Breeding for Quality Protein Maize (QPM)
Received: 01-Dec-2023 / Manuscript No. acst-23-122960 / Editor assigned: 04-Dec-2023 / PreQC No. acst-23-122960 / Reviewed: 18-Dec-2023 / QC No. acst-23-122960 / Revised: 22-Dec-2023 / Manuscript No. acst-23-122960 / Published Date: 29-Dec-2023
Abstract
In maize, zeins are the main protein components of seed stores. To alleviate protein deficiency, protein content can be increased to as high as 18% by increasing the prolamine (zein) fraction inmaize endosperm but unfortunately it consequently led to lysine and tryptophan deficiency. Zeins are the prolamins of maize grain which are soluble in an alcohol having one major class (α-zeins) and three minor classes (β, γ, and δ). These four types constitute about 50- 70% of maize endosperm and are essentially rich in glutamine, leucine and proline and poor in lysine and tryptophan causing malnutrition. The opaque-2 (o2)-a natural recessive mutation in maize led to nearly double the lysine and tryptophan content in endosperm due to a decrease in the synthesis of zein proteins and increase in the other seed protein bound lysine and tryptophan. The proportion of lysine and tryptophan in the total portion of protein were found to be almost double in QPM materials (4.1% and1%, respectively) than in non-QPM (2.7% and 0.6%, respectively). QPM also showed a corresponding increase in tryptophan content, which doubles the biological value of ordinary maize protein. Breeding for improved protein quality in maize commence in the mid-1960s with the discovery of mutants, such as opaque-2 (o2 However, successful utilization of these mutants is not achieved due to some adverse pleiotropic effects. So, researchers use two genetic systems Exploiting double-mutant combinations and Simultaneous use of o2gene and the genetic modifiers of the o2 locus. However, there was certain drawback like double mutant combination were not always vitreous and yield was severely affected due to the sum total of independent negative effects of two mutation. Combiningopaque-2 allele with its desirable genetic modifiers made it possible to breed QPM genotypes having hard kernel with high lysine and tryptophan content. Since, opaque-2 is a recessive mutation and endosperm specific, conventional backcross breeding alone is inefficient for the nutritional enrichment of maize. However, use of opaque-2 gene specific markers provided excellent opportunities for conversion of elite normal inbreds to homozygous o2/o2 forms through marker assisted selection (MAS)
Keywords
Maize opaque-2 zeian tryptophan breeding approaches
Introduction
Maize (Zea mays L.) is an important cereal crop of the World. It is a member of grass family poaceae and is highly cross pollinated crop. Maize (2n = 2x = 20) is the third most important cereal crop in the world after rice and wheat, and it is believed to have originated in Mexico, and to have been introduced to Ethiopia in the 1600s to 1700s (McCann, 2005). It is cultivated in a wider range of environments than wheat and rice because of its greater adaptability (Koutsika-Sotiriou, 1999) [1].
Maize contributes 15% of the world’s protein and 19% of the calories derived from food crops. Millions of people in the world, and particularly in developing countries, derive a part of their protein In Africa, maize supplies at least one fifth of total daily calories and accounts for 17% to 60% of the total protein supply per day of individuals who are more susceptible to risk of protein or essential amino acid deficiencies (Krivanek, et al. 2007). Millions of smallholder farmers in the major maize producing regions of Ethiopia depend on maize for their daily food throughout the year and they have almost no access to protein sources like meat, eggs and milk for their daily consumption (Dereje et al., 2001) [2].
Maize is the queen of cereal crops with highest grain yield potential. Endosperm is the store house of seed storage proteins. Maize grains contain around 9% protein. The major fraction (60%) of seed protein in maize is zeins (a prolamin group-alcohol soluble) (Leite et al. 1999) followed by glutelin (34%), while albumin and globulin occur in traces (3% each). A balanced protein is required to assist body building process and therefore, amino acid balance seems to be a determining factor for quality of any food and feed [3].
To alleviate malnutrition, protein content can be increased to as high as 18% by increasing the prolamine (zein) fraction in maize endosperm (Dudley & Lambert 1969), but unfortunately it consequently led to lysine and tryptophan deficiency. Many researchers around the globe have tried to address the problem using quality protein maize (QPM). In this pursuit, the research focus on the genetic basis and prospects of quality protein maize for amino acid amelioration and enhancing the productivity of maize through the development of heterotic hybrids using elite QPM introgression lines [4].
