Characterization of Climate Extremes on Plants and Soil Properties Using Nano-Dynamic Elastomechanic Response Analysis
Received: 30-Apr-2015 / Accepted Date: 29-May-2015 / Published Date: 08-Jun-2015 DOI: 10.4172/2157-7617.1000278
Abstract
This paper presents a nano dynamic plant response relationship by varying soil moisture content due to climate change. An advance soil condition monitoring technique using Laser Interferometry based biomechanical experiment is presented. Sensitive elasto-mechanic reversible R ΔL=0.0001mm and irreversible ( I ΔL=0.005mm) growth response of plants by multiple external environmental variables at nano level are measured. Such response is mathematically correlated with the mechanics of transpiration and soil physical properties (Dry and Wet) or more precisely water content and finally as a derivative of climate change. These dynamic changes in plant growth demonstrate the nano level effect of climate change is an alarming signature and it has been suggested that precipitation changes may have limited transpiration mechanism causing the lack of irreversible plant growth. It is also suggested that the change in plant diameter can be used to trigger alarming system for monitoring global warming.
Keywords: Mutable response; Reversible growth; Elastomechanics; External stimulant; Nanodynamics
11393Introduction
In biology, morphogenesis is climate-mediated phenological development in plant, which is subject of intensive research. Several experiments [1-10] have been conducted to study the periodic biological phenomena, such as flowering, breeding, and migration, in relation to the climatic condition. The complex relations of micro growth and climatic factors such as Light, Nutrients, Microbial, Soil, Water Level, pH value, Salinity, Soil moisture, etc., necessitates a reductionist approach to investigate plant growth characteristics at Nano level. It is well known that global surface temperatures increased by an estimated 0.74 UC over the past century, a change that is widely believed to result primarily from the effects of emissions of carbon dioxide and other greenhouse gases (International Panel on Climate Change, hereafter IPCC, 2007) and caused drought, landslides like a natural disaster. Many physical changes have been attributed to this warming, including sea level rise, melting of glaciers and ice sheets, decreased snow and ice cover, temperature, and low precipitation. Such changes are likely to have considerable biological effects and numerous studies have sought evidence of such biological effects in nature. Several recent papers summarize the results of these studies and conclude that biological effects are already evident and have affected numerous taxed in different geographical areas. Our goal in this paper is to summarize a specific sampling of field studies that have examined changes in plant response to the global worming relation to recent climate change. Several experiments and meta-analysis of the data has been conducted and are available in the literature. Our intention is to present a generalized experimental method which can be replicated by researchers to observe the change of plant response with the change of global warming at nano level. Prior study [2] demonstrates that several experiments [11] were conducted for micro level growth response analysis which is important in elementary and advanced biology. In the present research, we are analyzing the dynamics of morphogenic characteristics at Nanometer scale in relation with the water content in soil by using Interferometry that measures the precise expansion of plant stem in the presence of sunlight. Soil shrinkage caused by climate extremes, causing root and soil to pull away from each other. It reduces the hydraulic (Wet) linkage between root and soil and between soil granules causing dynamic variations in transpiration rate and growth characteristics. As soil dries, hydraulic conductivity of the soil–plant system declines due to several factors which are complex to measure and correlate with soil-plant-climate relation. Thus, as resultant effect, dynamic variables in growth characteristics are measured which is best observed by selecting mature stems or other cylindrical organs of various plants such as Dieffenbachia, Aglaonema etc. considering their sensitive morphogenic behavior.
Theoretical Descriptions
It is important to note that understanding the fundamental complex nature of the plant’s growth response with respect to their mode of cell enlargement due to transpiration is difficult, while considering the multiple variable climate factors responsible for controlling growth response.
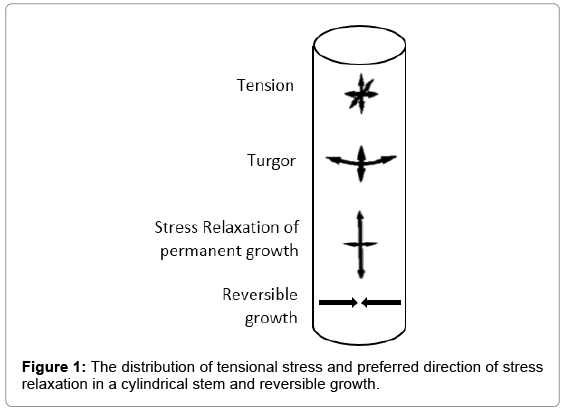
In a cylindrical stem, cells and micro fibrils matrix are deformed by the turgor and tension caused by fluid uptake pressure during transpiration. Figure 1 shows the direction of tension and stress relaxation inside the stem, causing reversible and permanent growth. A theoretical consideration for describing the growth as mechanohydraulic process is defined by Scientist Lockhart [3,4] in detail and further elaborated by the scientist Ray [5]. Theoretically, volumetric change with respect to the time 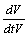 during cell growth is described as a result of a water transport process, which is driven by the potential difference Δψ between protoplast and apoplast and limited by water conductance coefficient L which is defined as
during cell growth is described as a result of a water transport process, which is driven by the potential difference Δψ between protoplast and apoplast and limited by water conductance coefficient L which is defined as
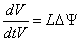 (1)
(1)
Volume increase during growth described as a material expansion of the cell wall is depicted by:
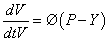 (2)
(2)
Whereby P −Y (P ≥ Y) is the turgor [5] above a yield threshold Y [6] and Ø is extensibility coefficient representing the time dependent yielding properties of the cell wall in the direction of growth. Yielding the general formula of mechano- hydraulic cell growth (Lockhart equation)
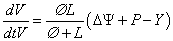 (3)
(3)
The equation (iii) describes the irreversible growth of stem [7] and it behaves as an elastic material [8]. Diametric strain produced in stem due to water uptake [9] is measured using interferometry in presence and absence of light and correlated with the existing Lockhart equation (3).
