Review Article Open Access
Bowel Endometriosis: An Overview
Abu Taiub Mohammed Mohiuddin Chowdhury*, Zhuo YueDepartment of Digestive Disease, School of Medicine, Jiamusi University, Jiamusi, China
- *Corresponding Author:
- Abu Taiub Mohammed Mohiuddin Chowdhury
Department of Digestive Disease
The 1st affiliated hospital of Jiamusi University
School of Medicine. Jiamusi University, Jiamusi, China
Tel: 008618745463632
E-mail: dr_mohiuddinchy@yahoo.com
Received date: March 19, 2014,; Accepted date: May 12, 2014; Published date: May 17, 2014
Citation: Mohammed Mohiuddin Chowdhury AT, Yue Z (2014) Bowel Endometriosis: An Overview. J Gastroint Dig Syst 4:186. doi:10.4172/2161-069X.1000186
Copyright: © 2014 Chowdhury ATMM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Endometriosis is defined as the presence of ectopic endometrial gland or stroma outside the endometrial cavity. When endometriosis involves organs outside the pelvis is characterized as extragenital endometriosis. Bowl involvement with endometriosis is not a common occurrence, but when it happens it stimulates various symptoms of inflammation and sometime local neoplastic conditions which are rare occasion and may lead to malignant changes that have been discussed in this literature. Though it is a benign & estrogen dependent gynecological condition it has certain gastroenterological manifestations that are commonly seen in the various gastrointestinal disorders which misleads or delays the proper diagnosis and lengthens the suffering of the patient.
Introduction
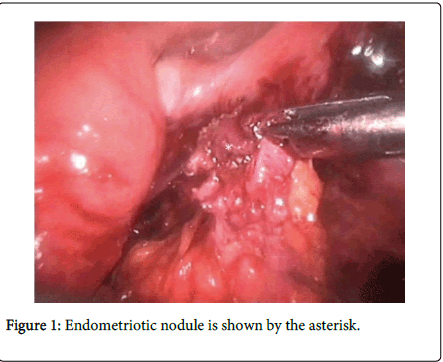
Though endometriosis predominantly affects the pelvic organs but the extra genital Endometriosis may involve the gastrointestinal tract at any site and can present with solitary or multiple areas of isolated satellite lesion or as nodule (Figure 1) with a strong association of pelvic endometriosis showing 15-35% cases of true multifocal involvement [1]. Though existence of independent bowl endometriosis has also been reported. Bowel endometriosis can be used to indicate the presence of endometrium like gland or stroma which infiltrate the intestinal wall involving the subserous fatty tissue along with the subserous part of the enteric plexus.
Presence of endometriotic foci in the serosa of the intestine without the evidence of involvement of subserous layer should be considered as peritoneal endometriosis [2]. Prevalence of bowl endometriosis in the general population is unknown but the highest frequency of involvement as endometriotic nodule is noted on the rectum including the rectosigmoid segment for about 70-80% of cases then followed by the sigmoid colon, ileum, the appendix and the cecum. The small intestine shows a less frequency of involvement [3]. Gastric and transverse colonic endometriotic involvement has also been reported. 0.53% of 7200 cases of small intestinal endometriosis have been presented in the Mayo Clinic over last 20 years [3]. According to autopsy studies incidence of appendeceal endometriosis has been estimated to be 0.054% of the general population [3]. Intestinal endometriosis is most commonly found within the subserosa as superficial serosal implants but may also be found infiltratingall the layer of the bowel wall. Under cyclical hormonal influences, these implants may proliferate and infiltrate the intestinal wall and cause a fibrotic reaction with formation of strictures and adhesions, which may lead to bowel obstruction and recurrent abdominal pain [4].
