Changes in Vascular and Immune Cell Content of an LPS Treated Rat Olfactory Bulb PD Model, Measured by Fluorescence Deconvolution Microscopy
Received: 26-Sep-2017 / Accepted Date: 12-Oct-2017 / Published Date: 20-Oct-2017 DOI: 10.4172/2576-3881.1000120
Abstract
Objective: We have previously shown changes in protein, immune cell, nitric oxide a neurotrophic factor content and distribution in the olfactory bulb of an endotoxin-treated rat model, as all these factors have been shown to be involved in neurodegeneration. We expanded our studies by fluorescently imaging smooth muscle actin and endothelial nitric oxide synthase (eNOS), both markers of blood vessels, to investigate loss of vasculature content, as well as imaging locations and quantities of immune cells. The work was performed to shed further light on associations between vessel integrity and immune cell initiated endothelial disruption. Our goal was to demonstrate that cytokine production, NOS induction and immune cell increases, are likely part of the process that leads to a loss of olfaction and dopaminergic signaling and includes vascular perturbations.
Methods: Rats were sacrificed following lipopolysaccharide (LPS) treatment. Olfactory bulbs were harvested, sectioned from top to bottom to include the tract and sensory neurons, and probed for markers of inflammation. Inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), eNOS, interleukin-1 beta (IL-1β), TNF-α, interleukin-6, glial cell derived neurotrophic factor (GDNF) and circulating nitric oxide (NO) were imaged together with tagged macrophages, T-cells, B-cells and neutrophils.
Results: Serum NO levels indicated that an inflammatory episode had occurred, being significantly higher in treated animals, with tissue levels of NOS elevated for an extended period of time. Immune cell clusters were seen in a number of areas and the localization of NOS isomers suggests that they have divergent roles in neurodegeneration. For instance, eNOS was associated with blood vessels, iNOS with glial and matrix cells and nNOS with glial cells and neurons. T and B-cell numbers showed a sustained increase; neutrophil numbers rapidly increased then returned to baseline levels; macrophage numbers increased and remained high; LAMP positive cell numbers (NK-cells) increased and remained high; GDNF content increased; IL-6, TNF-α and IL-1β levels all rapidly increased, before dropping to untreated levels, while circulating, NO levels increased dramatically. Of interest, the images of vascular content, immune cell content, eNOS and smooth muscle actin, allowed us to show detrimental interactions between cells, factors and vessels. Our data show that the majority of the vessels were intact, though sections of interest were ‘extracted’ to reveal possible leaky areas. Specific sites of IL-6 positive lymphocyte clustering were noted around vessels, suggesting that interactions are occurring that lead to disruptions of blood vessel tunicae, allowing the internalization of circulating cells and subsequent cytokine-initiated endothelial cell death.
Conclusion: Our findings suggest that protective GDNF and eNOS, which maintains vascular tone, are possibly synthesized too late to combat cytokine initiated neuron damage, glial activation and chronic loss of vascular integrity.
Keywords: Neurodegeneration; Olfaction; Blood-brain-barrier; Cytokines; Nitric oxide
Introduction
Many factors play a role in chronic neurodegeneration which leads to Parkinson’s disease (PD), including inflammation [1], cytokine syntheses and release [1], protein recycling-ubiquitination problems [2], glial cell activation [3], loss of astrocyte support [4], neurotrophic factors [2], nitric oxide synthase [5] and mitochondrial dysfunction [2]. However, recent reports have shown that circulating cells [6] are also involved in disease progression, which coupled with vascular damage [7], points to both peripheral and innate factors and cell involvement in the ensuing demise of neurons.
Vascular endothelial cells are thought to be damaged via interactions with not only reactive oxygen species (ROS), but also with increased cytokine concentrations, NO and LPS [8,9]. As multiple factors have been reported associated with neurodegeneration, the suggestion is that some have a role in loss of blood vessel integrity and ensuing damage to the blood brain barrier (BBB). This ‘neurovascular unit’ (endothelial cells, astrocytes, neurons, pericytes and matrix) [10-12]; is essential for the wellbeing of the brain and has become an area of major research regarding its role in neurodegenerative diseases, including a loss of tight junctions [13] and passage of toxic substances and circulating cells into the brain itself [14]. Thus, the disease process is multi-compartmentalized and requires therapies that do more than target the loss of dopamine [15]. For instance, therapies are needed to address functional alterations of the BBB in conjunction with elucidating the role of circulating T helper and B lymphocytes and alterations in leukocyte antigens. The immune system and associated inflammation and blood vessel integrity changes thus require focused research to elucidate new strategies [16].
