Carbon Stock of the Moist Afromontane Forest in Gesha and Sayilem Districts in Kaffa Zone: An Implication for Climate Change Mitigation
Received: 08-Nov-2018 / Accepted Date: 26-Mar-2019 / Published Date: 02-Apr-2019
Abstract
This study measures the carbon stock of the Moist Afromontane Gesha-Sayilem forest found in Gesha and Sayilem District in southwest Ethiopia. A stratified sampling method was used to identify the number of sampling point through the Global Positioning System. A total of 90 plots having nested plots to collect tree species and soil data were demarcated. The allometric and Walkley-Black method was used to estimate biomass and soil carbon stock, respectively. The carbon stock of trees was estimated using an allometric equation developed by Chave’s model. The results revealed that the total carbon stock of the forest was 362.4 ton per hectare (t/ha) whereas the above ground carbon stock was 174.95 t/ha, below ground litter, herbs, soil, and dead woods were 34.3, 1.27, 0.68, 128 and 23.2 t/ha (up to 30 cm depth) respectively. The carbon pools’ carbon stock variation with altitude and slope gradients were not significant (p>0.05) indicating the altitudinal influence was small due to similar topographic features. The Gesha-Sayilem Forest is a reservoir of high carbon and thus acts as a great sink of the atmospheric carbon. Thus conservation of the forest through introduction REDD+ activities is considered an appropriate action for mitigating climate change.
Keywords: Carbon sequestration; Carbon stock; Climate change; Allometric
Introduction
Forests play a major contribution in the global carbon balance. They act as both carbon sources and sinks and they have the latent to form an important constituent to fight global climate change. Forests sequester a substantial amount of carbon through photosynthesis and transform it into above and below ground biomass [1]. The forest sequesters a significant amount of carbon and stores it in plant tissues and soil accounting more than 80% of all terrestrial above ground carbon and 70% of all the carbon accumulated in the form of soil organic carbon [2,3]. The world’s forests are estimated to store more than 650 billion tons of carbon, of which 44% is stored in the aboveground living biomass, while 11% and 45% stored in dead wood and in the forest soil respectively [4]. Similarly, the tropical forests play a major role in the global carbon cycle, and it stores about 46% of the world’s terrestrial carbon pool and 11.55% of the world’s soil carbon pool, contributing for carbon reservoir and acting as a constant sink of atmospheric carbon [5]. According to Djomo et al. [6], moist tropical forests are important for carbon sequestration, because they typically have high carbon contents, nearly 110 tons per acre.
Ethiopia is one of the tropical countries with significant forest cover which can sequester a greater amount of carbon to mitigate climate change. The overall forest cover of Ethiopia is estimated to be around 13 billion hectare covering 11.4% of the total area of the country [4]. Based on the size of forest cover of the country, Ethiopian forests comprise about 2.8 billion tons of carbon [7]. The major stock of carbon is found in the woodland and shrub lands accounting for 45.7% and 34.4% respectively and constituting a larger area of the country. Relatively lesser amount is found in the high forests, plantations and in the lowland and highland bamboos accounting for 15.7%, 2.2% 1.92%, respectively. However the mitigation potential of Ethiopian forests is affected by deforestation and forest degradation. The major causes for low mitigation potential of are: land-use change to agricultural land, promotion of large-scale commercial farms, extraction of fuelwood and timber, expansion of settlement in forest areas and road construction. These consequences reduce the ecosystem services including carbon sequestration, the hydrological cycle and nutrient cycling which leads to cause climate change [8]. The impact of climate change on Ethiopian biodiversity is becoming a great challenge to the country. The extreme temperature and variability of rainfall have caused the extreme events such as drought and flood which significantly impacted the performances of the agricultural system particularly in the low lands of Ethiopia [9].
Therefore, the magnitude of climate change has critical on Ethiopian agriculture through maintaining the forest resources which requires mitigation and adaptation strategies for fulfilling the country’s food security and biodiversity conservation. The Gesha and Sayilem districts are potential forest areas designated as part of the Bonga National Forest Priority area (NFPA). This forest is found in the respective districts located in inaccessible remote areas and no study has been conducted on its carbon stock potential to sequester carbon in above and below ground biomass of forest. In addition the country is lacking periodic inventory data of carbon stocks for national carbon inventory for the REDD+ activities and thus the determination of the carbon stock potential of the Gesha and Sayilem forest will contribute to the national carbon database. Thus, the study aimed to determine the carbon stock potential of Gesha-Saiylem forest and to provide relevant information for National REDD+ programmed to inform policy and decision makers.
Materials and Methods
This study was conducted in the two districts of Gesha and Sayilem in Kaffa Zone of Southern Nations, Nationalities and Peoples Regional State (Figure 1). Gesha district is geographically located between 7°35.36’ N latitude and 35°45.27’ E longitude while Sayilem is located between, 70°49.57’ N latitudes and 35°49.32’ E longitude. The total area of the Gesha and Sayilem districts is 705.20 km2 and 856.60 km2, respectively [10]. The topography of the landscape is undulating, with valleys and rolling, plateau and some with flat plains. The elevation of Gesha and Sayliem districts ranges from 1600 to 3000 m. The mean monthly temperature ranges between 9.5-29.5°C and the mean annual rainfall of the area is 1578 mm. The vegetation types of the study area belong to Moist Evergreen Afromontane forest [11], dominated by upper story species such as Pouteria adolfi-friederici, Olea welwitschii, Cordia africana, Polyscias fulva, Croton macrostachyus, Albizia gummifera, Schefflera abyssinica, Ekebergia capensis, Prunus africana, and Arundinaria alpina.