Histology
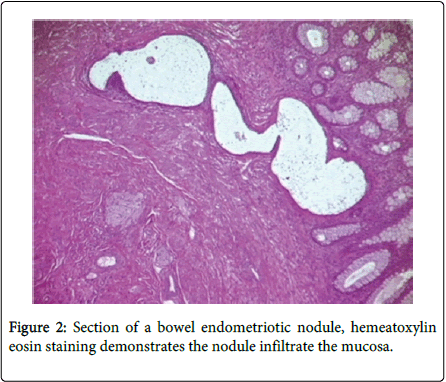
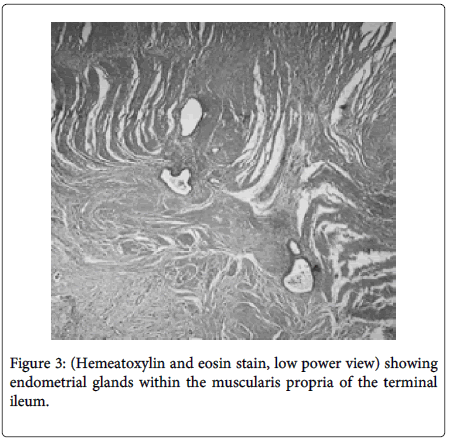
Bowel endometriosis shows variety in extent typically involving the serosa and the muscularis propria rarely shows involvement to the submucosa and mucosa (Figure 2). The implants of the endometrial stroma are usually found in the anti-mesenteric edge of the bowel. While microscopically the endometrial glands & stroma are seen to invade the bowel wall from serosa inwards. The extent varies from microscopic foci to large space occupying lesions invading the bowel wall causing significant narrowing of the lumen. Mural thickening and stenosis may be produced by smooth muscle hyperplasia and fibrosis surrounding the endometriotic nodule in case of larger lesions invading the muscular layer (Figure 3) [3,5,6].
Symptomology
Bowel symptoms are extremely common in patients with endometriosis. While 60% or more patients may have at least one symptom referable to their gastrointestinal tract though the exact percentage is difficult to pin down [7]. It remains unclear how bowel endometriosis causes intestinal symptoms. Endometriosis can cause bowel syndrome even when does not occur directly on the bowel. Inflammatory mediators released by the tissues by the response to inflammation include prostaglandin, tumor necrosis factor (TNF), interleukins and cytokines can affect the bowl and contribute to them [7]. They cause change with in the intestinal tissue by new vascularization, chemo taxis and scarring [7]. Diarrhea and intestinal cramping occurs due to increase smooth muscle contractility results by the release of Prostaglandin and likely other mediators that are released by the endometriosis implants and the uterus during menstruation. Occasionally, deep implants in adjacent structures such as the uterosacral ligaments or rectovaginal septum can also cause bowel symptoms. Large endometriotic nodules may contain extensive fibrosis and cause thickening of the bowel wall, resulting in a stenosis of the intestinal lumen. In addition they infiltrate and disrupt intestinal nervous plexus, damage interstitial cajal cells, reduce the density of intestinal sympathetic nerve fibers and thus, cause an alteration in bowel physiology [4,2]. Intestinal endometriotic lesions may have variable size and depth of infiltration in the bowel wall, therefore causes a wide range of symptoms [2]. Small endometriotic lesions affecting only the subserosal fatty tissue do not cause symptoms. A wide range of symptoms including dyschezia, constipation, diarrhea, abdominal bloating, painful bowel movements, passage of mucus in the stools and cyclical rectal bleeding can be produced by larger nodules infiltrating the intestinal muscular layer [2,6,8,9]. The symptoms associated with intestinal endometriosis often mimic irritable bowel syndrome and Inflammatory bowel disease Crohn’s Disease and Ulcerative Colitis. A part of the patients of endometriosis presenting with bowel symptoms may initially diagnosed as inflammatory bowel disease. Colonoscopic examination is usually required to confirm the diagnosis.
The extent of bowel involvement by endometriosis varies widely with a wide range of presenting symptoms. Disease limited to the bowel serosa may be asymptomatic [5]. The main manifestations are primary or secondary dysmenorrhea, bleeding disturbances, infertility, dysuria, pain on defecation (dyschezia), cycle-dependent or (later) cycle-independent pelvic pain, nonspecific cycle-associated gastrointestinal or urogenital symptoms. Most common in young and premenopausal women. With the presence of such manifestations, endometriosis should be considered in the differential diagnosis, and evidence for it should be sought. Patients presenting with mild or moderate symptoms, these symptoms become even more common. During their periods or pre-menstrually often these symptoms are more problematic. Women with symptoms similar to those of IBD but without any abnormalities on colonoscopy are often diagnosed with Irritable Bowel Syndrome (IBS). However after a series of GI tests a negative colonoscopy may be reassuring, indicating that endometriosis has not penetrated through the bowel wall. Unfortunately, bowel endometriosis may be an unexpected finding or may simply be overlooked if the possibility of bowel endometriosis was not fully entertained.
Bowel endometriosis includes following specific symptomatology: [3,2,7]
Painful defecation and tenesmus.
Cyclic hematochezia.
Change in bowel habits (diarrhea, constipation, hyperperistalsis, flatulence);
Rectal bleeding.
Rectal pain and painful defecation
Colonic endometriosis should be considered in women with chronic pelvic pain and intestinal bleeding, even if not cyclical with menstrual periods [10].