Our studies have focused on the olfactory bulb as an early loss of olfaction in Parkinson’s disease patients as a measurable change and could be the starting point to elucidate both the initiation of neurodegeneration and the subsequent chronic loss of neurons [17]. Therefore the ability of the olfactory bulb to maintain both vascular integrity and a functioning BBB becomes even more important due to intimate interactions with synaptic functions and α−synuclein [18]. As previously reported by Maon et al, α−synuclein changes have been reported as an early identifier of olfactory bulb and anterior olfactory nucleus related problems protein transmission through neurological and anatomic pathways [19].
Previous work in our lab has demonstrated the ‘toxicity’ of PD-CSF [20] including, the presence of non-resident cells and cytokine production in the olfactory bulb of an LPS rat model [21], specific localizations of PD-associated proteins and cytokines in LPS treated brain [22] and the induction of neurotrophins and nitric oxide [5]. We therefore continued our studies to further understand the progression of this chronic, debilitating disease, by correlating cytokine, protein and neurotrophic factor concentrations and localizations, together with immune cell numbers and a loss of vascular integrity. This focus was partly due to recent research into vascular integrity and blood vessel function during chronic inflammation [23-25]. Using fluorescent tags and deconvolution imaging we examined vascular content, vessel integrity and vessel types in images of LPS treated rat olfactory bulbs and concluded that, by and large, vessels are overall intact but with ‘suspect’ areas. These findings might indicate that even though protective GDNF is produced and nitric oxide is released to maintain vascular tone, subsequent inflammatory episodes are increasingly challenging due to the first assault making repair and rescue difficult due to a loss of BBB control, continuing increases in cytokine levels and reductions in normal cellular abilities [5].
Materials and Methods
i. Sectioning and staining of tissues
Adult rat brains (300 g male Sprague Dawley), from saline injected and LPS treated animals (i.v., 35 μg/kg), were removed and olfactory bulbs separated, fixed, sectioned (12 μm 3) and probed with antibodies and treated with secondary antibodies for deconvolution fluorescence microscopy. Images were acquired in a stepwise process (0.1-0.25 μm steps) following excision the guidelines of Paxinos and Watson [26]. The protocol for this work was approved by the animal welfare committee (AWC-14-0148 Title: Long-Term Effects of LPS in Rats: A Model of Neuro-Degenerative Disease).
ii. Imaging
Sections were mounted on 25 mm glass cover slips and fluorescently probed for the three NOS isoforms, iNOS, nNOS and eNOS. Tissue was rinsed with cold phosphate buffered saline (PBS) and fixed with 3.7% formaldehyde (1008B, Tousimis Research Corp., Rockville, MD). Fixation was halted by 3 rinses with PBS at room temperature and blocked with 10% goat serum, then incubated for 1 h at 37°C with antibodies (1:100 to 1:1000 dilutions). Sections were further incubated for 30 min at 37°C and rinsed 3 times with 0.05% Tween 20 in PBS (TPBS). Nuclei and F-actin were stained with phalloidin (Invitrogen, Grand Island, NY, USA) diluted 1:100 in 4',6-diamidino-2- phenylindole (DAPI; A-T rich region binding in DNA) solution. Specimens were covered with one drop of anti-fade (Elvanol, DuPont, Wilmington, DE, USA). Tissue sections were probed with antibodies for glial cell derived neurotrophic factor (GDNF; sc-9010, Santa Cruz Biotech, CA, USA) and lysosomal associated membrane protein (LAMP-1; sc-19992; Santa Cruz Biotech, CD107a, a marker for lymphocytic cells), a CD3 antibody (sc-20047, Santa Cruz Biotech; T-cell marker), a CD20 antibody (Polyclonal B-Cell marker, sc 15361, Santa Cruz; B-lymphocyte marker), a CD44 monoclonal neutrophil marker (sc-9960, Santa Cruz Biotech) and a CD68 polyclonal macrophage marker, (sc-9139, Santa Cruz Biotech). Secondary fluorescent antibodies were purchased from Jackson ImmunoResearch (West Grove, PA, USA). Fluorescence intensity measurements were made using pixel counts of RGB deconvoluted images, as previously described [22,27,28] and 3D reconstructions and rotations were made [28]. NOS antibodies were from Abcam (Cambridge, MA, USA; nNOS-ab5586, iNOS-ab3523; eNOS-M221).
