Case Report Open Access
Case Report: A Rare and Unusual Cause of Acute Liver Failure
Nwe Ni Than1*, Frederick Chen2, Simon Olliff3 and Dhiraj Tripathi1
1Liver Unit, University Hospital NHS Trust, Birmingham, UK
2Department of Haematology, University Hospital NHS Trust & NHS Blood and Transplant, Birmingham, UK
3Interventional Radiology Department, University Hospital NHS Trust, Birmingham, UK
- *Corresponding Author:
- Nwe Ni Than
Liver unit, University Hospital NHS Trust
Birmingham, UK, B15 2WB
Tel: +44 7738628170
E-mail: nwenithan@gmail.com
Received date: April 14, 2015; Accepted date: May 11, 2015; Published date: May 18, 2015
Citation: Than NN, Chen F, Olliff S, Tripathi D (2015) Case Report: A Rare and Unusual Cause of Acute Liver Failure . J Gastrointest Dig Syst 5:287. doi:10.4172/2161-069X.1000287
Copyright: © 2015 Than NN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Budd-Chiari syndrome (BCS) is a rare condition caused by hepatic outflow obstruction. Typical clinical features include right upper quadrant pain, ascites and hepatosplenomegaly. We report a case of a 26 year old female who presented with jaundice, worsening abdominal swelling and abdominal pain. She subsequently developed acute liver failure and spontaneous bacterial peritonitis (SBP) with significant renal impairment requiring organ support. Computed tomography (CT) scan showed congested liver and occlusion of three hepatic veins consistent with Budd- Chiari Syndrome. Transjugular intra hepatic portosystemic stent/shunt (TIPSS) was inserted which resulted in complete resolution liver and renal function. She was subsequently found to be positive for the JAK-2 V617F mutation suggesting an underlying myeloproliferative disorder. This is a rare case in which a patient developed spontaneous bacterial peritonitis (SBP) in the context of BCS. The patient had excellent outcome from TIPSS procedure, which rescued her from undergoing liver transplantation.
Keywords
Ascites; Acute liver failure; Spontaneous bacterial peritonitis; Budd-Chiari syndrome; Transjugular intrahepatic portosystemic stent-shunt; JAK2V617F mutation; Myeloproliferative disorder
Background
Budd-Chiari syndrome (BCS) is a rare condition caused by obstruction of hepatic venous outflow system from the level of the small hepatic veins to the junction of inferior vena cava and right atrium [1]. We present an unusually severe case of BCS presenting with overwhelming sepsis and acute liver failure. Despite her poor condition, TIPSS rescued the patient from acute liver failure requiring liver transplantation. This case illustrated the need to hunt for additional risk factors for BCS, since the initial assumption was that it due to the contraceptive pill only. However, only later the positive test for the JAK2 mutation comes to light.
Case History
A 26 year old lady presented to local hospital with two weeks history of abdominal pain and swelling. She was seen several times over six months before admission with abdominal bloating and pain and was diagnosed with irritable bowel syndrome. There was no significant past medical history. She was on oral contraceptive pill (OCP). She smoked 10 cigarettes per day and alcohol intake was two units per week.
Clinical examination revealed tender right upper quadrant and moderate ascites. Admission blood tests are shown in Table 1, which showed raised white cell count (WCC), neutrophil count, raised alanine aminotransferase (ALT) and international normalized ratio (INR) with normal renal function. Pregnancy test was negative.
| Admission Days | WCC (109/L) |
Neutrophil (109/L) |
Haemoglobin (g/dL) |
Platelet (109/L) |
Urea (mmol/L) |
Cr (umol/L) |
ALT (U/L) |
Bilirubin (umol/L) |
Albumin (g/L) |
INR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 20.8 | 17 | 17 | 233 | 5.7 | 78 | 590 | 38 | 40 | 1.8 |
| 4# | 30.4 | 23.19 | 14.3 | 166 | 13 | 245 | 1606 | 67 | 35 | 5.4 |
| 5** | 14.4 | 12.8 | 11.7 | 86 | 11.3 | 182 | 1418 | 79 | 32 | 1.8 |
| 7 | 14.1 | 9.2 | 10.8 | 111 | 11.9 | 228 | 239 | 61 | 31 | 1.4 |
| 13## | 7.3 | 4.1 | 9.6 | 200 | 4.5 | 128 | 48 | 28 | 34 | 2 |
Table 1: Trends of blood tests during admission. *Admission bloods from local hospital #Bloods when admitted to liver unit, Birmingham,**Bloods on the day of TIPSS insertion, ##Bloods on day of discharge.