Diagnosis
Diagnosis of bowel endometriosis is still a clinical challenge as the clinical examination and the evaluation of the symptoms are inadequate for the accurate diagnosis of intestinal endometriosis. Although there is no gold standard universally accepted method but Magnetic resonance imaging (MRI) is one of the most commonly used techniques. Though Multidetector computerized tomography enteroclysis (MDCT-e) has recently been suggested for the diagnosis of bowel endometriosis [11,12]. On the other hand though the diagnostic accuracy of ultrasonography depends on the experience of the operator, several studies have demonstrated that rectosigmoid endometriosis can not only be accurately diagnose by transvaginal ultrasonography it also may detect the depth of infiltration of the nodule in the intestinal wall [8,13-16]. During transvaginal ultrasonography after a proper bowel preparation by adding water contrast to the rectum may facilitate the identification and characteristic evaluation of the endometriotic nodular lesion [17-19]. Rectal endoscopic ultrasonography has been widely used for the diagnosis of intestinal endometriosis [20-22]. This permits to estimation of the precise depth of infiltration of endometriosis lesion in the intestinal wall, the maximum diameter of the lesions and the distance of the lesions from the anus [22].
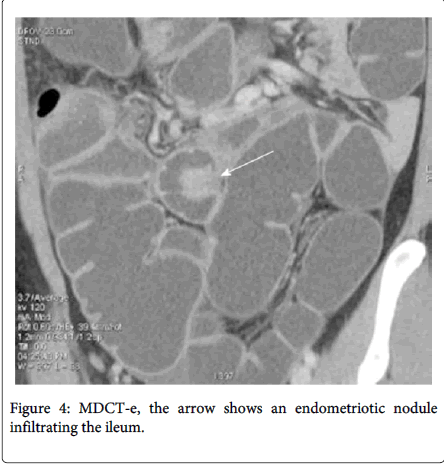
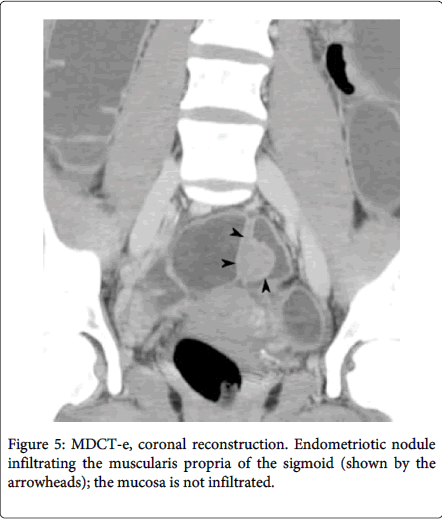
Multidetector computerized tomography enteroclysis (MDCT-e) can identify the presence of endometriosis foci and also evaluate the characteristic, depth and infiltration of the nodule, by the presence of nodule adjacent to the bowl loop resulting in irregular intestinal outline the infiltration of the endometriosis can be characterized (Figure 4). Multilayered stratification of the thickened bowel wall is observer when the muscularis propria is infiltrated (Figure 5). Presence of a hypodense layer between the lumen and the muscularis propria indicate the full thickness infiltration of the bowel wall involving the submucosa and the mucosa [11,12]. Use of iodinated contrast medium and ionizing radiations is the major disadvantage of MDCT-e [2]
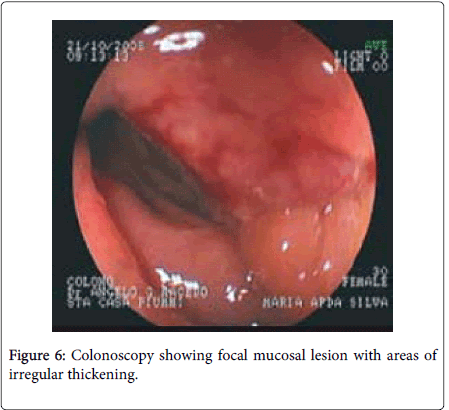
Endoscopic diagnosis of intestinal endometriosis is difficult because the lesion hardly reaches the intestinal mucosa, though it can be evaluated by the presence of localized pathological foci, growth, presence of mucosal lesion (Figure 6) [23,24,10]. Since endometriosis is located in the bowel wall or in muscle layer as implants, narrowing, spasm, discoloration, and hyperemia in the affected bowel wall can be seen through colonoscopy. As the biopsies from the suspected area do not contain submucosa and muscle layers, they do not allow for diagnosis [25]. A case reported by A.G.Macedo on 27th Feb. 2008 revels a 5 cm long laterally spreading lesion in the recto-sigmoid junction occupying half of its lumen. The lesion was characteristically friable in nature slightly harden with irregular surface. The histopathology of the biopsy sample obtained from the lesion confirms intestinal endometriosis [10]. Another study on diagnostic colonoscopy shows detection of multifocal lesion in the colonic mucosa revealing authentic signs of colorectal endometriosis which include polypoid growth over the endometriotic foci, endometrioid heterotopias in the colonic mucosa presence of ulceration in the mucosal membrane in the endometriotic projection and geoidal mucosa [23].