Quality protein maize (QPM) was developed in the late 1960s (Prasanna, B.M 20010) and it produces 70% to 100% more lysine and tryptophan than ordinary modern and traditional varieties of tropical maize (Bjarnason, M et al., 1992) Additionally, nutritional evaluation of QPM in various locations has proved the stability of lysine and tryptophan content within the prescribed range for QPM, in spite of quite diverse types of environmental conditions (Zaidi 2008). The nutritional quality of the protein in QPM grain approaches that of protein derived from cow’s milk (Prasanna, B.M et al 2010). The adoption of QPM can contribute immensely to alleviation of malnutrition in maize-based economies in developing countries (Zaidi 2008). In this pursuit, this paper deals with the prominent series of events accompanied with the development of QPM, nutritional and improvement of protein quality mechanism of o2 mutant and problem associated with o2mutant, breeding, genetics and molecular approach that could used to get efficient QPM cultivars [ 5-7].
Objective
To review the quality traits, measurements and possible breeding methods to improve protein quality of maize.
Literature Review
Structure of maize kernel
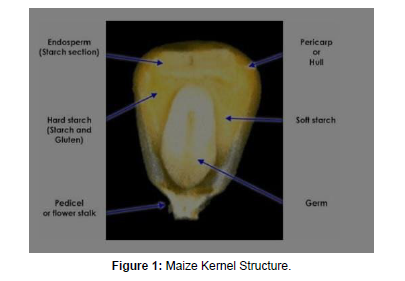
Maize kernel mainly consists of three parts: pericarp (6%), embryo (12%) and endosperm (82%). The pericarp is the outer covering of the kernel that protects and preserves the nutrient value inside of it. A thin, suberized nucellar membrane acquired from the outer epidermal wall of the nucellus persists as a continuous covering between the aleurone and the pericarp [8]. The embryo is located in one face of the basal part of the kernel. A mature embryo is comprised by the embryo axis and the scutellum. Both the embryo and endosperm contain proteins but the germ proteins are superior in quality as well as quantity. Most of the volume and weight of the kernel is accomplished by the endosperm. It can be divided into three parts: starchy endosperm, aleurone layer, and the basal transfer layer. The aleurone layer is the outer most layers secreted by specialized cells, rich in hydrolytic enzymes. Starch-rich endosperm is present within the aleurone layer having vitreous and starchy regions [9-12].
The zein proteins form insoluble accretions which are acquired in a vitreous region called protein bodies in the lumen of rough endoplasmic reticulum and it is densely packed between starch grains towards maturity (Gibbon, and Larkins, 2005). Zeins are the prolamins of maize grain which are soluble in an alcohol having one major class (α-zeins) and three minor classes (β, γ, and δ). These four types constitute about 50-70% of maize endosperm and are essentially rich in glutamine, leucine and proline and poor in lysine and tryptophan (Nelson,.1969) [13,14].
Higher proportion of leucine (18.7%), phenylalanine (5.2%) isoleucine (3.8%), valine (3.6%) and tyrosine (3.5%) are normally present in zein fraction, while other essential amino acids such as threonine (3%), histidine and cysteine (1%), methionine (0.9%), lysine (0.1%) are in smaller amounts and is significantly deficient in tryptophan as it is devoid from the major prolamin fraction (α-zeins) of maize kernel. Non-zeins include other proteins such as globulins (3%), glutelins (34%) and albumins (3%). The nonzein protein fraction is balanced and rich in lysine and tryptophan (Vasal,.2002) [15-17] (Figure 1).
Nutritional quality and impact of QPM
Malnutrition is a persistent problem in Africa, especially in rural areas where poor people depend on staple foods and have limited access to a diverse diet. Bio-fortified crops bred for improved nutritional quality can alleviate nutritional deficiencies if they are produced and consumed in sufficient quantities (De Groote et al. 2010) several studies in controlled settings have indicated the positive impact of QPM on the nutritional status of human consumption and animal feeds (Twumasi- Afriye, et al. 2016) [18].
A natural, spontaneous, recessive mutation of maize with soft opaque grains was first described by Jones and Singleton in the early 1920s (Emerson, et al. 1935). However, the nutritional significance of the mutation was first discovered by Mertz and coworkers (Nelson, 1965). In 1963, researchers at Purdue University in USA discovered that opaque-2 (op2) created grain proteins in the endosperm nearly twice as those found in normal maize (Mertz, et al. 1965) [19].