Ascitic tap performed on admission was culture negative although no cell count performed. She underwent paracentesis of 8 liters two days after admission. Ultrasound scan (USS) scan showed a bulky liver with ascites, patent portal vein and no splenomegaly. There was no comment on hepatic vein patency.
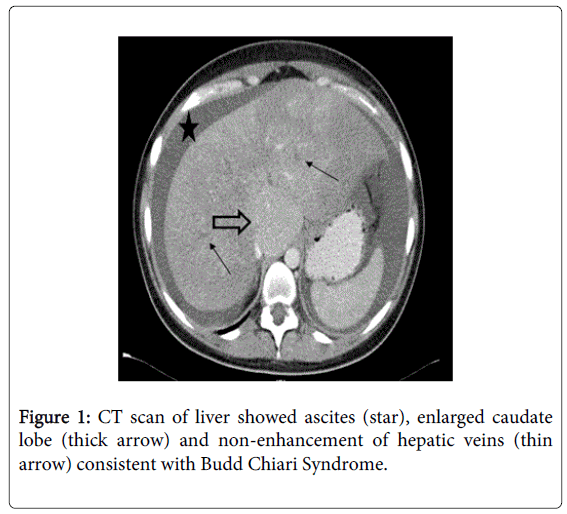
Four days later, she became unwell with nausea and vomiting and her temperature was noted to be 37°C. Repeat blood test showed acute renal failure with worsening liver function (Table 1). Arterial gas showed features of metabolic acidosis with a high lactate of 15. Computed tomography (CT) scan was arranged due to raised lactate and vomiting. This showed a congested liver, moderate ascites with enlarge caudate lobe and there were no contrast seen in all three hepatic veins consistent with BCS (Figure 1).
She was transferred to the liver unit and upon arrival, she was pyrexial (38°C) and repeat ascitic tap showed polymorph counts of 532 cells mm3 consistent with spontaneous bacterial peritonitis (SBP) with no evidence of bacterial growth in culture of ascites fluid.
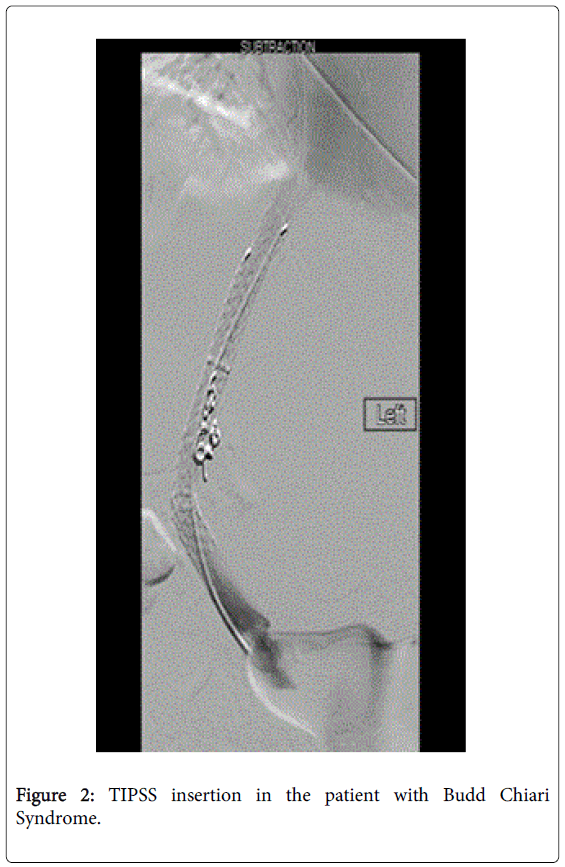
She was treated with intravenous antibiotics (Piperacillin/Tazobactam). Patient was put on haemofiltration due to underlying acute kidney injury and metabolic acidosis. TIPSS was arranged and two covered stents were inserted 24 hours later with correction of coagulopathy (Figure 2).
Portal pressure pre TIPSS was 44 mmHg and post TIPSS was 31mmHg, right atrial pressure of 24 mmHg with final gradient of 7 mmHg. Clinical and biochemical parameters improved slowly after TIPSS insertion (Table 1) with rapid resolution of ascites.