Intestinal obstruction
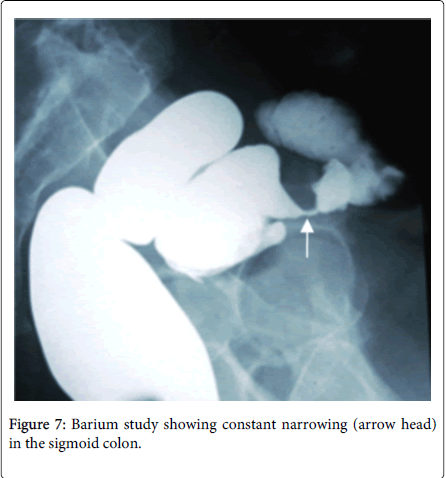
Partial of complete intestinal obstruction can be produced by endometriosis by various manifestations like angulation of the bowl or localized endometriosis causing pressure over the lumen interrupting the flow or causing intussusceptions. Commonly observed phenomena is stenosis of bowl lumen (Figure 7) caused by smooth muscle hyperplasia and fibrosis by benign ectopic glandular tissue and localized repeated inflammation of the bowl wall due to cyclical hemorrhage [26]. Case reported by Unalp HR shows acute intestinal obstruction to a 45 year old lady due to ileal endometriosis (Histology reveals endometriosis with annular stricture showed foci of ectopic glandular tissue) where laparotomy evaluated an ulcerated, edematous and fragile segmental lesion above the iliocecal valve [27]. Case reported by A Alhumidi describes two free constricting mass in the sigmoid colon involving the serosal surface of the lumen causing symptoms to a 41 year old lady with 18 years of known history of endometriosis.
Presented with cyclical history of fresh bright red bleeding per rectum with abdominal pain associated with dyschezia. Colonoscopy reveals stricture in the sigmoid colon and barium study showed area of constant narrowing at the sigmoid colon.
Intestinal perforation
As mostly the affected cases of bowel endometriosis the intestinal mucosa remains intact, a transmural intestinal wall involvement causing perforation of the intestinal tract is a very rare complication. 42.8% among these reported cases are occurred in pregnancy. Sigmoid colon is the most common site of perforation among the published case. Although endometriosis improves during pregnancy under the effect of progesterone the ectopic endometrium becomes decidualized with a progressive reduction in size [28,29].
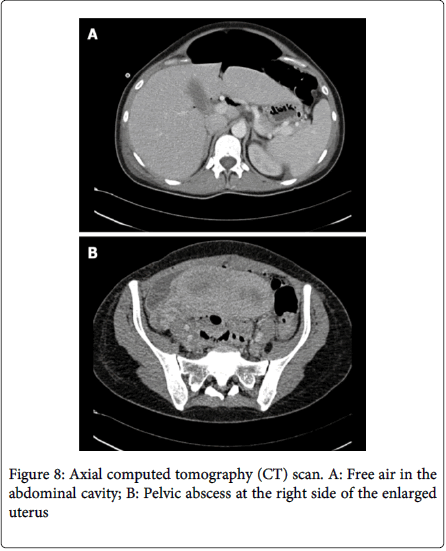
A case reported by Adolfo Pisanu [Which is believed to be the first reported case of spontaneous rectal perforation from endometriosis in pregnancy] shows perforation of rectum in the 30th week of pregnancy where symptoms were suggestive of pyelonephritis, later evaluated as a 3 cm wide perforation of rectal wall which shows Histopathological findings were consistent with decidualization of the rectal wall. Intestinal wall becomes weaken by the reduction of the transmural endometriotic lesion under the influence of progesterone during pregnancy, particularly in the third semester. Perforation may also facilitate by progressive traction of the enlarged uterus on the strictly adherent loops of intestine [29]. Haufler described a jejunal perforation due to rupture of an endometriotic cyst during the sixth month of pregnancy in a 30 years old woman. In 1955, Henriksen briefly mentioned a case of sigmoid perforation in his series of 1000 cases of endometriosis. Clement in 1977, Rud in 1979, and, most recently, Schweitzer also reported similar cases of sigmoid colonic perforation secondary to endometriosis during pregnancy [3,30-32]. Colonic perforation by endometriosis generally presents with asymptomatic or painful pelvic mass (Figure 8). A case reported by Neeraj Kumar Garg [30,31] shows a perforation of sigmoid colon by a large endometriotic mass arising from the left fallopian tube and ovary, which had adhered to the sigmoid colon in a previously fit 44years old patient with previous known history of mild endometriosis (Figure 9). Presented with pyrexia, 10 days history of worsening colicky pain in the left flank and iliac fossa with a mass.