Endosperm of maize grain is the store house of storage proteins. Homozygous maize varieties with two copies of the mutation (recessive o2 allele) have substantially higher lysine (69%) in grain endosperm compared to normal maize. The proportion of lysine and tryptophan in the total portion of protein were found to be almost double in QPM materials (4.1% and1%, respectively) than in non-QPM (2.7% and 0.6%, respectively) (Table 1) [20].
| Total Protein | Lysine | Tryptophan | |
|---|---|---|---|
| QPM(%) | >9 | 4.1 | ####### |
| Non-QPM(maize flour) | >9 | 2.7 | ####### |
| FAO recommendations(g/100g total protein ) | |||
| Children | 6.6 | ####### | |
| adult | 1.6 | ####### | |
Table 1: Proportion of lysine and tryptophan in the total protein of non-QPM, QPM material, and the recommended protein proportion for children and adults
Additionally, QPM also showed a corresponding increase in tryptophan content, which doubles the biological value of ordinary maize protein. To classify the maize grain as QPM, the quality index, which is the tryptophan-to-protein ratio in a given sample, must be higher or equal to 0.8%. Consumption of QPM could go a long way in reducing the growing challenge of malnutrition in various parts of the world (.Akuamoa-Boateng 2002, Menkir et.at,. 2008) The nutritional benefits of QPM have been demonstrated numerous times in feeding experiments involving livestock(Mpofu et.ai,.2012) and children (Akuamoa-Boateng,2002). For instance, children who ate porridge made from QPM had fewer sick days relative to those who ate porridge from common maize (CM). Consumption of QPM instead of CM resulted in a 12% increase in the rate of growth in weight and 9% increase in the rate of growth in height in infants and young children with mild to moderate under nutrition. QPM germplasms have different maturity periods, as well as different grain color and texture. Some QPM varieties have high levels of carotenoids which are a precursor of vitamin A (Pillay, et al. 2011) [21-23].
Improving protein quality in maize
Breeding for improved protein quality in maize commence in the mid-1960s with the discovery of mutants, such as opaque-2 (o2), Researchers discovered that protein present in endosperm of o2maize is nearly twice nutritious compared to normal maize (Mertz,.et al. 1964) due to superior levels of lysine and tryptophan that are the two amino acids deficient in maize endosperm proteins. However, successful utilization of these mutants is not achieved due to some adverse pleiotropic effects. So, researchers use two genetic systems by exploiting double-mutant combinations and. Simultaneous use of o2gene and the genetic modifiers of the o2locus [24]. However, there was certain drawback like double mutant combination were not always vitreous (Paez, 1973) and yield was severely affected due to the sum total of independent negative effects of two mutation. While the second approach was most successfully adopted. In this, the conservative approach was accepted at the beginning in which after getting certain increment in the level of lysine maintenance rather than further enhancement was adopted and then research diverted towards the development of grain texture. After that QPM donor stock generated by using two strategies: The first was intra population selection for genetic modifiers in o2backgrounds elucidates a higher frequency of modified o2kernels. In the initial cycle controlled full-sib pollination was executed followed by modified ear-to row system (Vasal, S. K. 2002). A selection was accomplished at all stages for modified ears and modified kernels (Vasal, S. K. 2002, Bjarnason, M. and Vasal, S. K. 1992) [25].The second approach includes recombination of superior hard endosperm o2families.The yellow and white families were recombined separately to develop „Yellow H.E.o2‟ (yellow, hard endosperm o2) composite and „White H.E.o2‟ composite, respectively. After that large-scale QPM germplasm developed for different zones but standard back cross programme might not work due to the complexity and nature of kernel modification trait. Therefore, an innovative breeding procedure, modified [25].