Measurement of carbon stock in the field
Stratified random sampling methods were employed to collect vegetation data due to large size of the study forest and unknown patterns as recommended by Smartt [12], Kent and Coker [13]. In order to increase precision and accuracy of measuring carbon stock, the area was divided into substrata [14]. For this purpose, altitudinal stratification was taken as criterion to divide the study area into different strata to get homogenous sampling units. Based on this stratification, the study area was divided into five elevational strata and the elevation distribution was extracted from the Digital Elevation Model (DEM). Contour lines were generated from the LiDAR Image Services, used to represent the elevations of a land surface above sea level. The altitudinal classification was made starting from the lower to the highest altitude at intervals of 200 m. The total number and distribution of sample plots for each stratum of the forest varied with the size of the strata. The size of the strata was calculated for each stratum and the numbers of plots were proportionally allocated based on the size of the stratum. In this sampling design, more sample plots were taken, from the strata having large area coverage of the forest and low numbers of sample plots were taken when the size of the forest was small.
For sampling of carbon stock of the forest, a nested plot design was used for the sampling of various carbon pools from different strata. Accordingly trees whose diameters were greater than 50 cm DBH were measured in 35 × 35 m plots, whereas those trees and shrubs with DBH 20-50 cm were measured in 25 × 25 m subplots and small sized trees with 5-20 cm DBH were measured in 7 × 7 m subplots [14]. The DBH and height were measured for woody plant species encountered in the sample plots with a DBH>5 cm. Those individuals with DBH below 5 cm were not measured but destructively sampled for estimation of the biomasses of saplings and seedlings. The measurements of DBH and height were made using a measuring tape. The model developed by Chave et al. [15] was used to estimate the AGB due to its accuracy and the similarity of climatic zone of the study area. Thus, the equation developed by Chave et al. [15] was used to calculate the aboveground biomass and presented below:
AGBest=0.0559 × ρD2H
Where, AGB: Above-Ground Biomass (kg); ρ: Wood specific gravity (g cm-³); H: Height of the plant and D: Diameter of the tree.
The wood density was obtained from different wood density databases developed for tropical forest by Reyes et al. [16] and Chave et al. [17]. The wood density data for Ethiopian species was obtained from, the Ministry of the environment and climate change. When the wood density for a species was not recorded in the data base, an average default value of 0.5 was taken as endorsed by Chave et al. [17] for trees of tropical species. The sum of all plant species’ biomass was calculated for each sample plot and the biomass density was expressed in kg/m2 and finally converted to tons per hectare. The biomass stock density was converted to carbon stock densities after multiplication of 0.47 with the IPCC default value [18]. The below ground biomass (BGB) of woody plant species was estimated from its aboveground biomass [19], the BGB of the study forest was estimated from the AGB using the following equation developed for the tropics:
BGB=AGB × 0.2
Where, BGB: Below Ground Biomass, AGB: Above Ground Biomass, 0.2 is conversion factor (20% of AGB).
Measurement of litter carbon
The litter biomass was estimated using a simple wooden frame having 1 × 1 m (1 m2) was established at the center of each plot. Then samples of the litter were collected at five spots four from a corner of the plots and one from the center. The fresh weight of samples was weighed and recorded using a spring balance. From this fresh sample a well-mixed sub-sample of 100 g was brought to the laboratory and oven dried for 48 hours at 65°C using dry-ashing method as suggested by Allen et al. [20].
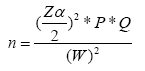
Where, LB: Litter biomass (t/ha); W field: Weight of wet field sample of litter sampled within an area of size 1 m2 (Kg); A: Size of the area in which litter were collected (ha); Wsub-sample, dry: Weight of the oven-dry sub-sample of litter taken to the laboratory to determine moisture content (g), W sub-sample, fresh: Weight of the fresh subsample of litter taken to the laboratory to determine moisture content (g).
For carbon content determination of litter then oven-dried samples were taken in pre-weighed crucibles and ignited at 550°C for three hours in the furnace. After cooling, the crucibles with ash were weighed and percentage of organic carbon was calculated. Litter biomass (t/ ha) and Carbon stock in litter biomass was then determined using the following formula:
CL=LB × %C
Where, LB: Litter biomass (t/ha); CL: Total carbon stock in the litter in t ha-1; %C: The carbon content in forest litter was calculated by multiplying it with the carbon fraction analyzed in the laboratory.