Experimental Methodology
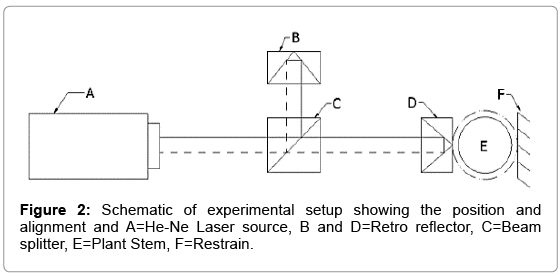
A Dieffenbachia plant was kept on vibration proof platform at normal environmental condition (20-30°C, 760 mm of Hg, RH 50%) to stabilize its motion till forty hours and monitored. Michelson Morley interferometer was positioned co-linearly with laser source, beam splitter and plant stem and kept apart on the vibration proof platform. Retro reflector (D) is placed in contact with the surface of a stem (E) which is restrained by fixed support (F). Layout of the experimental setup is given in Figure 2.
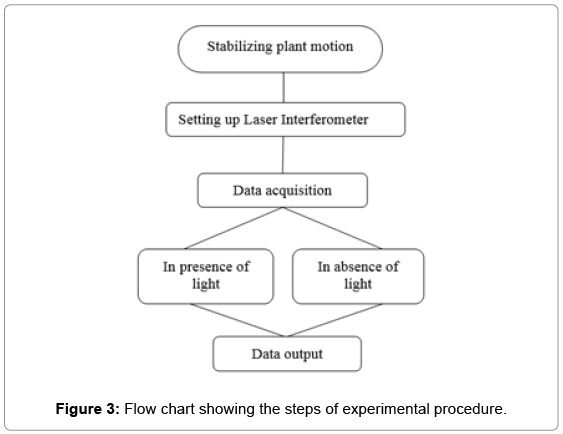
Figure 2 shows the displacement of retro-reflector (D) attached to stem (E) was measured by the combination of He-Ne (1 mW) laser source (A), beam splitter (C) and retro reflector (B) for 0 to 90 seconds and 90.1 to 180 seconds in the presence and absence of light respectively and the same experiment was repeated 32 times on a variety of plants such as Dieffenbachia, Aglaonema etc. Figure 3 shows the experimental methodology with a flow chart that includes stabilizing the plant motion, setting up the laser interferometer and data acquisition steps. The same experiment was conducted in absence of water in soil which causes instauration in hydraulic conductivity similar to extreme draught condition.
The experiment was performed (Figure 4) within a closed chamber to avoid undue vibration in plant stem and error in data accusation technique. Initially the experiment was conducted in presence and absence of light which was considered as external stimulant. Figure 4 shows the experimental setup and the arrangement of laser optics and Figure 2 shows its alignment in a straight line A to D.
Experimental Results and Discussion
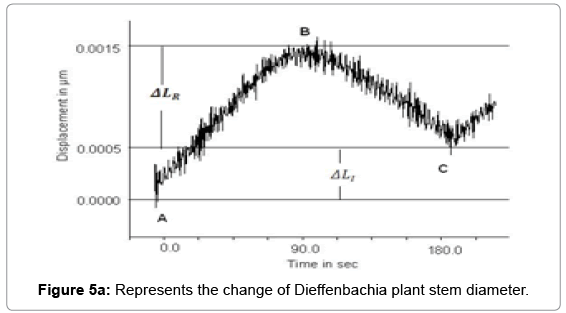
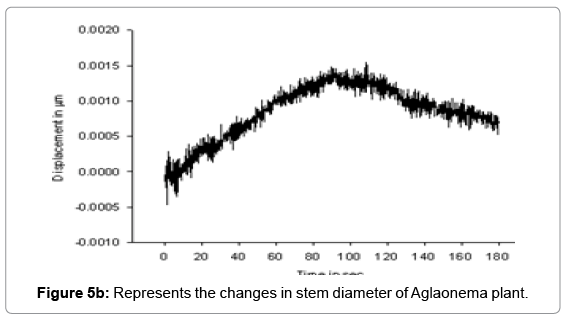
Laser Interferometry method is used for measuring mutable change in stem diameter with one nanometer resolution. We observed that in the absence of light, stem diameter was decreasing while in the presence of light it started increasing. Physical changes in cell volume of stem under the effect of climatic factors such as light and water uptake pressure resulted change in the size of the stem. The experiment continued for a period of 180 sec. with data sampling time interval of 0.1 s. Table 1 shows the data of the mutable changes that recorded in presence and absence of light. Figures 5a and 5b represents the mutable changes in diameter of Dieffenbachia and Aglaonema plant from point A to point B during the time period 0 to 90 sec. in presence of light and displacement from B to C during the time period 90.1 to 180 s in the absence of light.