Several natural maize mutants conferring higher lysine and tryptophan were identified in the 1960s and 1970s, viz., opaque-2 (o2), floury-2 (fl2), opaque-7(o7), opaque-6 (o6), and floury-3 (fl3. Of these, the o2 mutation, originally identified in a maize field located in the State of Connecticut, USA was found to be the most suitable for genetic manipulation in breeding programs aimed at developing maize high in lysine and tryptophan. Maize homozygous for the o2 (recessive) mutation was shown to have substantially higher lysine and tryptophan content (Table 2) than maize that was either heterozygous (O2o2) or homozygous dominant (O2O2) for the opaque-2 locus (Vivek, 2008) [27]. (Vivek 2008) showed that increased concentration of these two amino acids in the grain endosperm can double the biological value of maize protein.2However, the amount of protein in such maize remains at about 10%, the same as that of common (or normal endosperm) maize. In other words, the amount of common maize that needs to be consumed to achieve amino acid equilibrium is more than twice as much as the amount of opaque-2 maize (FAO, 1992). The nutritive value of milk protein is considered to be higher than that of maize protein; however, milk is a protein source that very few people can afford. Maize homozygous for the o2 mutant has a quality value equivalent to 90% that of milk [28-30] (Tables 2-4).
| Gene | Allele | Researchers | Year of discovery |
|---|---|---|---|
| Opaqua-2 | o2 | Mertz, Bates and Nelson | 1964 |
| Floury-2 | fl2 | Nelson, Mertz and Bates | 1965 |
| Opaque-6 | o6 | McWhirter | 1971 |
| Opaque-7 | o7 | Ma and Nelson | 1975 |
| Floury3 | fl3 | Ma and Nelson | 1975 |
Table 2: High lysine mutants of maize
| Normal | Opaque-2 | |
|---|---|---|
| g/100 g protein | ||
| Lysine | 2.6 | 4.2 |
| Tryptophan | 0.4 | 0.9 |
| Sources Bressani et al. 1969b; Viteri et al. 1972 | ||
Table 3: Comparative average percentages of lysine and tryptophan In opaque-2 and normal (non opaque-2) maize
| Quality as % of milk | |
|---|---|
| Normal maize | 39 |
| o2 maize | 90 |
Table 4: Comparison of the protein value of normal and opaque-o2 maize with milk.
Mechanism of o2 mutant
The binding site for the o2 protein (o2) in the promoter of 22kDaα zein genes are identified and that sequence is similar to the target site recognized by “basic leucine zipper” (bZIP) proteins (Hartings,et.at., 1989). The promoter regions contain an ACGT core that is necessary for DNA binding and is placed in the-300region respective to the translation initiate. It remains in the highly conserved zein gene sequencemotif about 20 bp downstream known as the “prolamin box” () Lopes and Larkins, 1993). When the mutation occurs by o2 mutant expression of 22kDa zein is reduced, that is majorly present in the central region of protein body and this ultimately reduced the size of protein bodies and give soft kernel texture. (Bass, 1992) [31].
The lysine-ketoglutarate reductase (LKR) enzyme activity was examined in two maize inbred lines which having homozygous normal and opaque-2 endosperms. By examining the pattern of LKR activity outcome was that LKR is correlated with therate of zein accumulation during endosperm development that was recognized in theopaque- 2and normal endosperm for the LKR activity. Both were two to three times lowerinopaque-2 compared to the normal. Due tothe reduction in the enzyme activity it ultimately increases the free amino acid in the endosperm. Opaque-2 gene may be implicating the regulation of the lysine ketoglutarate reductase gene in maize endosperm. In accession, lysine concentration was increased in part in which reduction in the reductase activity induced by the opaque-2 mutation was detected (Brochetto-Braga, 1992) [32].
Problems associated with o2 mutants
Opaque-2 mutant having high lysine content brought about enormous interest and eagerness for their possible use in developing maize with superior protein quality. Even though its superior quality, its extensive acceptance is limited and it is also not commercially utilized because of its negative pleiotropic effects include reduced yield than normal maize, low grain consistency and afarinaceous endosperm that retains water (Sofi, 2009). These features result in a soft, chalky endosperm that dried slowly making it prone to damage, a thick pericarp, more susceptibility to diseases and pests, higher storage losses and also affects harvest ability [33]. Since the kernel weight is reduced due to less density per unit volume as starch is loosely packed with abundant air spaces, there is an equivalent decrease in the yield (Singh, and Venkatesh, 2006). Especially in developing countries, where farmers are habituated to hard flint and dent grains, the kernel appearance of such mutants formed it less ideal for large-scale utilization and acceptance in target areas. The mutations that alter grain protein synthesis cause changes in the texture of grains. The early opaque-2 (o2) mutants had reduced levels of α-zeins resulting in small unexpanded protein bodies (Wu, Y., Holding, and Messing, J.2010), whereas, o15 that reduces γ-zeins leads to asmaller number of protein bodies. Othermutations such as floury-2(fl-2), Mucronate (Mc) and defective endosperm (De B30) resultin irregularly shaped protein bodies. (Coleman,.1997) [34].