Determination of herb and shrub carbon
Herbs and shrubs were collected within plots of 2 × 2 m and 5 × 5 m respectively by clipping all the plants in sample plots and weighed in the field using a spring balance. A well-mixed subsample of 150 g was taken from the field and oven dried until a constant mass is achieved for the dry biomass determination. The carbon fraction of the sample was determined by taking the oven-dried samples in pre-weighed crucibles and it was ignited at 550°C for one hour in the muffle furnace. The ash was weighed after the ash containing crucible has been cooled and percentage of organic carbon was calculated.
The DBH of the standing dead trees with leaves and branches were measured as live trees using the allometric equation, when a tree has no branches and just the bole, volume was estimated using truncated cone formula:
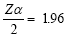 as developed by Pearson et al. [14]
as developed by Pearson et al. [14]
Where, h: The height in meters; r1: The radius at the DBH of the tree; r2: The radius at the top of the tree.
Measurement of carbon in dead wood
Dead wood lying on the ground was measured using the lineintersect method [21]. To measure dead wood a subplot size of 10 × 10 m was laid within the main plot. The length of the lines intersecting a piece of down dead wood and its diameter was measured. The three density classes (sound, intermediate and rotten wood) were determined in the field using the machete test [22]. The samples of dead wood were collected from each density classes and brought to the laboratory and their volume was measured using the floatation method [23]. The wood was oven dried for 48 hours at temperature of 105°C. For each density class the volume was calculated using the formula developed by Brown et al. [24].
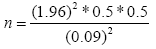
Where d1, d2, dn: Diameters of intersecting pieces of dead wood in cm; L: Length of the transect line in m.
Biomass of laying dead wood (t/ha)=volume × density.
Measurement of soil organic carbon
The soil samples were collected from five sub-plots in the same way as litter collection. A soil sample was collected on two layers 0-15 cm and 15-30 cm from five spots and mixed homogeneously and a composite subsample of 100 g from each plot was taken to the laboratory for analysis. The Walkley-Black method was used to determine SOC after it had been grounded and passed through a 2 mm sieve. For the bulk density, a core sampler was used to collect soil samples and bulk density was calculated from the oven dry weight of soil after it has been dried at 105°C and divided by volume of the core sampler. The soil organic carbon was computed using the formula:
SOC=BD × D × %C
Where, SOC: Soil organic carbon stock per unit area (t/ha); BD: Soil bulk density (g cm-3); D: The total depth at which the sample was taken (30 cm) and %C: Carbon concentration (%).
Statistical analysis
The mean values of tree DBH, height, density, soil organic carbon, litter carbon were managed using Microsoft Excel 2010 and one-way analysis of variance (ANOVA). The Pearson correlation was performed between aboveground carbon and species diversity, disturbance, altitude, slope and aspect.
Results
Estimation of above and below ground biomass of trees
A total of 78 woody plant species were recorded for the above ground biomass estimation. The aboveground biomass and carbon stock-density of the woody species vary among the plant species. The highest aboveground carbon density per tree was obtained for a few woody species having higher DBH values. The highest carbon stock densities were recorded for Schefflera abyssinica (58.75 t/ha), Ekebergia capensis (31.71 t/ha), Prunus africana (5.6 t/ha), Olea welwitschii (42.12 t/ha), Pouteria adolfi-friederici, (10.62 t/ha), Syzygium guineense (15.80 t/ha), Elaeodendron buchananii (7.04 t/ha), Sapium ellipticum (7.8 t/ ha) and Croton macrostachyus (7.48 t/ha) (Table 1). These species contributed 65% of the total carbon stock density in the forest. The least AGC were recorded for Rhamnus prinoides, Hypericum revolum and Nuxia congesta.
| Species name | Carbon in AGB t/ha | Carbon in BGB t/ha | Total carbon t/ha/tree | CO2 t/ha/tree |
|---|---|---|---|---|
| Schefflera abyssinica | 58.75 | 27.61 | 86.36 | 316.9 |
| Ekebergia capensis | 31.71 | 14.91 | 46.62 | 171.1 |
| Prunus africana | 27 | 5.4 | 32.4 | 118.908 |
| Olea welwitschii | 42.12 | 19.8 | 61.92 | 227.2 |
| Pouteria adolfi-friederici | 10.62 | 4.99 | 15.61 | 57.3 |
| Syzygium guineense | 15.8 | 7.43 | 23.23 | 85.3 |
| Elaeodendron buchananii | 7.04 | 3.31 | 10.35 | 38 |
| Sapium ellipticum | 7.8 | 3.66 | 11.46 | 42.1 |
| Croton macrostachyus | 7.48 | 3.51 | 10.99 | 40.3 |
| Elaeodendron buchananii | 7.04 | 3.31 | 10.35 | 38 |
| Apodytes dimidata | 5.33 | 2.51 | 7.84 | 28.8 |
| Ficus sur | 5.33 | 2.51 | 7.84 | 28.8 |
| Ilex mitis | 3.15 | 1.48 | 4.63 | 17 |
| Macaranga capensis | 2.71 | 1.27 | 3.98 | 14.6 |
| Euphorbia ampiphylla | 2.58 | 1.21 | 3.79 | 13.9 |
| Macaranga capensis | 2.71 | 1.27 | 3.98 | 14.6 |
| Euphorbia ampiphylla | 2.58 | 1.21 | 3.79 | 13.9 |
| Allophyllus abyssinicus | 1.52 | 0.71 | 2.23 | 8.2 |
| Dracena afromontana | 0.97 | 0.46 | 1.43 | 5.2 |
| Gallineria saxifarga | 0.65 | 0.31 | 0.96 | 3.5 |
| Rytignia neglecta | 0.64 | 0.3 | 0.94 | 3.4 |
| Millettia ferruginea | 0.61 | 0.28 | 0.89 | 3.3 |
Table 1: Carbon stock (t C ha-1) of above and below ground biomass of tree species with the highest IVI Values in Gesha-Sayilem forest.