iii. Deconvolution fluorescence microscopy
3D image reconstructions of NOS isoforms, actin and nuclei were generated [5,21,29] and images were saved as TIFF files. Comparative statistical analyses (one way ANOVA) of fluorescence densities (pixel numbers) were made using Corel (Ottawa, Ontario, Canada) and SigmaStat software (SPSS, Chicago, IL, USA).
iv. Circulating cytokine and nitric oxide level determinations
Blood samples from LPS treated and control animals were assayed for plasma cytokines using a commercial, enzyme-linked immunosorbent assay ELISA kit (R&D Systems, MN, USA). Plasma NO levels were quantified by a modified chemiluminescence method [30] using a Sievers NO analyzer 270B (GE Analytical, CO, USA). Nitrite concentrations were quantified by comparing measured versus sodium nitrate standards (20-400 pmol).
Results
Some of the results discussed are from previous reports, but are crucial to constructing a global picture of what we believe is occurring in the early stages of neurodegeneration and with vascular integrity.
Cytokine and immune cell content in olfactory bulb
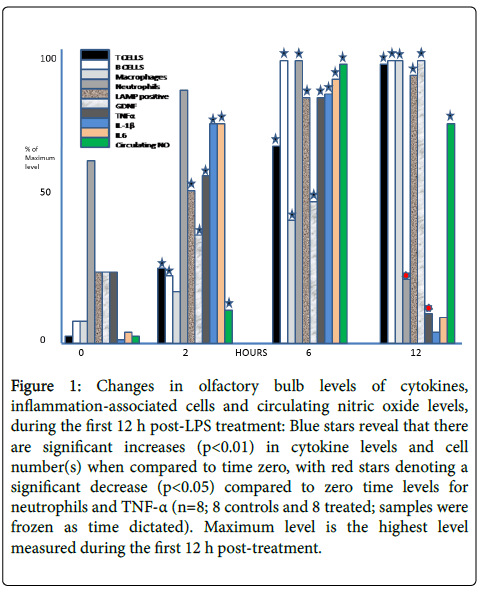
Measurements of cytokine and immune cell content in the olfactory bulb are shown in Figure 1. A rapid, and sustained, rise in T-cell, B-cell and macrophage content, as well as small increases in the number of neutrophils, were observed. IL-6 and TNF-α, which are frequently associated with inflammation and cell death pathways, were increased over the first 12 h post treatment and remained elevated for at least 48 h (data not shown). The data indicate that an inflammatory episode has taken place, and justifies this model for our studies and identifies cytokines and cells involved in the process.
Figure 1: Changes in olfactory bulb levels of cytokines, inflammation-associated cells and circulating nitric oxide levels, during the first 12 h post-LPS treatment: Blue stars reveal that there are significant increases (p<0.01) in cytokine levels and cell number(s) when compared to time zero, with red stars denoting a significant decrease (p<0.05) compared to zero time levels for neutrophils and TNF-α (n=8; 8 controls and 8 treated; samples were frozen as time dictated). Maximum level is the highest level measured during the first 12 h post-treatment.
Imaging of immune cell content in olfactory bulb
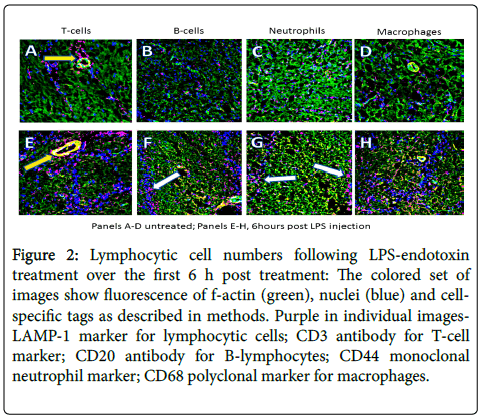
Increases in immune cell numbers were noted as shown in low magnification images (Figure 2), visualizing T cells, B cells and macrophage numbers over the first 6 h. These findings suggest the peak response time of the acute phase of the inflammation, when enough GDNF is present to overcome at least some of the endotoxin assault, cytokine-initiated cell death and glial cell activation [5]. Of note, is the reduction in the number of macrophages and neutrophils after an initial increase, while B-cell numbers remain high at 6 h and T-cell content continues to increase. Figure 2 shows immune system cell content in the olfactory bulb illustrated in the colored panels (all the probes are visualized) and in black and white split screen images of fluorescence of only the immune cell tag. The colored images contain green f-actin, blue nuclei and red for the designated cell type (B-cells, Macrophages, T-cells, and neutrophils, top to bottom.