She was discharged on long term warfarin two weeks after admission. She had a bone marrow examination after haematology review which was non-diagnostic, however further haematological investigations revealed her to be JAK2V617F mutation positive indicating an underlying myeloproliferative neoplasia (MPN).
Discussion
BCS is not a primary condition of the liver parenchyma; it is the result of partial or complete obstruction of hepatic venous outflow [1]. The obstruction can occur anywhere from the small hepatic veins to the right atrium of the heart [1]. It is a rare condition and usually occurs in 1 in 100,000 populations worldwide [2]. BCS is classified as primary when the obstruction to hepatic venous outflow is related to a primary venous problem, such as thrombosis, stenosis, or webs and as secondary when it is related to extrinsic compression, such as abscess, tumor, cyst, or hyperplastic nodules [2]. In western countries, it is most often caused by thrombosis, as a result of inherited and acquired hypercoagulable states [2]. Often, no clear causes can be identified for cause of BCS.
Details of risk factors and causes are mentioned in Table 2. The presence of the JAK2V617F mutations is diagnostic of MPN and accounts for 50% of patients with BCS. JAK2V617F mutation is found in 97% of patients with polycythaemia Vera as well as in approximately 60% of essential thrombocytopenia and myelofibrosis [3-5]. Forty percent of cases with BCS are JAK2V617F positive, although 17.1% of these do not have haematological features of MPN as defined by World Health Organisation (WHO) diagnostic criteria [6,7]. Inherited deficiencies of protein C, protein S and anti-thrombin are increasingly being reported in BCS [3,5]. It is important to note that interpretation of low protein C, protein S and anti-thrombin levels in the setting of BCS is challenging as they are consumed in acute thrombosis and in liver dysfunction.
| Prothrombotic Condition (%) | Investigations |
|---|---|
| Myeloproliferative disease (49%) | Peripheral blood JAK2(V617F) mutation |
| Bone marrow biopsy (aspirate cytology, cytogenetic studies, trephine histology) | |
| Paroxysmal nocturnal haemoglobinuria (19%) | Flow cytometry of peripheral blood cells showing CD55 and CD59 deficient clones |
| Factor V Leiden (12%) | Leiden mutation (R506Q) by Peripheral blood molecular analysis (Functional Clotting assays during acute thrombosis not reliable) |
| Prothrombin gene mutation (3%) | Molecular analysis for G20210A mutation |
| Inherited protein C deficiency (4%) | Qualitative or quantitative defect in Protein C. The assay is a generally a functional one and identifies both qualitative and quantitative deficiency (after acute thrombosis and off warfarin) |
| Inherited protein S deficiency (3%) | Qualitative or quantitative defect in Protein S. ELISA for free Protein S antigen. Functional Protein S test also available. (after acute thrombosis and off warfarin) |
| check for AT3 levels- again functional and needs to be off heparin (part of thrombophilia screening) | |
| Behcet’s disease (4%) | Oral ulcers at least 3 times within one year period along with 2 out of the following 4 "hallmark" symptoms: |
| 1) Genital ulcers | |
| 2) Skin lesions | |
| 3) Ocular inflammation | |
| 4) Pathergy reaction (papule >2 mm, 24-48 hours or more after needle-prick). | |
| Antiphospholipid syndrome (25%) | Diagnosis of APS requires one clinical manifestation |
| 1)Arterial, venous, or small vessel thrombosis | |
| 2) One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation and/or 3 or more unexplained consecutive spontaneous abortions before the 10th week of gestation. | |
| and one laboratory manifestation on 2 or more occasions | |
| 1)Anti-cardiolipin Immunoglobulin (Ig) G and/or IgM | |
| 2)Anti-β2 glycoprotein I Ig G and/or Ig M | |
| 3) Lupus anticoagulant assay | |
| Oral contraceptives or pregnancy (33%) | The oral contraceptive pill (OCP) is associated with an additional risk factor in the majority, and the direct relationship with BCS is unclear. |
| Ulcerative Colitis (8%) | Colonoscopy if symptoms suggestive |
| Coeliac disease | Anti-TTG and Anti-EMA antibodies, Endoscopic D2 biopsy |
Table 2: Prothrombotic conditions associated with Budd Chiari Syndrome [3].
BCS commonly occurs in women and young adults. Patients may present with acute signs and symptoms with the classic triad of abdominal pain, ascites and hepatomegaly or more chronic symptoms such as ascites, variceal bleeding related to long-standing portal hypertension [1]. In half of BCS patients as in this case, symptom duration is less than one month [2]. Clinical presentations may be variable from asymptomatic in 15 to 20% of cases, fulminant in 5%, acute presentation in 20% to sub-acute/chronic in 60% of cases [2].