Carbon stock in above and below ground biomass
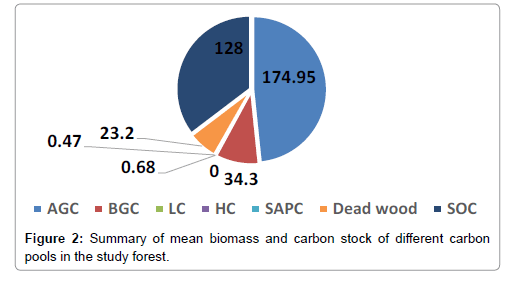
The minimum and maximum aboveground biomass per plot was found to be 0.92 and 2266.3 t/ha, respectively with the mean value of 349.9 t/ha . The minimum below ground carbon was 0.08 t/ha whereas the maximum was 213 t/ha with mean value of 34.3 t/ha (Figure 2). The analysis of litter carbon concentration per sample plot gave a minimum of 0.01 t/ha and a maximum of 6 t/ha with a mean value of 1.27 t/ ha indicating a variation between sample plots. The minimum and maximum carbon content of the herbs was 0.06 and 2.04 t/ha and with mean value of 0.68 t/ha. Similarly, the minimum and maximum shrub carbon stock density per plot was 0.01 and 4.2 t/ha, respectively with mean value of 0.46 t/ha. The greater amount of carbon stored in above ground carbon and soil organic carbon while lesser amount stored in litter and non woody plants.
| Forest type | AGC | BGC | LC | SOC | Carbon stock ton ha-1 | References |
|---|---|---|---|---|---|---|
| Egdu forest | 278.08 | 55.62 | 3.47 | 277.56 | 614.73 | Feyissa et al. [26] |
| Simien mountain National park | 57.83 | 13.88 | 0.85 | 92.7 | 165.26 | Assaye et al. [27] |
| Gera forest | 217.27 | 43.54 | 5.08 | 172.62 | 440.71 | Hassen [28] |
| Anbessa forest | 169.02 | 34 | 1.15 | 149 | 353 | Yilma et al. [29] |
| Masha forest | 155 | 31 | - | - | 186 | Yilma et al. [29] |
| Selected church forest in Addis Ababa | 122.85 | 25.97 | 4.95 | 135.94 | 289.6 | Tolla [35] |
| Congo forest | 168.60 | 39.55 | - | - | 207 | Ullah and Al-Amin [30] |
| Bangladesh forest | 96.48 | 14.61 | 4.21 | 168.15 | 283.45 | Ullah and Al-Amin [30] |
| Gesha-Sayilem forest | 164.5 | 32.9 | 1.27 | 137.67 | 362.4 | Present study |
Table 2: Carbon stock comparison of the study forest with other forest types in Ethiopia.
Soil organic carbon
Laboratory analysis of soil for soil organic carbon showed that, the mean soil organic carbon of Gesha-Sayilem forest was 6.7% with a minimum value of 2.28% to the maximum of 17%. The soil bulk density ranged from 0.5 g cm-3 to 1.4 g/cm-3 while the average soil bulk density was 0.7 g/cm-3 demonstrating the presence of high soil organic matter in mineral soil. The mean soil carbon stock density was 128 tons of carbon ha-1, while the minimum and maximum soil carbon stock densities were 44.16 and 302.94 ton of carbon ha-1, respectively. This soil carbon pool sequestered a minimum and maximum CO2 value of 162 t/ha and 1020.85 t/ha, respectively.
Carbon pools in dead wood
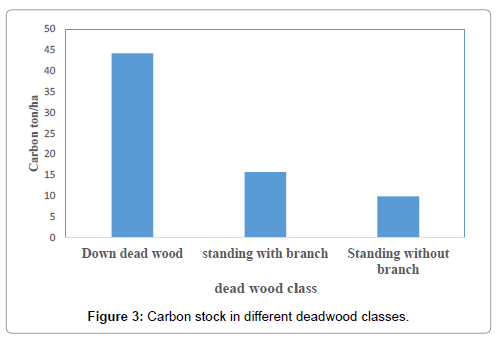
The collection of dead wood from sample plots indicated that the study forest had abundance of dead wood on the forest floor. The average carbon stock density of dead wood per plot was 23 t/ha. The carbon density of dead wood lying on the forest floor was higher compared to that of carbon density of standing dead wood (Figure 3).