Figure 2: Lymphocytic cell numbers following LPS-endotoxin treatment over the first 6 h post treatment: The colored set of images show fluorescence of f-actin (green), nuclei (blue) and cell-specific tags as described in methods. Purple in individual images- LAMP-1 marker for lymphocytic cells; CD3 antibody for T-cell marker; CD20 antibody for B-lymphocytes; CD44 monoclonal neutrophil marker; CD68 polyclonal marker for macrophages.
Localization of immune cells using fluorescence imaging
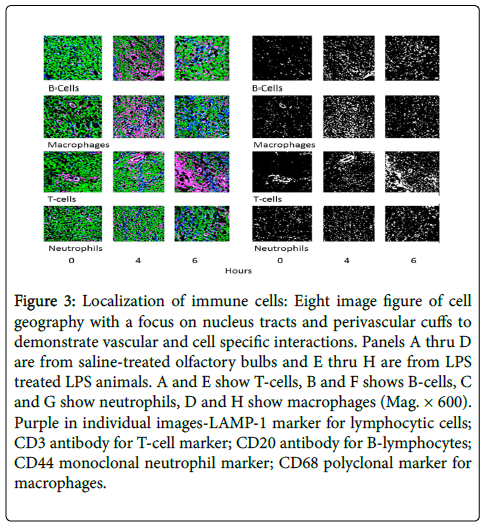
Figure 3 shows representative images from olfactory bulb sections. Saline-treated (Panels A-D) is compared to LPS-treated tissues (Panels E-H), and focuses on the localization of cell types. Note in Panels A and E the vessel-associated ‘cuff’ of T-cells, with increased numbers in the treated sample (E). While in panels B and F, the white arrows point to B-cells and neutrophils (Panels C and G) revealing a specificity of being located along nuclei tracts. Macrophages are obviously increased in treated v control (H v D) at 6 h, but do have a homogenous distribution. The findings strongly implicate the interaction of T-cells and vascular endothelium, as well as some the occurrence of some cell death and/or the presence of non-resident as supported by the large number of macrophages in the tissue.
Figure 3: Localization of immune cells: Eight image figure of cell geography with a focus on nucleus tracts and perivascular cuffs to demonstrate vascular and cell specific interactions. Panels A thru D are from saline-treated olfactory bulbs and E thru H are from LPS treated LPS animals. A and E show T-cells, B and F shows B-cells, C and G show neutrophils, D and H show macrophages (Mag. × 600). Purple in individual images-LAMP-1 marker for lymphocytic cells; CD3 antibody for T-cell marker; CD20 antibody for B-lymphocytes; CD44 monoclonal neutrophil marker; CD68 polyclonal marker for macrophages.