Quality protein maize genetics and breeding strategies
Genetics of high lysine and tryptophan maize: The breeding of QPM involves the manipulation of three distinct genetic systems. The simple recessive allele of the opaque-2 gene, Modifiers/enhancers of the o2o2-containing endosperm to confer higher lysine and tryptophan, and Genes that modify the opaque-2-induced soft endosperm to hard endosperm [35].
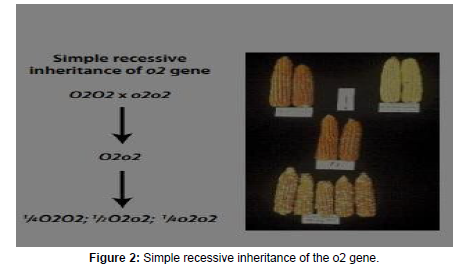
The simple recessive allele of the opaque-2 gene: This is a central component of the genetic system that confers higher levels of lysine and tryptophan in maize endosperm protein. The o2 allele is inherited in a simple recessive manner (Figure 2). The presence of o2 in the homozygous recessive (o2o2) state is a pre-requisite for the entire process of obtaining high lysine/tryptophan maize, discussed in the following sections. The most abundant proteins in the grain endosperm are the zeins and, particularly, alpha zein which are poor in lysine and tryptophan (Gibbon and Larkins, 2005) [36].
The homozygous o2 mutant causes a decrease in the production of alpha-zein fraction of endosperm protein and a corresponding increase in the proportion of non-zein proteins (Fractions I, IV, and V) that naturally contain higher levels of lysine and tryptophan (Gibbon and Larkins, 2005). Therefore, in a given quantity of protein from o2o2 maize, the proportion of non-zeins is higher, which predisposes o2 maize to have higher lysine and tryptophan. Protein fraction distribution of endosperm samples of normal and soft endosperm (o2) [37] (Table 5).
Alleles of endosperm hardness modifier genes: The second distinct genetic system managed within a QPM breeding program is comprised of the alleles of endosperm hardness modifier genes (termed here “en-modifiers”) which convert the soft/opaque mutant endosperm to a hard/vitreous endosperm with little loss of protein quality. It has been shown that increased levels of gamma zein likely contribute to the recovery of a hard endosperm phenotype as the o2 modified (QPM) grains have approximately double the amount of gamma zein in the endosperm relative to the o2 only mutants (Wallace et al., 1990) [38]. These en-modifiers along with the o2 mutant allele can be selected for using a rapid and low cost method of selection, whereby light is projected through the vitreous grains or blocked by the opaque grains respectively. Grain endosperm opaqueness is rated on a scale from 1(completely hard/vitreous) to 5 (soft/opaque). All grains with a score of 2-5 are homozygous for the o2 allele, but only grains with score 2-3 have sufficiently modified hard endosperm to be selected as QPM grains. Using this semi quantitative measure, two genetic loci which affect the modification of the endosperm hardness in o2o2 backgrounds have been mapped to the long arm of chromosome 7 (Lopes etal., 1995) and interestingly one endosperm modifier locus maps near a gamma zein gene gzr1’ (Maize Genetics/Genomics Database www.maizegdb. org). Genetic variance accounted for by these two major loci was not calculated in the study but it is likely that other en-modifier loci are also involved in the endosperm hardness modification [39-41].
Maize is a cross pollinated crop. The major breeding approach for increasing productivity is production of hybrids using heterosis breeding. The success of this method depends on development and identification of suitable inbred lines using an appropriate mating design; and selection of most promising heterotic normal maize hybrid (Figure 3).
Modifiers/enhancers of the o2o2-containing endosperm to confer higher lysine and tryptophan this is the second essential genetic system that confers higher lysine/tryptophan in maize. It consists of minor modifying loci that affect lysine and tryptophan levels in the endosperm. Lysine levels in normal and o2 maize average 2.0% and 4.0%, respectively, of total protein in whole grain flour. However, across diverse genetic backgrounds, these levels range from 1.5-2.8% in normal maize to 2.6-5.0% in their o2 converted counterparts (Moro et al., 1996). Therefore, if lysine or tryptophan levels are not monitored while developing new cultivars, one could end up with a maize cultivar having the o2o2 genotype and lysine and tryptophan levels equivalent to those in normal maize, since the lower limits of lysine and tryptophan in o2o2 maize overlap with the upper limits in normal maize [42,43].