Total carbon stock
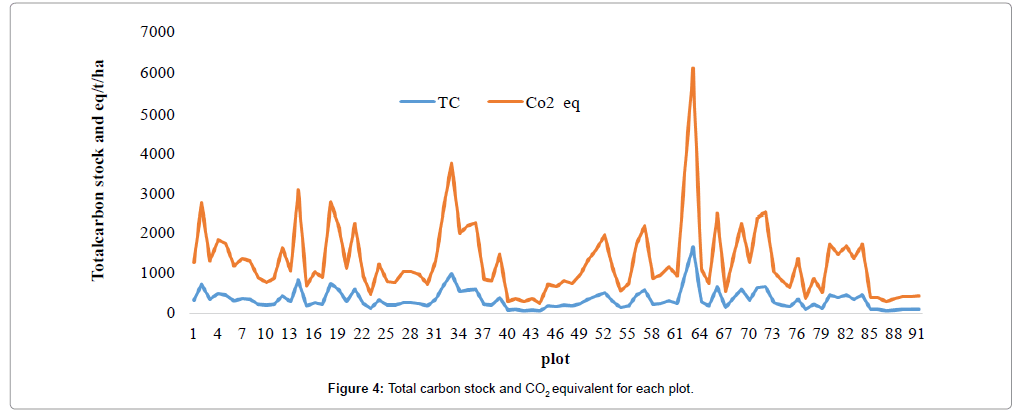
The total carbon stock of the study forest was calculated by adding up of all the carbon stock values of each carbon pool for all plots. The carbon stock ranged from a minimum of 69 t/ha in plot 17 to a maximum of 1665.69 t/ha in plot 63 with a corresponding minimum value of 2265.53 and maximum value of 6113.0823 CO2 equivalents (Figure 4) and the mean carbon density in all carbon pools of the study forest was 362.4 t/ha.
Comparison of carbon stock with other forest
When the carbon stock of the study forest is compared with that of different forest types (dry and moist Afromontane forests) in Ethiopia, the mean carbon stock in the above and below ground biomass is greater than Menagesha Suba State Forest, selected church forests in Addis Ababa, Semien mountain, Masha forest and lower than Gergeda and Anbessa forests, Egdu forest (Table 2) [25]. The above ground carbon of this study also falls within ranges reported for the global above ground carbon stock in tropical dry and wet forests which range between 13.5-122.85 t/ha and 95-527.85 t/ha, respectively. The variation in carbon stock between different forest types can probably be due to inaccurate measurements of tree variables, in efficiency of allometric models, presence of bigger sized trees with higher basal area, a higher density of woody species and anthropogenic disturbances. This agrees with Lasco et al. [31]; Moges et al. [7] who reported that different types of models used for biomass estimation would have an impact on the value of carbon stock estimated in a given forest.
The effect of altitude on carbon pools of the Gesha-Sayilem forest
The grouping of the carbon stock into lower, middle and higher altitudes classes indicated that higher carbon stock was recorded from the middle and higher altitudes, being 180.61 ± 26.48 and 158.83 t/ha, respectively as compared to lower altitudes (48.75 t/ha) of the study forest. The litter carbon density of the forest did not show a significant variation between upper, lower and middle altitudes but it is relatively higher in the middle and higher altitudes with the mean carbon density of 3.75 t/ha and 0.86 t/ha, respectively (Table 3). The soil organic carbon density of the study forest was low in lower altitude as compared to the middle and higher altitudes. The lower altitude had a soil carbon density of 98.18 t/ha but middle and higher altitude stored carbon density of 127.6 t/ha and 128 t/ha respectively (Table 4). The overall carbon density of the forest significantly varies (p<0.05) between lower, middle and higher altitudes. The maximum total carbon density was recorded at middle (729.76 t/ha) and higher (741 t/ha). The lower carbon stock density was recorded in lower altitudes with the mean value of 261 t/ha.
| Carbon pools | Lower | Middle | Higher | F | p-value |
|---|---|---|---|---|---|
| AGB | 103.72 ± 35.5 | 384.47 ± 56.3 | 337.9 ± 42.3 | 6.23 | 0.003 |
| AGC | 48.75 ± 16.7 | 180.61 ± 26.48 | 158.83 ± 19.8 | 6.24 | 0.003 |
| BGC | 9.75 ± 3.34 | 36.13 ± 5.2 | 36.72 ± 6.4 | 4.507 | 0.014 |
| LC | 0.60 ± 0.114 | 3.75 ± 3.12 | 0.86 ± 0.087 | 0.730 | 0.485 |
| SOC | 98.18 ± 8.52 | 127.61 ± 4.5 | 128.00 ± 9.11 | 3.232 | 0.044 |
| HC | 0.38a ± 0.17 | 0.44a ± 0.11 | 0.19a ± 0.2 | 0.52 | 0.595 |
| SPC | 0.56a ± 0.36 | 0.81a ± .61 | 0.59a ± 0.27 | 1.797 | 0.172 |
| Total Carbon density | 261.7 ± 30.6 | 729.76 ± 88.1 | 741.01 ± 110 | 4.94 | 0.009 |
Table 3: Mean biomass and carbon stock (t ha-1) in different pools and altitudinal gradient.
| Variables | AGC | BGC | LC | SOC |
|---|---|---|---|---|
| Slope | 0.033 | 0.033 | 0.037 | 0.154 |
| Aspect | -113 | -113 | -172 | -0.176 |
| Disturbance | -0.249* | -0.249* | -0.150 | -0.300** |
| Species diversity | 0.405** | 0.405** | 0.172 | 0.176 |
| Species richness | 0.371** | 0.371** | 0.312 | 0.103 |
| *P=0.05; **P=0.001; ***P=0.0001. | ||||
Table 4: Person correlation coefficients (r) between Carbon stock and environmental variables.