Images to detail comparison and quantification of vascular content
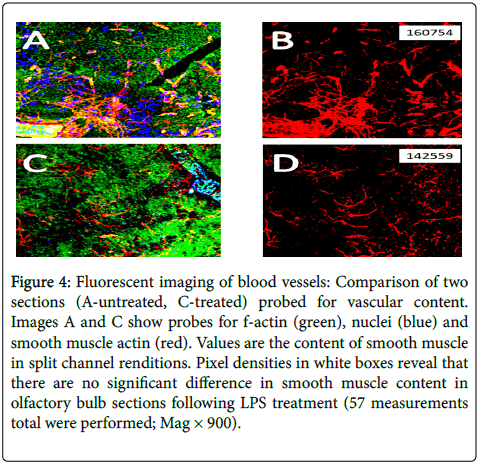
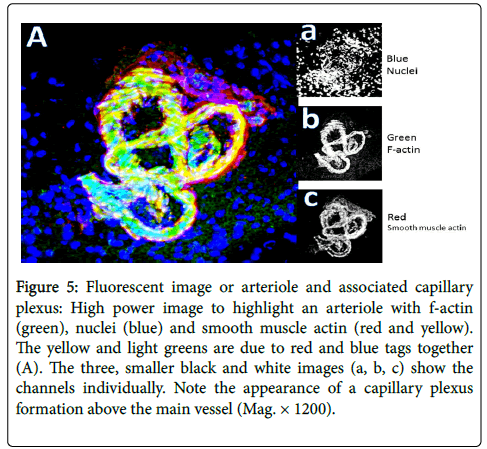
Figure 4 compares two random sections (A-saline-treated, C-LPS treated) to estimate vascular content by fluorescence pixel numbers. Values show content using single channels from RGB images following channel splitting. A count of 3 areas from each of 16 treated animal olfactory bulb sections revealed no significant difference in vessel content, specifically with eNOS (red tag; green=f-actin, blue=nuclei). Similar results when smooth muscle actin was imaged. As an example of fluorescence imaging, Figure 5 shows an arteriole, once again with factin (green), nuclei (blue) and smooth muscle actin (red). The yellow and light greens are due to red and blue tags overlapping/merging, while the small black and white images show the channels individually. Note the capillary plexus formation above the main vessel. There is thus a suggestion that angiogenesis has occurred, probably not due to LPS treatment, and that loss of vasculature can be overcome for a short period of time.
Figure 4: Fluorescent imaging of blood vessels: Comparison of two sections (A-untreated, C-treated) probed for vascular content. Images A and C show probes for f-actin (green), nuclei (blue) and smooth muscle actin (red). Values are the content of smooth muscle in split channel renditions. Pixel densities in white boxes reveal that there are no significant difference in smooth muscle content in olfactory bulb sections following LPS treatment (57 measurements total were performed; Mag × 900).
Figure 5: Fluorescent image or arteriole and associated capillary plexus: High power image to highlight an arteriole with f-actin (green), nuclei (blue) and smooth muscle actin (red and yellow). The yellow and light greens are due to red and blue tags together (A). The three, smaller black and white images (a, b, c) show the channels individually. Note the appearance of a capillary plexus formation above the main vessel (Mag. × 1200).
High Power images to indicate possible endothelial discontinuity
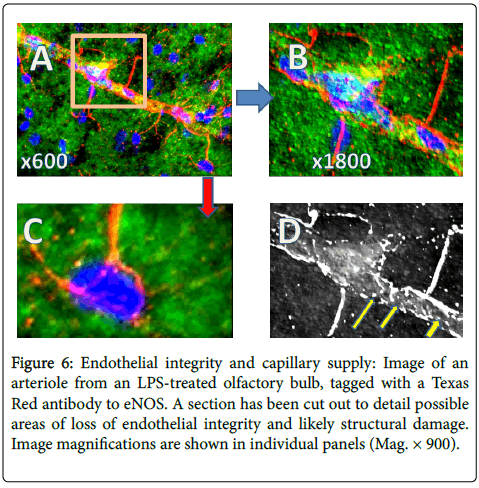
Figure 6 is an image of an arteriole from an LPS-treated olfactory bulb, with a section shown at higher magnification to detail possible areas of loss of integrity/structural damage. Panel A is at medium power and Panel B is a selected area at higher power. Panel C is an interesting observation of capillaries seeming to go to specific cells, not all cells, while panel D is a black and white, embossed image that gives a clearer rendition of what appears to be a discontinuous epithelium (yellow arrows). The data points to the fact that there might be leakiness in vessels, but whether this is due to LPS treatment, whether this is an acute event, or whether this is a ‘natural’ phenomenal, cannot be resolved.
Figure 6: Endothelial integrity and capillary supply: Image of an arteriole from an LPS-treated olfactory bulb, tagged with a Texas Red antibody to eNOS. A section has been cut out to detail possible areas of loss of endothelial integrity and likely structural damage. Image magnifications are shown in individual panels (Mag. × 900).