Hepatic venous outflow obstruction increases hepatic sinusoidal pressure which results in portal hypertension with formation of ascites, liver congestion, and decreased liver perfusion [1,2]. Hepatocytes then undergo hypoxic damage which eventually evolves into non-inflammatory centrilobular cell necrosis, which is found in 70% of cases of BCS [2,8]. Obstruction of the portal vein is also seen in 10%-20% of cases of BCS, due to underlying thrombophilic disorder and stagnant blood flow [8].
Having a high clinical suspicion is important in making a diagnosis; otherwise there may be delays in diagnosis and treatment as in the presented case. Serum transferase levels may be more than five times the upper limit of the normal range, especially in the fulminant and acute forms of BCS [8]. Serum alkaline phosphatase and bilirubin levels also increase but serum albumin level decreases moderately [8]. In all cases diagnostic imaging is required to outline the pathological anatomy of the hepatic vein (HVs), IVC, and portal venous system because this information will determine therapeutic options [9,10]. Imaging also plays an important role in postoperative evaluation and surveillance of shunts [9].
This is accomplished by ultrasound with Doppler and supplemented by CT and/or magnetic resonance imaging (MRI) [10]. Doppler ultrasound, often the first study obtained, has a sensitivity and specificity as high as 85%. Other radiographic findings seen in BCS include inhomogeneous parenchymal enhancement, presence of intrahepatic collaterals, hyper vascular nodules and caudate lobe hypertrophy [9]. The caudate lobe has direct venous drainage into the IVC and therefore, often undergoes compensatory hypertrophy. Liver biopsy is rarely necessary now to diagnose BCS due to advancements in imaging. Liver biopsy may reveal a wide range of histological findings from sinusoidal congestion, inflammation to cirrhosis and parenchymal necrosis [9]. Extravasation of red cells into the liver-cell plate and space of Disse is a characteristic feature of BCS [9].
Management requires a multidisciplinary team approach with hepatology, haematology, surgical, and radiology expertise [10]. Restoring HV blood flow is the most physiological method of treatment of BCS [10]. The management of BCS can be divided into three main categories: medical, surgical, and endovascular aiming to relive the obstructed venous flow. Treatment options include medical management for pain, ascites and anticoagulation as well as the treatment of the underlying myeloproliferative condition of using cytoreductive therapy [11]. Hepatic vein dilatation or re-canalisation, surgical porto-systemic shunt, TIPSS and liver transplantation are other treatment options for patients with BCS to relieve the obstructed flow. In recent years, in cases where HV flow cannot be restored or where the approach fails (usually because the remaining patent veins are too small or have insufficient flow), TIPSS is used as a decompressing nonsurgical portocaval shunt [10]. Two series with a total of 34 patients showed improved patency and less dysfunction with polytetrafluoroethylene (PTFE)-covered stents compared with bare stents [9,12,13]. One of the largest series of TIPSS for BCS reported excellent outcomes even in high risk patients with 1-year and 5-year transplant free survival of 88 and 78%, respectively [14].
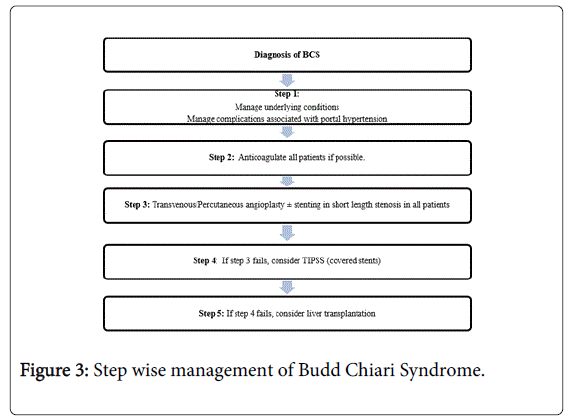
Surgery is now reserved for those few cases where all radiological procedures fail or the severity of liver failure is such that liver transplantation may be required [10]. A surgical shunt may thus rescue a patient in whom TIPS cannot be performed [10]. Liver transplant may be the choice for severe fulminant liver failure or used if a patient does not improve significantly or develops serious encephalopathy after TIPSS [10]. Liver transplantation is needed in up to 15-20% of patients where other treatment modalities have failed [3]. Fulminant presentation of BCS merits urgent liver transplantation. A stepwise approach to managing patient with BCS has been shown in Figure 3.