Pearson correlation
The analysis between carbon stock of different pools and environmental factors (altitude, slope, aspects and disturbance and species diversity and richness indicated significant positive correlation between altitude and AGC, BGC but negative correlation with SOC. The slope is positively correlated with all carbon pools while aspect is negatively correlated but the correlation was not significant. Disturbance is negatively correlated for carbon pools (AGC, BGC, and SOC). A strong positive correlation was obtained for species diversity and richness with AGC and BGC (Table 4).
Discussion
Aboveground biomass
Sixty five percent aboveground was contributed by eight tree species (Schefflera abyssinica, Pouteria adolfi-friederici, Olea welwitschii, Ekbergia capensis, Syzygium guineense, Elaeodendron buchananii, Ilex mitis and Croton macrostachyus). These species are dominant in the study forest and have relatively high IVI values. They comprise a few large-sized individuals that contributed the larger proportion of the total biomass of the forest. The high carbon stock potential of trees in the forest was attributed to the density, age, and DBH of the tree species. Similar study by Singh et al. [32] reported that the size and age of trees could affect the carbon stock in the forest ecosystem. The carbon stored in the aboveground living biomass of trees is impacted by deforestation and degradation [33]. Forest disturbance significantly affects the carbon storage of the forest and it is the major contributor to carbon emission in the tropics and an important contributing factor to climate change. The variation of belowground biomass and carbon is due to variation in tree size, density and productivity of the forest area.
Litter carbon
Litter carbon concentration per sample plot recoded in this study is comparable to those reported for tropical rainforests (1.4 t/ha) [34] and tropical secondary forest in Philippines (1.9 ton/ha) [31]. However, the mean carbon stock in the litter pool of the study area was less compared to values recorded for selected church forests in Addis Ababa [35] and tropical dry forests (2.1 t/ha) [36]. The low carbon stock in litter can probably be attributed to the high decomposition rate and with less amount of litter fall. Since the area is found in tropical climate, the rate of decomposition is relatively fast and all the litter carbon could have converted to soil carbon [37]. In addition, the tree stands in the forest may not be matured and this could result in a low amount of litter fall leading to less amount of carbon.
Non-tree carbon biomass (herbs and shrubs)
The amount of herbaceous and shrub carbon in this study was low when compared to a mean 0.57 t/ha from a dipterocarp forest in the Philippines. However, it was higher than that in a secondary forest in the Philippines [31] and a tropical forest in Eastern Panama [38] which had the mean values of 0.07 and 0.11 t/ha, respectively. The decrease in non-tree biomass and carbon may be attributed to the shading effect of the canopy of trees which reduces light penetration and significantly affects the physical and chemical soil properties for the growth of herbs.
Soil organic carbon
The average amount of soil organic carbon in the study forest was 128 tons/ha, which is lower than the carbon density estimates for the Afromontane Rain Forests of the Eastern Arc Mountains in Tanzania, which was found to be between 252 and 581 tons/ha [39,40]. The variation in SOC between different vegetation types is attributed to various factors including presence of different tree species, moisture contents, soil nutrient levels, climate zones, topographic areas and disturbance regimes [41]. The soil bulk density ranged from minimum of 0.32 g cm-3 to maximum of 1.1 g cm-3 equivalent with a mean value of 0.64 g cm-3 which is an indication of high soil organic matter in mineral soils [42].
The effect of altitude on carbon stock
The analysis of altitude variation between lower, middle and higher altitudes indicated higher carbon stock levels in the middle and higher altitudes and low carbon stock at lower altitudes. This can be explained by the fact that most woody plant species with higher DBH class were recorded in the middle altitudes. Reyes et al. [16] showed that the presence of large individuals of trees can contribute to larger proportion of the above and below ground carbon. The carbon stock density of the forest decreased beyond 2300 m due to reduction in forest trees and dominance of wetlands and bamboos thickets. Litter carbon was high at low and mid altitudes and this indicated that at high-altitude, there were a few large trees accounting for the lower litter accumulation. The Soil Organic Carbon negatively correlated with altitude and it was relatively high at lower and middle altitudes but low at higher altitudes. This can be explained by the fast that decomposition of organic matter due to high temperature at low and mid altitudes. However, it decreased at higher altitude due to low temperatures and increasing precipitation which prevent decomposition of organic matter [43].