Discussion
Studies in our lab with cultured microglia, cultured astrocytes and LPS-treated rats, have suggested that the chronic nature of idiopathic PD might be due to a gradual process associated with protein folding, increasing exposure to high cytokine levels, perivascular immune cell invasion, a breached blood barrier and a loss of astrocyte adhesion, is eventually overwhelmed. The LPS model has been used by us and other researchers [31-33] being a naturally occurring substance, as opposed to MPTP and rotenone for example, and has produced a robust inflammatory response in the olfactory pathways. The endotoxin model has also been shown to elicit effects in the olfactory bulb, with subsequent loss of neuronal function leading to full-blown degradation, which includes detrimental astrocyte function, reduced phagocytosis and reduced neurotrophic factor (NGF, GDNF, BDNF) synthesis and release.
While it is now accepted that PD is a results of an inflammatory episode and subsequent microglia activation, increasing loss of functioning dopaminergic neurons [34], synthesis and release of multiple cytokines, accumulation of toxic substances, subcellular organelle dysfunction and increased effects of invading cells, a loss of BBB integrity, vascular endothelium integrity and astrocyte damage, are strongly implicated in a continuing loss of protective abilities [7,35-39].
Fluorescence imaging has yielded a more detailed insight into explaining the unremitting progression to loss of function and quality of life for the patient as well as for loved ones. Overall, our findings suggest the recognition of a potential time point from our work with the olfactory bulb and the idea that loss of olfaction is an early-enough marker in neurodegeneration to recognize that the initiation of strategies which will overcome a continuing loss of cognitive abilities will need to be considered and implemented [40-42].
Multiple ‘non-resident’ cell types are present in the olfactory bulb and their number increases following LPS treatment, with colocalization to specific areas and structures. These increases become important in understanding the rapid events leading to cell destruction and loss of normal function. For example, neutrophils which are present in increasing numbers in the first 6 h post LPS treatment in our experiments, are known to be both protective and damaging [36]. The same can be said for astrocytes, with ‘misplacement’ of the cells in PD and Alzheimer’s disease and their release of deadly molecules [39]. Thus, protective cells can become harmful during inflammatory episodes and, coupled with an influx of immune cells, causes disruption of the BBB which is exacerbated by usually beneficial astrocytes that are mechanistically altered and interact with invading cells [37-40]. Indeed, our previous research has shown cuffs of T-cells around vessels after endotoxin treatment, as well as ubiquitin and TNFα in vascular endothelial cells, suggesting that perivascular disruption occurs and that cells respond in both self-preservation and destructive manners [5,43]. Previous results also indicated that astrocytes, which are multi-functional glia cells and an integral part of the BBB, synthesize peripherally localized α-synuclein, suggesting that the protein has adhesive properties, and the adhesion is disrupted via glial activation causing a loss of astrocytic support of vascular endothelial cells and an ensuing, leaky BBB [20]. Imaging the localizations of diverse immune cells as there is increasing evidence for their involvement in neurodegeneration, loss of blood vessel integrity and age-related problems [44,45], suggests roles for both circulating-invading cells as well as resident cells.
In these experiments in our study, we did not find a decrease in vasculature content after LPS treatment although there was some evidence for loss of vessel integrity suggested by a lack of continuity of the tagged smooth muscle and associated endothelium. Increases in NOS isomers (not shown; Ref 5) suggested that they might each have a different role in neurodegeneration. NOS induction, increased levels of protective cytokines and GDNF synthesis, might be an adequate, initial, group of protective mechanisms. However, with chronic inflammation due to either a massive single event or repetitive/additive episodes, the mechanisms are overcome, with ensuing, sustained microglial activation, prolonged and continuous protein misfolding, lasting damage to the BBB [27] and detrimental effects of previously protective cytokines and immune cells. As Wyss-Coray and Mucke [46] stated, “Even cytokines that likely fulfill primarily beneficial functions when expressed during acute phases of wound repair can have complex, including detrimental, effects if overexpressed for prolonged period” and, “Altered expression of different inflammatory factors can either promote or counteract neurodegenerative processes. Since many inflammatory responses are beneficial, directing and instructing the inflammatory machinery may be a better therapeutic objective than suppressing it”. Loss of olfaction might be the time, and the olfactory bulb could be the place, to realize that impending neurodegeneration is at its acute stage, a stage that might respond to immune manipulation, neuropeptide therapy and nitric oxide regulation [47-49].