Asymptomatic patients carry a good prognosis, however symptomatic patients usually have a poor course and it has been estimated that 90% of untreated patients will die within 3 years [3]. There are many prognostic scoring indexes for BCS. Among them, BCS-TIPS PI score showed adequate accuracy in predicting mortality in the overall cohort of patients and better predictive capacity than the Rotterdam score [15].
A patient with a BCS-PI of >7 has a poor outcome and early liver transplantation should be considered. In the case of this patient the BCS-TIPS PI was 1.87 suggesting better outcome. However, none of the prognostic scores had sufficient accuracy for use in individual patients. With TIPSS, the outcomes are excellent and comparable to liver transplantation [14]. It is unusual for a patient with BCS to require surgery where there is access to expertise in interventional radiology.
Myeloproliferative disorder (MPD) or Myeloproliferative Neoplasm (MPN) is a spectrum of diseases in which there is an abnormal and increased production of red blood cells, white blood cells and platelets in the bone marrow. MPN include polycythemia vera (PV), essential thrombocytopenia and myelofibrosis (MF) [16]. The treatment is variable depends on the type of underlying conditions and it involves general medical therapy such as aspirin or warfarin, more specific therapy either in the form of cytoreductive agent such as hyroxyurea, JAK inhibitors such as Pacritinib or allogenic stem cell transplantation [16]. The primary goals of therapy for both PV and ET include prevention of thrombotic and bleeding complications in addition to minimizing the risk of progression to MF or acute leukemia [16]. In contrast, MF treatment goals are based on assessment of both disease burden (symptoms, cytopenias, splenomegaly) and impact of disease on survival [16].
Conclusion
In conclusion, patients with BCS should be screened for underlying myeloproliferative disorder (MPD) since it is common and can be found in half of the patients. Once JAK2 mutation is confirmed, bone marrow examination is necessary to exclude underlying other causes of MPD such as polycythaemia vera or essential thrombocytopenia. If there are no associated haematological malignancies such as in our case, the patient can be managed with medical therapy such as anticoagulation to prevent further venous occlusion. However, if there is underlying hematological malignancies, targeted therapies should be initiated after discussion with Haematologist.
References
- Cura M, Haskal Z, Lopera J (2009) Diagnostic and interventional radiology for Budd-Chiari syndrome. Radiographics 29: 669-681.
- Ferral H, Behrens G, Lopera J (2012) Budd-Chiari syndrome. AJR Am J Roentgenol 199: 737-745.
- MacNicholas R, Olliff S, Elias E, Tripathi D (2012) An update on the diagnosis and management of Budd-Chiari syndrome. Expert Rev Gastroenterol Hepatol 6: 731-744.
- Sekhar M, McVinnie K, Burroughs AK (2013) Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 162: 730-747.
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, et al. (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365: 1054-1061.
- Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, et al. (2012) Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 120: 4921-4928.
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779-1790.
- Aydinli M, Bayraktar Y (2007) Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol 13: 2693-2696.
- Horton JD, San Miguel FL, Membreno F, Wright F, Paima J, et al. (2008) Budd-Chiari syndrome: illustrated review of current management. Liver Int 28: 455-466.
- Beckett D, Olliff S (2008) Interventional radiology in the management of Budd Chiari syndrome. Cardiovasc Intervent Radiol 31: 839-847.
- Neumann AB, Andersen SD, Nielsen DT, Holland-Fischer P, Vilstrup H, et al. (2013) Treatment of Budd-Chiari syndrome with a focus on transjugular intrahepatic portosystemic shunt. World J Hepatol 5: 38-42.
- Hernández-Guerra M, Turnes J, Rubinstein P, Olliff S, Elias E, et al. (2004) PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology 40: 1197-1202.
- Gandini, R., D. Konda, and G. Simonetti, Transjugular Intrahepatic Portosystemic Shunt Patency and Clinical Outcome in Patients with Budd-Chiari Syndrome: Covered versus Uncovered Stents. Radiology, 2006. 241(1): p. 298-305.
- Garcia-Pagán JC1, Heydtmann M, Raffa S, Plessier A, Murad S, et al. (2008) TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology 135: 808-815.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15670
- [From(publication date):
June-2015 - Aug 03, 2025] - Breakdown by view type
- HTML page views : 11073
- PDF downloads : 4597