Breeding for quality protein maize (QPM)
In the 1920s in a Connecticut USA maize field, a natural spontaneous mutation of maize with soft, opaque grains was discovered and delivered to the Connecticut Experiment Station (Vietmeyer, 2000). This maize mutant was eventually named opaque-2 (o2) by a Connecticut researcher (Singleton, 1939) and in the 1960’s at Purdue University USA, the geneticist Dr. Oliver Nelson, (who began his career as a graduate student at the Connecticut. Experiment Station (Crow et al. 2002), provided to Dr. Edwin Mertz seeds of opaque-2 maize to be included in his group’s systematic effort to identify maize accessions with improved protein quality (Paes and Bicudo, 1994). Soon after the discovery of the nutritional benefits of the o2 mutation, it began to be incorporated into many breeding programs worldwide, with a major emphasis on conversion of normal endosperm populations and inbred lines to o2 versions through a direct backcross approach (Prasanna et al., 2001). However, enthusiasm over the direct use of the o2 mutation in breeding programs soon subsided after the discovery of serious negative secondary (pleiotropic) effects of this mutation [44]. The soft endosperm of o2 genotypes initially caused up to a 25% yield loss due to the lower density of the opaque grains, as well as increased susceptibility to fungal ear rots and storage pests (Vasal, 2000). The soft endosperm texture also is not acceptable to many in the developing world who are accustomed to harder grain types. Such negative secondary effects severely limited practical use of the mutation in the field. Fortunately, during the process of converting normal maize populations to o2 versions, partially harden do sperm (i.e. vitreous) or modified grains had been observed by many researchers including breeders at CIMMYT in Mexico. Separation of such grains when encountered began as early as 1969 by Dr. John Lindquist (Vasal, 2000). Besides, the first published report highlighting the importance of such grain modification in reducing the negative pleiotropic effects of the o2 mutation was published in 1969 (Paezet al., 1969). Selection for hard endosperm modification was rapidly incorporated into o2 breeding schemes. Occurring at the beginning QPM breeding efforts at CIMMYT focused on conversion of a range of sub-tropical and tropical lowland adapted, normal endo-sperm populations to o2 versions through a backcross-recurrent selection procedures, with a focus of accumulating the hard endosperm phenotype, maintaining protein quality and increasing yield and resistance to ear rot (Villegas et al. 1992). The improved populations were released for direct use in the field as open pollinated varieties (OPV’s), or individual plants were self-pollinated to for mind red lines used in hybrid formation. Similar programs with sustained breeding of QPM also continued at the University of KwaZulu-Natal (previously University of Natal), South Africa and the Crow’s Hybrid Seed Company at Milford, Illinois USA (Prasanna etal., 2001) [45].
Marker-assisted breeding in QPM
There is a need of marker-assisted selection because of mainly three reasons:
• each backcross generation needs to be selfed to identify the opaque-2 recessive gene and a minimum of six backcross generations are required to recover satisfactory levels of recurrent parent genome
• To maintain the homozygous opaque-2 gene, multiple modifiers must be selected.
• Rigorous biochemical tests to ensure enhanced lysine and tryptophan levels in the selected materials in each breeding generation require. After the sequencing of the maize genome has been completed, a large number of the market system are now available that are associated with o2and endosperm modification phenotype (Singh,et al. 2017). A convenient utilization of such markers will greatly enhance the efficacy of selection for improvement of grain protein in maize furthermore reduce the cost and time. Both foreground MAS and background MAS can be efficiently utilized for selecting o2phenotypemoreoverassuring maximum recovery of the recurrent parent. MAS used for development of QPM parental lines and developed QPM hybrid in less than half the time required through conventional breeding (Singh, et al. 2017).
Various markers are used to introgress o2gene into elite maize inbred lines by rapid backcross conversion programme. They found that using a marker for QPM and endosperm modification can enormously improve the selection efficiency for isolating fully modified kernels in QPM background (Singh,et al. 2017) [46].