Inflammatory Bowl Disease
A study report published by Tine Jess on unselected nationwide Danish cohort of 37,661 women hospitalized with endometriosis during 1977–2007 shows that the risk of IBD in women with endometriosis was increased even in the long term, hence suggesting a genuine association between the diseases. Women with endometriosis had a increased risk of Inflammatory Bowel Disease overall (Standardized Incidence Ratio=1.5; 95% CI 1.4 to 1.7) and of Ulcerative Colitis (Standardized Incidence Ratio=1.5; 95% CI 1.3 to 1.7) and Crohn’s Disease (Standardized Incidence Ratio=1.6; 95% CI 1.3 to 2.0) separately, even 20 years after a diagnosis of endometriosis (Ulcerative Colitis: Standardized Incidence Ratio=1.5; 95% CI 1.1 to 2.1; Crohn’s Disease: Standardized Incidence Ratio=1.8; 95% CI 1.1 to 3.2). Restricting analyses to women with surgically verified endometriosis suggested even stronger associations (Ulcerative Colitis: Standardized Incidence Ratio=1.8; 95% CI 1.4 to 2.3; Crohn’s Disease: Standardized Incidence Ratio=1.7; 95% CI 1.2 to 2.5). [33]
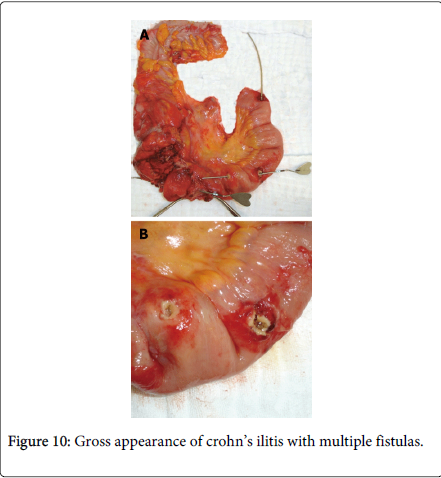
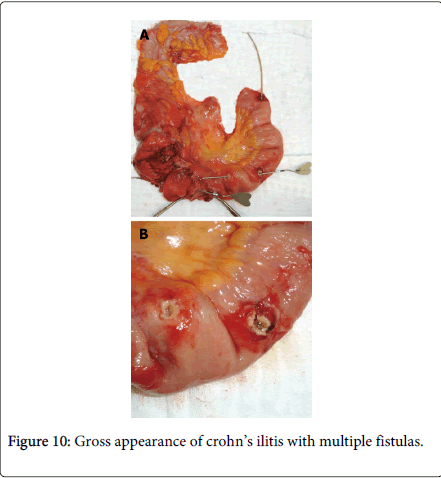
Zafer Teke reported a case of complicated Crohn’s disease clinically mimicking ileal endometriosis in a 31 year old woman due to her premenstrual symptoms of mechanical subileus for 3 years; at first monthly, but later continuous, and gradually increasing in severity. A right hemicolectomy and partial distal ileum resection were performed but histopathology revealed Crohn’s disease without endometrial tissue. The differential diagnosis of Crohn’s disease with intestinal endometriosis is difficult as they share overlapping clinical, radiological and pathological features. They both are characterized grossly by patchy involvement of small intestine with intervening uninvolved skip areas. Moreover transmural process with chronic inflammatory changes results in formation of strictures, adhesion, mucosal thickening, mural fibrosis and obstruction and shares common manifestation such as perianal abscess or fistulas (Figure 10) and inflammatory pseudo tumor involving the cecum terminal ilium and the appendix. In rare case endometriosis may cause deep fissures even fistulous tracts, further mimicking regional enteritis [33].