References
- Honh H, Kim BS, Im HI (2016) Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int Neurourol J 20: 2-7.
- Sarkar S, Raymick J, Imam S (2016) Neuroprotective and therapeutic strategies against Parkinson’s disease: Recent perspectives. Int J Mol Sci 17: 904-935.
- Su P, Zhang J, Wang D, Zhao F, Cao Z, et al. (2016) The role of autophagy in modulation of neuroinflammation in microglia. Neurosci 319: 155-167.
- Wang Q, Yingjun L, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19.
- Doursout MF, Schiess MC, Liang Y, Padilla A, Poindexter BJ, et al. (2016) Are temporal differences in the induction of GDNF and NOS isoforms contributors to neurodegeneration? A fluorescence microscopy study. Open Neurol J 10: 67-76.
- Grozdonov V, Bliederhaeuser C, Ruf WP, Roth V, Clemens FK, et al. (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128: 651-663.
- Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, et al. (2013) Vascular degeneration in Parkinson’s disease. Brain Pathol 23: 154-164.
- Vandenbrouke E, Mehta D, Minshall R, Malik AB (2008) Regulation of endothelial junction permeability. Annals NY Acad Sci 112: 135-145.
- Cahill-Smith S, Li JM (2014) Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: a role of NADPH oxidase 2. Brit J Pharmacol 78: 441-453.
- Lee H, Pienaar IS (2014) Disruption of the blood-brain barrier in Parkinson’s disease: curse or route to cure?. Front Biosci 19: 272-280.
- Al-Bachari S, Parkes LM, Vidyasagar R, Hanby MF, Tharaken V, et al. (2014) Arterial spin labelling reveals prolonged arterial arrival time in idiopathic Parkinson’s disease. Neuroimae Clin 6: 1-8.
- Zhang R, Tang S, Huang W, Liu X, Li G, et al. (2015) Protection of the brain following cerebral ischemia through attention of the PARP-1-induced neurovascular unit damage in rats. Brain Res 1624: 9-18.
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Courard PO, et al. (2012) Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9: 23.
- Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the bloodbrain barrier. J Neural Transm (Vienna) 113: 477-485.
- Takeshita Y, Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier (BBB): chemokine regulation and in vitro models. Immunol Rev 248: 228-239.
- Stevens CH, Roew D, Morel-Kopp MC, Orr C, Russell T, et al. (2012) Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol 15: 95-99.
- White TL, Sadikot AF, Djordjevic J (2016) Metacognitive knowledge of olfactory dysfunction in Parkinson’s disease. Brain Cogn 104: 1-6.
- Wang C, Zhao C, Li D, Tian Z, Lai Y, et al. (2016)Versatile structures of α-synuclein. Front Mol Nerosci 9: 48.
- Mason DM, Nouraei N, Pant DB, Miner KM, Hutchinson DF, et al. (2016) Transmission of α-synucleinopathy from olfactory structures deep in the temporal lobe. Mol Neurodegener 11: 49.
- Schiess, MC, Barnes, J, EllmoreTM, Poindexter, BJ, Dinh K, et al. (2010) CSF from Parkinson disease patients differentially affects cultured microglia and astrocytes. BMC Neurosci 11: 151.
- Doursout MF, Schurdell MS, Young LM, Osuagwu U, Hook DM, et al. (2013) Inflammatory cells and cytokines in the Olfactory Bulb of a rat model of neuroinflammation; Insights into neurodegeneration?. J Interfer Cyto Res 7: 376-382.
- Doursout MF, Schiess MC, Schurdell MS, Osuagwu U, Hook DM, et al. (2013) Specific localizations of cytokines and Parkinson’s disease-associated proteins revealed by fluorescence deconvolution microscopy in brain tissues of an LPS treated rat model. Curr Trends Neurol 2: 39-49.
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH, et al. (2016) Microgliablood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol 131: 347-363.
- Lecuyer MA, Kebir H, Prat A (2016) Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim Biophys Acta 1862: 472-482.
- Hansson O (2015) Formation of new blood vessels may explain intractable symptoms of Parkinson’s disease. Science Daily.
- Paxinos G, Watson C (1997) The rat brain in sterotaxic coordinates. 4th ed. New York, NY: Academic Press.