Correlation of carbon stock with environmental variables
There was weak a positive correlation (R2=0.03) between aboveground biomass and altitude. This can be attributed to variation in altitude, species richness and presence of large sized trees and soil variation at plot level. In this study, SOC and litter biomass decreased with increasing altitude. This decrease in soil organic carbon is due to a decrease in canopy cover, litter biomass and species richness and diversity. The amount of above ground carbon stock also varied with different disturbance levels. The highest amount of AGC stock was recorded at less disturbed forest. This is due to limited anthropogenic influences such as collection of firewood, logging as well as forest encroachment. This is in agreement with Wekesa et al. [44] who reported that natural disturbance and logging practice are strongly affect the forest carbon stocks. The result of correlation analysis between carbon stock density and species diversity in Gesha-Sayilem forest indicated that there is positive correlation between carbon stock and species diversity. This is in agreement with Hicks et al. [45] that found a positive relationship between carbon stocks and biodiversity globally. This to say a forest, having diverse plant species may produce more biomass resulting in higher carbon stock.
Conclusion
The five dominant species with higher IVI value for source as carbon sinks found in the forest are Schefflera abyssinica, Syzygium guineense, Pouteria adolfi-friederici, Gallineria saxifarga, Ilex mitis, Lepidotrichilia volkensii and Dracena afromontana. These plant species need to be protected for their economical, ecological and social services. The Gesha-Sayilem Afromontane Forest has high potential to store carbon stock of 362.46 ton/ha and this carbon stock should be included in the National Carbon Stock Database for the implementation of REDD+ with relevant organizations to benefit the local communities from carbon trade.
Conflict of Interests
The authors have not declared any conflict of interests.
Acknowledgment
The authors highly acknowledged for the financial support provided by thematic research division of Addis Ababa University. We also thank the Ethiopian Agricultural Research Institute for soil analysis and letting to utilize the available facilities including oven and furnace for ashing the samples.
References
- Karousakis K (2009) Promoting biodiversity co-benefits in REDD. OECD Publishing, Paris.
- Perschel RT, Evans AM, Summers MJ (2007) Climate change, carbon, and the forests of the Northeast. Forest Guild.
- Sundquist E, Robert B, Stephen F, Robert G, Jennifer H, et al. (2008) Carbon sequestration to mitigate climate change. US Geological Survey, Science for a Changing World, New York, USA, pp: 1-4.
- FAO (2010) Global forest resource assessment. Country Report Ethiopia. FRA2010/065. Food and Agriculture Organization, Rome.
- Brown S, Lugo AE (1982) The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropia 14: 161-187.
- Djomo AN, Ibrahima A, Saborowski J, Gravenhorst G (2010) Allometric equations for biomass estimations in Cameroon and pan moist tropical equations including biomass data from Africa. Forest Ecol Manag 260: 1873-1885.
- Moges Y, Eshetu Z, Nune S (2010) Ethiopia forest resources: Current status and future management options in view of access to carbon finances: A report prepared for the Ethiopian Climate Research and Networking and the United Nations Development Programme (UNDP); UNDP: Addis Ababa, Ethiopia.
- Beniston J, Lal R (2012) Improving soil quality for urban agriculture in the North Central US. In: Carbon sequestration in urban ecosystems. Springer, Dordrecht, pp: 279-313.
- Deressa T, Hassan RM, Ringler C (2008) Measuring Ethiopian farmers' vulnerability to climate change across regional states. Intl Food Policy Res Inst.
- Kebede A (2011) Delimiting the interface between garden coffee expansion and forest coffee conservation and its implication for protected area management: The Case of KafaCoffee Biosphere Reserve. M. Sc. thesis, University of Klagenfurt, Austria.
- Friis I, Demissew S, van Bruegel P (2010) Atlas of the potential vegetation of Ethiopia. The Royal Danish Academy of Science and letters, Denmark. Det Kongelige Danske Videnskabernes Selskab Biol Skr 58: 1-307
- Smartt PF (1978) Sampling for vegetation survey: A flexible systematic model for sample location. J Biogeogr 5(1): 43-56.
- Kent M, Coker R (1992) Vegetation description and analysis: A practical approach. CRC Press, Inc, London.
- Pearson T, Walker S, Brown S (2013) Source book for land use, land-use change and forestry projects. World Bank, Washington, DC
- Chave J, Réjou-Méchain M, Búrquez A, Chidumayo E, Colgan MS, et al. (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Change Biol 20: 3177-3190.
- Reyes G, Brown S, Chapman J, Lugo AE (1992) Wood densities of tropical tree species. General Technical Report SO-88. USDA Forest Service, Southern Forest Experiment Station, New Orleans, USA.
- Â Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, et al. (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145(1): 87-99.
- Eggleston S, Buendia L, Miwa K, Ngara T, Tanabe K (2006) IPCC guidelines for national greenhouse gas inventories. Hayama, Japan: Institute for Global Environmental Strategies.
- Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world's upland forests. Oecologia 111: 1-1.
- Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. Methods in plant ecology. 2ndend. Back well scientific publications, Oxford.
- Brown JK (1974) Handbook for inventorying downed woody material. Gen Tech Rep INT-16. Ogden, UT: US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station 16.