Summary and Conclusion
QPM could have an impact in areas where maize constitutes a large proportion of the diet, especially as a source of protein, and where children and lactating mothers, suffer protein deficiency. with the discovery of opaque-2 mutation. This natural recessive mutation causes alteration in amino acid composition and opaque phenotype of endosperm by regulation of specific zein genes and combined use of the o2 gene and genetic modifiers. Modified marker assisted back cross breeding used to develop QPM versions of normal maize inbreeds with desirable endosperm characteristics and seed yield. These QPM introgression lines may be united to develop QPM hybrids. There may be increasing use of molecular genetic tools in QPM research in the future.
References
- Akalu G, Taffesse S, Gunaratna NS, De Groote H (2010) The effectiveness of quality protein maize in improving the nutritional status of young children in the Ethiopian Highlands. Food Nutr. Bull 31: 418-430.
- Bass HW, Webster C, Roberts JK, Boston RS (1992) A maize ribosome-inactivating protein is controlled by the transcriptional activator opaque-2. The Plant Cell 4: 225-234.
- Bjarnason M, Vasal SK (1992) Breeding of quality protein maize (QPM). Plant breeding reviews 9:181-216.
- Bjarnason M, Vasal SK (1992) Breeding for quality protein maize. Plant Breed. Rev 9:181-216.
- Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001) Quality protein maize. Curr Sci 81: 1308-1319.
- Bressani R (1991) Protein quality of high-lysine maize for humans. Cereal Foods World 36: 806-811.
- Brochetto-Braga MR, Leite A, Arruda P (1992) Partial purification and characterization of lysineketoglutarate reductase in normal and opaque-2 maize endosperms. Plant Physiology 98: 1139-1147.
- Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, et al. (1997) Expression of a mutant α-zein creates the floury2 phenotype in transgenic maize. Proceedings of the National Academy of Sciences 94:7094-7097.
- De Groote H, Gunaratna N, Ergano K, Friesen D (2010) Extension and adoption of biofortified crop: Quality protein maize in East Africa. In Proceedings of the African Agricultural Economics Association Meetings, Cape Town, South Africa 19-23.
- Dereje B, Mosisa W, Hadji T, Wonde A, MandefroN, et al. (2001) On-Farm Evaluation of CIMMYT’S Quality Protein Maize Varieties in Ethiopia. Seventh Eastern and Southern Africa Regional Maize Conference held in Nairobi Kenya 77-79.
- Dudley JW, Lambert RJ (2004) 100 Generations of selection for oil and protein in corn. Plant Breeding Review 24: 79-110.
- Emerson RA, Beadle GW, Fraser AC (1935) A Summary of Linkage Studies in Maize; Cornell University Agricultural Experiment Station Memoirs. Ithaca NY USA 180: 1-80.
- Gibbon BC, Larkins BA (2005) Molecular genetic approaches to developing quality protein maize. Trends in Genetics 21: 227-233.
- Gibbon BC, BA Larkins (2005) Molecular genetics Approaches to develop quality protein maize.
- Gunaratna NS, De Groote H, Nestel P, Pixley KV, Mccabe GP, et al. (2010) A meta-analysis of community-based studies on quality protein maize. Food Policy 35: 202-210.
- Hartings H, Maddaloni M, Lazzaroni N, Di Fonzo N, Motto M, et al. (1989) The o2 gene which regulates zein deposition in maize endosperm encodes a protein with structural homologies to transcriptional activators. The EMBO journal 8: 2795-2801.
- Ignjatovic-Micic D, Kostadinovic M, Stankovic G, Markovic K, Vancetovic J (2013) Biochemical and agronomic performance of quality protein maize hybrids adapted to temperate regions. Maydica 58:311-317.
- Koutsika-Sotiriou M (1999) Hybrid seed production in maize. In Basra AS (Ed.) Heterosis and Hybrid Seed Production in Agronomic Crops. Food Products Press New York pp: 25-64.
- Krivanek AF, Groote HD, Gunaratna NS, Diallo AO, Friesen D (2007) Breeding and disseminating quality protein maize (QPM) for Africa. Afr J Biotechnol 6, 312-324.
- Leite A, Neto GC, Vettore AL, Yunes JA, Arruda P (1999) The prolamins of sorghum, Coix and millets. In: Shewry PR & Casey R (Eds) Seed proteins. Dordrecht. Kluwer Academic Publishers 141-157.
- Lonnquist JH (1964) A modification of the ear-to-row procedure for the improvement of maize populations. Crop Science 4: 227-228.
- Lopes MA, Takasaki K, Bostwick DE, Helentjaris T, Larkins BA, et al. (1995) Identification of 2 Opaque2 Modifier Loci in Quality-Protein-Maize. Mol Gen Genet 247: 603-613.
