2722
- Shirota H, Gursel M, Klinman DM (2004) Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol 173: 5002–5007.
- Dong L, Ito SI, Ishii KJ, Klinman DM (2004) Suppressive oligonucleotides protect against collagen-induced arthritis in mice. Arthritis Rheum 50: 1686–1689.
- Hagiwara E, Okubo T, Aoki I, Ohno S, Tsuji T, et al. (2000) IL-12-encoding plasmid has a beneficial effect on spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. Cytokine 12: 1035–1041.
- Mageed RA, Prud'homme GJ (2003) Immunopathology and the gene therapy of lupus. Gene Ther 10: 861-874.
- Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, et al. (2001) A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol 167: 5338–5347.
- Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, et al. (1999) IL-12 drives IFN-?-dependent autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol 163: 6884–6891.
- Park MC, Park YB, Lee SK (2004) Elevated interleukin-18 levels correlated with disease activity in systemic lupus erythematosus. Clin Rheumatol 23: 225–229.
- Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa-Jedrzejowska A (2006) Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 15: 268–275.
- Neumann D, Tschernig T, Popa D (2006) Injection of IL-12- and IL-18-encoding plasmids ameliorates the autoimmune pathology of MRL/Mp-Tnfrsf6lpr mice: synergistic effect on autoimmune symptoms. Int Immunol 18: 1779–1787.
- Paradowska-Gorycka A, Sowinska A, Stypinska B, Grobelna MK, Walczyk M, et al. (2016) Genetic Variants in IL-12B and IL-27 in the Polish Patients with Systemic Lupus Erythematosus. Scand J Immunol 84: 49-60.
- Guo Y, Chai Q, Zhao Y, Li P, Qiao J, et al. (2015) Increased activation of toll-like receptors-7 and -8 of peripheral blood mononuclear cells and upregulated serum cytokines in patients with pediatric systemic lupus erythematosus.
- Koenig KF, Groeschl I, Pesickova SS, Tesar V, Eisenberger U, et al. (2012) Serum cytokine profile in patients with active lupus nephritis. Cytokine 60: 410-416.
- Doria A, Briani C (2008) Lupus: improving long-term prognosis. Lupus 17: 166-170.
Citation: Wang B, Lu JS, Wang ZH (2017) Biological Features of IL-12 and IFN-γ in the Pathogenesis of Systematic Lupus Erythematosus. J Clin Exp Pathol 7: 323. DOI: 10.4172/2161-0681.1000323
Copyright: © 2017 Wang B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6936
- [From(publication date): 0-2017 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 5827
- PDF downloads: 1109