- Brown S, Gillespie AJ, Lugo AE (1989) Biomass estimation methods for tropical forests with applications to forest inventory data. Forest Science 35: 881-902.
- Maniatis D, Saint André L, Temmerman M, Malhi Y, Beeckman H (2011) The potential of using xylarium wood samples for wood density calculations: A comparison of approaches for volume measurement. iForest-Biogeosciences and Forestry 4: 150.
- Brown S, Shoch D, Pearson T, Delaney M (2004) Methods for measuring monitoring forestry carbon projects in California. Winrock International, for the California Energy Commission, PIER Energy-Related Environmental Research, pp: 500-504.
- Yohannes T (2016) Plant diversity and carbon stock analysis along environmental gradients: The case of Gergeda and Anbessa Forests in Western Ethiopia.
- Feyissa A, Soromessa T, Argaw M (2013) Forest carbon stocks and variations along altitudinal gradients in Egdu Forest: Implications of managing forests for climate change mitigation. Sci Technol Arts Res J 2: 40-46.
- Assaye H, Asrat Z (2016) Carbon storage and climate change mitigation potential of the forests of the simien mountains national park, Ethiopia. Agriculture, Forestry and Fisheries 5: 8-17
- Hassen N (2015) Carbon stock estimation along an altitudinal gradient in Gera moist ever green afromontane forest, Southwest Ethiopia. M.Sc. Thesis, Addis Ababa University.
- Yilma Z, Yohannes T, Peter Sutcliffe (2010) Forest inventory in Ethiopian Montane Cloud Forest. MizanTeferi, Ethiopia
- Ullah MR, Al-Amin M (2012) Above- and below-ground carbon stock estimation in a natural forest of Bangladesh. Journal of Forest Science 58: 372-379.
- Lasco RD, MacDicken G, Pulhin F, Guillermo IQ, Sales RF, et al. (2006) Carbon stocks assessment of a selectively logged dipterocarp forest and wood processing mill in the Philippines. Journal of Tropical Forest Science, pp: 212-221.
- Singh V, Tewari A, Ram J, Singh C (2009) Aspect related changes in biomass stocks and carbon sequestration rates of Shorea robusta (Sal) forest of Central Himalaya. Report and Opinion 1: 56-60.
- Gibbs HK, Brown S, Niles JO, Foley JA (2007) Monitoring and estimating tropical forest carbon stocks: making REDD a reality. Environ Res Lett 2: 045023.
- Lü XT, Yin JX, Jepsen MR, Tang JW (2010) Ecosystem carbon storage and partitioning in a tropical seasonal forest in Southwestern China. Forest Ecol Manag 260: 1798-1803.
- Tolla T (2011) Estimation of carbon stock in church forests: implications for managing Church forest for carbon emission reduction. Unpublished MSc thesis, Addis Ababa University, Ethiopia.
- IPCC (2003) Good Practice Guidance for land use, land-use change and forestry. Intergovernmental Panel on Climate Change. National Greenhouse Gas Inventories Programme.
- Fisher RF, Binkley D (2000) Ecology and management of forest soils. John Willey and Sons, Inc. New York, USA.
- Kirby KR, Potvin C (2007) Variation in carbon storage among tree species: Implications for the management of a small-scale carbon sink project. Forest Ecology and Management Forest Ecol Manag 246: 208-221.
- Munishi PKT (2001) The Eastern Arc Mountains Forests of Tanzania: Their Role in Biodiversity and Water Resources Conservation and Net Contribution to Atmospheric Carbon. PhD Thesis, North Carolina State University, Raleigh, NC USA, p: 128
- Munishi PK, Shear TH (2004) Carbon storage in Afromontane rain forests of the Eastern Arc mountains of Tanzania: Their net contribution to atmospheric carbon. J Trop For Sci 16(1): 78-93.
- Houghton RA (2005) Tropical deforestation as a source of greenhouse gas emissions. Tropical Deforestation and Climate Change, p: 13.
- Zhu B, Wang X, Fang J, Piao S, Shen H, et al. (2010) Altitudinal changes in carbon storage of temperate forests on Mt Changbai, Northeast China. J Plant Res 123: 439-452.
- Wekesa C, Leley N, Maranga E, Kirui B, Muturi G, et al. (2016) Effects of forest disturbance on vegetation structure and above-ground carbon in three isolated forest patches of Taita Hills. Open J For 6: 142.
- Hicks C, Woroniecki S, Fancourt M, Bieri M, Garcia RH, et al. (2014) The Relationship between biodiversity, carbon storage and the provision of other ecosystem services: Critical Review for the Forestry Component of the International Climate Fund. Cambridge, UK.
Citation: Addi A, Demissew S, Soromessa T, Asfaw Z (2019) Carbon Stock of the Moist Afromontane Forest in Gesha and Sayilem Districts in Kaffa Zone: An Implication for Climate Change Mitigation. J Ecosys Ecograph 9: 261.
Copyright: © 2019 Addi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5209
- [From(publication date): 0-2019 - Oct 22, 2025]
- Breakdown by view type
- HTML page views: 4034
- PDF downloads: 1175