Malignant transformation
The phenomenon of malignant transformation of endometriosis was first described by Sampson in 1925. The gastrointestinal tract involvements were classified as endometriosis associated intestinal tumors (EAITs). Development of a malignancy is a relatively common complication of endometriosis and may occur in up to 5.5% of female patients with endometriosis and malignant transformation of gastrointestinal endometriosis with out pelvic involvement is uncommon [34]. However malignant transformation of the intestinal tract not been reported as frequently [35]. An exogenous estrogen is the widely accepted factor for the development of premalignant or malignant transformation of endometriosis [36,37]. Endometrioid carcinoma is a common histologic type confuse with colorectal carcinoma. Colon carcinomas always involve the mucosa and in advanced case and extend through the bowl wall in to serosa or adjacent fat. While endometriosis shows an opposite extramural growth pattern invading in to the bowl mucosa from the serosa [38,39]. The colon carcinoma can be differentiated from the endometrioid adenocarcinoma by the presence of a background of benign endometriosis [40]. As practically accurate diagnosis is difficult so for confirmation of immunochemistry is the recommended technique for all cases of EAITs [41,42]. Nine cases of primary EAITs arising in the rectosigmoid colon in patients using unopposed estrogen for several years prior to the development of carcinoma was reported by Jones where they concluded that their findings supported a casual, cancer causing roll of unopposed estrogen [43].
Case reported by Joaquin Marchena-Gomez described a 45 year old lady with past medical history of ovarian endometriosis treated with bilateral oophorectomy received treatment with medroxyprogesterone and transdermal estrogens presented with paroxistic abdominal pain and vomiting. Intestinal obstruction was confirmed by an X-ray and laparotomy revealed an ileal mass protruding in to the lumen where morphologic and immunohistochemical features were typical of an endometrioid carcinoma. Several endometriosis foci were identified adjacent to the mass. The prognosis for estrogen-stimulated EAIT arising in any gastrointestinal site is highly favorable with a survival rate of 82 per cent at 5 years.
Another case reported in Red Orbit in Sept 2005 describes a primary endometrioid carcinoma arising in the rectal wall endometriosis in a 60 year old woman presented with hematochezia with history of extensive endometriosis and total abdominal hysterectomy with bilateral salpingo-oophorectomy 10 years earlier. She underwent colonoscopy which revealed a 3.5 to 4 cm sessile polyp in the anterior rectal wall which was partially excised. Pathologic specimens of hysterectomy and oophorectomy specimen was reexamined and found to contain benign endometriosis.
Management
Management of patient with bowel endometriosis depends on the patient’s symptoms, size of the nodule and the degree of luminal stenosis. Large endometriotic lesion causing symptoms can be treated by hormonal therapy or surgery or both. In case of uncomplicated intestinal endometriosis treatment depend patient’s age and intention to conceive, If malignancy cannot be excluded with symptoms of obstruction or bleeding in case of child bearing age resection of the involved bowl followed by hormonal therapy is the choice otherwise hysterectomy with bilateral oophorectomy is indicated and with a past history of endometriosis or co-existent gynecological symptoms a laparoscopy prior to laparotomy should be considered [30,31]. Though in case of estimated bowl stenosis <60% hormonal therapy can be safely offered but because of ovulation inhibition, patients willing to conceive are not good candidates for this therapy [2]. Options for pharmacotherapy include Gestagens, Oral contraceptive drugs, GnRH analogues and analgesics [44]. Progestins are most commonly used which is in general well tolerated and effectively reduce the severity of symptoms caused by pelvic endometriosis. More recently, aromatase inhibitors have been suggested for the treatment of endometriosis [2]. Colorectal endometriotic nodules infiltrating the intestinal muscularis propria can be excised by either by partial or full thickness nodulectomy or segmental bowel resection by laparoscopy or laparotomy, though women with colorectal endometriosis, segmental bowel resection improves pain, intestinal symptoms and quality of life [45,46]. However nodulectomy may have the advantage of a lower incidence of postoperative unpleasant functional digestive outcomes compares with the segmental bowl resection [47,48]. Several studies have showed that good pregnancy rates are achieved after surgical excision of intestinal endometriosis. A study of 288 women wishing to conceive demonstrated that nodulectomy is associated with an 84% pregnancy rate obtained either naturally or after assisted reproductive technologies, after a median follow-up of 3.1 years [2].
Conclusion
infectious etiology, ischemic enteritis/colitis, inflammatory bowel disease, and neoplasm’s [48].unopposed estrogen hormone replacement and currently
References
- Redwine DB (1999) Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. FertilSteril 72: 310-315.