- Lopes MA, Larkins BA (1993) Endosperm origin, development, and function. The Plant Cell 5: 1383-1393.
- Menkir A, Liu W, White WS, Maziya-Dixon B, Rocheford T, et al. (2008) Carotenoid diversity in tropical-adapted yellow maize inbred lines. Food Chem 109:521-529.
- Mertz ET, Bates LS, Nelson OE (1964) Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145:279-280.
- Mpofu IDT, Sibanda S, Shonihwa A, Pixley K (2012) The nutritional value of quality protein maize for weaner pigs. J Pet Environ Biotechnol 3:129.
- Nelson OE (1969) Genetic modification of protein quality in plants. Advances in Agronomy 21:171-194. Academic Press.
- Nelson OE, Mertz ET, Bate LS (1965) Second mutation gene affecting amino acid pattern of maize endosperm proteins. Science 150: 1469-1470.
- Nuss ET, Tanumihardjo SA (2011) Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv Nutr 2:217-224.
- Paes MCD, MH Bicudo (1994) Nutritional Perspectives of Quality Protein Maize; Quality Protein Maize: Proceedings of the International Symposium on Quality Protein Maize. Sete Lagoas 1-3: 65-68.
- Paez AV, Helm JL, Zuber MS (1969) Lysine content of opaque-2 maize kernels having different phenotypes. Crop Sci 9: 251-252.
- Paez AV (1973) Protein quality and kernel properties of modified opaque- 2endosperm corn involving a recessive allele at the sugary-2 locus 1. Crop Science 13: 633-636.
- Pillay K, Derera J, Siwela M, Veldman FJ (2011) Consumer acceptance of yellow provitamin a-bio fortified maize in Kwazulu-Natal. S Afr J Clin Nutr 24: 186-191.
- Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001) Quality protein maize. Current science 1308-1319.
- Sarika K, Hossain F, Muthusamy V, Baveja A, Zunjare R, et al. (2017) Exploration of novel opaque16 mutation as a source for high-lysine and-tryptophan in maize endosperm. Indian J Genet 77: 59- 64.
- Shewry PR (2007) Improving the protein content and composition of cereal grain. J Cereal Sci 46:239-250.
- Singh NN, Venkatesh S (2006) Development of quality protein maizeinbred lines. Research Book Center New Delhi 102-113.
- Singh R, Ram L, Singh RK, Singh Jakhar D (2017) SSR marker aided introgression for opaque-2 allele for development of quality protein maize inbreds. Cereal Research Communications 45: 466-477.
- Singleton WR (1939) Recent Linkage Studies In Maize: V. Opaque endosperm-2 (o2). Connecticut Experiment Station, New Haven. Genetics 24:61.
- Sofi PA, Wani SA, Rather AG, Wani SH (2009) Quality proteinmaize (QPM): genetic manipulation for the nutritional fortification of maize. Journal of Plant Breeding and Crop Science 1: 244-253.
- Twumasi-Afriye S, Palacios Rojas N, Friesen D, Teklewold A, Gissa DW, et al. (2016) Guidelines for the Quality Control of Quality Protein Maize (QPM) Seed and Grain. CGIAR CIMMYT Addis Ababa, Ethiopia p 38.
- Vasal SK (2002) Quality protein maize: overcoming the hurdles. Journal of crop production 6: 193-227.
- Vietmeyer ND (2000) A drama in three long acts: the story behind the story of the development of quality protein maize. Diversity 16: 29-3.
- Vietmeyer ND (2000) A drama in three long acts: The story behind the story of the development of quality-protein maize. Diversity 16: 29-32.
- Wu Y, Holding DR, Messing J (2010) γ-Zeins are essential forendo sperm modification in quality protein maize. Proceedings of the National Academy of Sciences 107:12810-12815.
- Zaidi PH, Vasal SK, Maniselvan P, Jha GC, Singh RP, et al. (2008) Stability in performance of quality protein maize under abiotic stress. Maydica 53: 249-260.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Girma BT, Siyoum YM (2023) Breeding for Quality Protein Maize (QPM).Adv Crop Sci Tech 11: 646.
Copyright: © 2023 Girma BT, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2546
- [From(publication date): 0-2024 - Dec 11, 2025]
- Breakdown by view type
- HTML page views: 2190
- PDF downloads: 356