- Bick RJ, Poindexter BJ, Kott MM, Liang YA, Dinh, K, et al. (2008) Cytokines disrupt intracellular patterns of Parkinson’s disease-associated proteins alpha-synuclein, tau and ubiquitin in cultured glial cells. Brain Res 1217: 203-212.
- Dinh K, Poindexter BJ, Barnes JL, Schiess MC, Bick RJ, et al. (2009) Fluorescence microscopy and 3D image reconstruction of cytokine initiated disruption of the Parkinson disease associated proteins alpha-synuclein, tau and ubiquitin in cultured glial cells. Cytokine 45: 179-183.
- Merritt TM, Bick R, Poindexter BJ, Alcorn JL, Hecht JT, et al. (2007) Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am J Pathol 170: 293-300.
- Radomski MW, Palmer RM, Moncada S (1987) Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2: 1057-1058.
- Ota A, Mori K, Kaneko YS, Nakashima A, Nagatsu I, et al. (2008) Peripheral lipopolysaccharide administration affects the olfactory dopamine system in mice. Ann NY Acad Sci 1148: 127-135.
- He Q, Yu W, Chen C, Lou Z, Zhang Q, et al. (2013) Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLOS One 8: e78418.
- Herbert, RP, Harris, J, Chong, KP, Chapman, J, West, AK, et al. (2012) Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised olfactory pathway. J Neuroinflamm 9: 109.
- McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Move Dis 23: 474- 483.
- Xu B, Zhang Y, Du JL (2016) Progress in the study of the blood-brain-barrier. Sheng Li Xue Bao 68: 306-322.
- Parkos CA (2016) Neutrophil-epithelial interactions: A double edged sword. Amer J Pathol 186: 1404-1416.
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481-487.
- Xie L, Yang SH (2015) Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res 1623: 63-73.
- Gimsa U, Mitchinson NA, Brunner-Weinzieri MC (2013) Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell mediated neuroinflammation. Mediators Inflamm 2013: 320519.
- Masaoka Y, Pantelis C, Phillips A, Kawamura M, Mimura M, et al. (2013) Markers of brain illness may be hidden in your olfactory ability: a Japanese perspective. Neurosci Lett 549: 182-185.
- Ottaviano G, Frasson G, Nardello, Martini A (2016) Olfaction deterioration in cognitive disorders in the elderly. Aging Clin Exp Res 28: 37-45.
- Vengalil S, Agadi JB, Raghavendra K (2016) University of Pennsylvania smell identification test abnormalities in Parkinson’s disease. J Assoc Physicians India 64: 32-36.
- Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, et al. (2013) Vascular degeneration in Parkinson’s disease. Brain Pathol 23: 154-164.
- Guerriero F, Sgarlata C, Francis M, Maurizi N, Faragli A, et al. (2016) Neuroinflammation, immune system and Alzheimers disease: searching for the missing link. Aging Clin Exp Res 29: 821-831.
- Saxena S, Caroni P (2011) Selective neuronal vulnerability in neurodegenerative disease: from stressor thresholds to degeneration. Neuron 71: 35-48.
- Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease–a double- edged sword. Neuron 35: 419-432.
- Gualtierotti R, Guarnaccia L, Beretta M, Navone SE, Campanella R, et al. (2017) Modulation of neuroinflammation in the central nervous system: role of chemokines and sphingolipids. Adv Ther 345: 396-420.
- Tome D, Fonseca CP, Campos FL, Baltazar G (2016) Role of neurotrophic factors in Parkinson’s disease. Curr Pharm Des 23: 809-838.
- Lourenco CF, Ledo A, Barbosa RM, Laranjinha J (2017) Neurovascular-neuroenergetic coupling axis in the brain: master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic Biol Med 108: 668-682.
Citation: Bick RJ, Poindexter BJ, Doursout MF (2017) Changes in Vascular and Immune Cell Content of an LPS Treated Rat Olfactory Bulb PD Model, Measured by Fluorescence Deconvolution Microscopy. J Cytokine Biol 2: 120. DOI: 10.4172/2576-3881.1000120
Copyright: © 2017 Bick RJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5891
- [From(publication date): 0-2017 - Dec 11, 2025]
- Breakdown by view type
- HTML page views: 4901
- PDF downloads: 990