- Bowel endometriosis: Recent insights and unsolved problems (2011) Simone Ferrero, Giovanni Camerini, Umberto Leone Roberti Maggiore, Pier L Venturini, EnnioBiscaldi,lentinoRemorgida. World journal of Gastrointestinal Surgery.
- Laparoscopic management of intestinal endometriosis by DoronKopelman; Edited by Louise P. King MD, JD, CamranNezhat MD. Prevention & management 3rd edition
- Garg NK, Bagul NB, Doughan S, Rowe PH (2009) Intestinal endometriosis--a rare cause of colonic perforation. World J Gastroenterol 15: 612-614.
- Remorgida V, Ferrero S, Fulcheri E, Ragni N, Martin DC (2007) Bowel endometriosis: presentation, diagnosis, and treatment. ObstetGynecolSurv 62: 461-470.
- Yantiss RK, Clement PB, Young RH (2001) Endometriosis of the intestinal tract: a study of 44 cases of a disease that may cause diverse challenges in clinical and pathologic evaluation. Am J SurgPathol 25: 445-454.
- Endometriosis and Bowel Symptoms by Ken Sinervo.
- Bazot M, Thomassin I, Hourani R, Cortez A, Darai E (2004) Diagnostic accuracy of transvaginalsonography for deep pelvic endometriosis. Ultrasound ObstetGynecol 24: 180-185.
- Marcoux S, Maheux R, Bérubé S (1997) Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med 337: 217-222.
- Case report: Intestinal endometriosis diagnosed through colonoscopy. A G Macedo, J.Sausa, G>P.Pena, K.R.Bertges, L.C.Bertges, CLIGED.
- Biscaldi E, Ferrero S, Fulcheri E, Ragni N, Remorgida V, et al. (2007) Multislice CT enteroclysis in the diagnosis of bowel endometriosis. EurRadiol 17: 211-219.
- Biscaldi E, Ferrero S, Remorgida V, Rollandi GA (2007) Bowel endometriosis: CT-enteroclysis. Abdom Imaging 32: 441-450.
- Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, et al. (2007) Accuracy of transvaginalsonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound ObstetGynecol 30: 994-1001.
- Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, et al. (2009) Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod 24: 602-607.
- Hudelist G, Tuttlies F, Rauter G, Pucher S, Keckstein J (2009) Can transvaginalsonography predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Hum Reprod 24: 1012-1017.
- Goncalves MO, Podgaec S, Dias JA Jr, Gonzalez M, Abrao MS (2010) Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod 25: 665-671.
- Menada MV, Remorgida V, Abbamonte LH, Fulcheri E, Ragni N, et al. (2008) Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. FertilSteril. FertilSteril: 89: 699-700.
- ValenzanoMenada M, Remorgida V, Abbamonte LH, Nicoletti A, Ragni N, et al. (2008). Does transvaginal ultrasonography combined with water-contrast in the rectum aid in the diagnosis of rectovaginal endometriosis infiltrating the bowel? Hum Reprod 23: 1069-1075.
- Morotti M, Ferrero S, Bogliolo S, Venturini PL, Remorgida V, et al. (2010) Transvaginal ultrasonography with water-contrast in the rectum in the diagnosis of bowel endometriosis. Minerva Ginecol 62: 179-185.
- Chapron C, Vieira M, Chopin N, Balleyguier C, Barakat H, et al. (2004) Accuracy of rectal endoscopic ultrasonography and magnetic resonance imaging in the diagnosis of rectal involvement for patients presenting with deeply infiltrating endometriosis.UltrasoundObstetGynecol 24:175-179.
- Chapron C, Dumontier I, Dousset B, Fritel X, Tardif D, et al. (1998) Results and role of rectal endoscopic ultrasonography for patients with deep pelvic endometriosis. Hum Reprod 13: 2266-2270.
- Bazot M, Detchev R, Cortez A, Amouyal P, Uzan S, et al. (2003) Transvaginalsonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod 18: 1686-1692.
- MatronitskiÄ RB, Mel'nikov MV, Chuprynin VD, Askol'skaia SI, Khabas GN, et al. (2013) Endoscopic diagnostics of colorectal endometriosis. EkspKlinGastroenterol : 11-14.
- Paksoy M, Karabiçak I, Ayan F, Aydoğan F (2005) Intestinal obstruction due to rectal endometriosis. Mt Sinai J Med 72: 405-408.
- Meyers WC, Kelvin FM, Jones RS (1979) Diagnosis and surgical treatment of colonic endometriosis. Arch Surg 114: 169-175.
- A Alhumidi, M Hamodat (2008). Colonic Endometriosis Mimicking Colonic Carcinoma. The Internet Journal of Pathology 8:2
- Unalp HR, Akguner T, Yavuzcan A, Ekinci N (2012) Acute small bowel obstruction due to ileal endometriosis: a case report and review of the most recent literature. Vojnosanit Pregl 69: 1013-1016.
- McArthur JW, Ulfelder H (1965). The effect of pregnancy upon endometriosis. ObstetGynecolSurv. 20:709-733.
- Faucheron JL, Pasquier D, Voirin D (2008) Endometriosis of the vermiform appendix as an exceptional cause of acute perforated appendicitis during pregnancy. Colorectal Dis 10: 518-519.
- Pisanu A, Deplano D, Angioni S, Ambu R, Uccheddu A (2010) Rectal perforation from endometriosis in pregnancy: case report and literature review. World J Gastroenterol 16: 648-651.
- Endometriosis of the Appendix. Nasser S Al Oulaqi, Ashraf F Hefny, [...], and Fikri M Abu-Zidan.\ African Health Sciences Sept 2008, Makerere University Medical School. 34. Haufler F. Ungewöhnlicheomplikation der SchwangerschaftinfolgeendometrioiderHeterotopien am Dünndarm. Virchows Arch. 280: 822-828.
- HENRIKSEN E (1955) Endometriosis. Am J Surg 90: 331-337.
- Jess T, Frisch M, Jørgensen KT, Pedersen BV, Nielsen NM (2012) Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut 61: 1279-1283.
- Heaps JM, Nieberg RK, Berek JS (1990) Malignant neoplasms arising in endometriosis. ObstetGynecol 75: 1023-1028.
- Stern RC, Dash R, Bentley RC, Snyder MJ, Haney AF, et al. (2001) Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J GynecolPathol 20: 133-139.
- Reimnitz C, Brand E, Nieberg RK, Hacker NF (1988) Malignancy arising in endometriosis associated with unopposed estrogen replacement. ObstetGynecol 71: 444-447.
- Chang HY, ChangchienCC,Chen HH, Lin H, Huang CC et al. (2005) Extrauterine müllerian adenosarcoma associated with endometriosis and rectal villotubular adenoma: Report of a case and review of the literature. Int J Gynecol Cancer 15: 361-365.
- Slavin RE, Krum R, Van Dinh T (2000) Endometriosis-associated intestinal tumors: a clinical and pathological study of 6 cases with a review of the literature. Hum Pathol 31: 456-463.
- Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, et al.(2007) Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: A Gynecologic Oncology 25: 526-531.
- Heaps JM, Nieberg RK, Berek JS (1990) Malignant neoplasms arising in endometriosis. ObstetGynecol 75: 1023-1028.
- Han AC, Hovenden S, Rosenblum NG, Salazar H (1998) Adenocarcinoma arising in extragonadal endometriosis: an immunohistochemical study. Cancer 83: 1163-1169.
- Lagendijk JH, Mullink H, Van Diest PJ, Meijer GA, Meijer CJ (1998) Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol 29:491-497.
- Jones KD, Owen E, Berresford A, Sutton C (2002) Endometrial adenocarcinoma arising from endometriosis of the rectosigmoid colon. GynecolOncol 86: 220-222.
- The Diagnosis and Treatment of Deep Infiltrating Endometriosis. Gülden Halis, Dr. med.,1,2 Sylvia Mechsner, Dr. med.,3 and Andreas D. Ebert, Prof. Dr. med. Dr. phil. Dr. h. c
- Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, et al. (2006) Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod 21: 1243-1247.
- Ferrero S, Camerini G, Menada MV, Biscaldi E, Ragni N, et al. (2009) Uterine adenomyosis in persistence of dysmenorrhea after surgical excision of pelvic endometriosis and colorectal resection. J Reprod Med 54: 366-372.
- Roman H, Loisel C, Resch B, Tuech JJ, Hochain P, et al. (2010) Delayed functional outcomes associated with surgical management of deep rectovaginal endometriosis with rectal involvement: giving patients an informed choice. Hum Reprod 25:890-899.
- Crohn’s disease complicated by multiple stenoses and internal fistulas clinically mimicking small bowel endometriosis. ZaferTeke, FarukOnderAytekin, [...], and NeseCalliDemirkan. World Journal of Gastroenterology Jan 7 2008.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 27347
- [From(publication date):
June-2014 - Aug 13, 2025] - Breakdown by view type
- HTML page views : 22538
- PDF downloads : 4809