| Soil Condition (Wet) | Duration (Second) | Stem Diameter (mm) ÄLR |
|---|---|---|
| In presence of light | 10.0 | 0.0006 |
| 30.0 | 0.0007 | |
| 60.0 | 0.0012 | |
| 90.0 | 0.0014 | |
| In absence of light | 90.1 | 0.0015 |
| 145.0 | 0.0008 | |
| 165.0 | 0.0008 | |
| 180.0 | 0.0005 |
Table 1: The averages data of multiple records showing the mutable changes in plant stem diameter.
In graphical representation, we found:
Displacement AB>Displacement BC
Total displacement (ΔLT ) = AB
Reversible elastic displacement (ΔLR ) = BC
Irreversible displacement
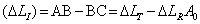 (4)
(4)
We observed that in Figure 5a, non-linear change ΔLT instem diameter is dynamic at Nano level. This change in the cell wall is an accumulation of elastic and plastic expansions. Major part of this change ΔLI is reversible in the absence of light during 90.1 to 180 sec., which is elastic in nature and rest part is irreversible ΔLI which is lastic in nature. In another experiment with Aglaonema plant Figure 5a also reveals the nonlinear response between -0.0005 to 0.0015 mm.
Mathematically the relation is expressed as bellow,
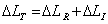 (5)
(5)
To find the cause of reversible change (ΔLR ) we measured ΔLI . Total displacement increases with increase in water uptake dynamic pressure (P) in the presence of water (rain fall) which affected the transpiration rate JV (equation 6), is given by:
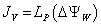 (6)
(6)
Where, JV=volume of water crossing the membrane per unit area and per unit time (m3/m/s or equivalently, m/s). Here JV has the physical meaning of velocity.
Pressure force due to water uptake is given by
F=wa/g (7)
Where, F=Pressure force (N)
W=weight of uptake water (N)
g=gravitational acceleration (m/s2)
a=water uptake acceleration (m/s2)
Equation (vii) may be expressed as bellow,
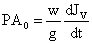 (8)
(8)
Here, P = Water uptake dynamic pressure (Pa)
A0 = Cross sectional area of stem (m2)
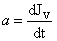 = physical meaning of a rate velocity
= physical meaning of a rate velocity
Redial stress (σr) for thin walled cylindrical xylem is given by
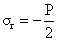 (8a)
(8a)
Dependent factors of Radial stress is found by solving both the equation (viiiA) and (viii) as follows,
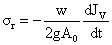 (9)
(9)
Radial Strain of thin walled cylindrical stem is
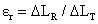 (10)
(10)
Here Young's modulus,
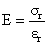 (11)
(11)
Solving equation 9, 10 and 11
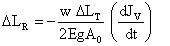 (12)
(12)
The equation (7) proves inconsistent flow ( JV) of water developing dynamic nature in ΔLT and elongation property (E) resulting the reversible change ΔLR in stem diameter during 90.1s to 180 s detected by laser interferometry, which is a key phenological response of plant at Nano level.
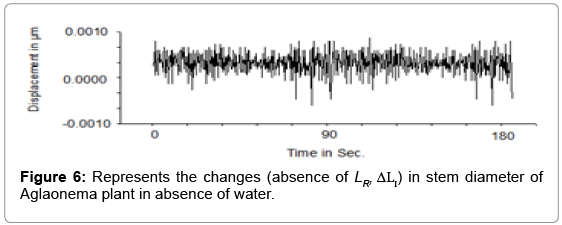
Another sequence of experiment has been conducted in absence of water (rain fall) in soil similar to extreme draught condition. No such significant growth response (Figure 6) was identified in absence of JV).
Equation (ix) shows the radial stress JV of steam is proportional to the water flow ( JV). We are able to detect the nano-dynamic irreversible and reversible change due to changing radial stress in stem by precise measurement technique using Laser interferometry. It is also evidencing that permanent growth is an effect of incomplete dynamic stress relaxation (ΔLI) and depends on ΔLR (Figure 5a). Result (Figure 6) shows, in absence or lack of soil moisture due to drought, the response to the diameter of stem becomes almost linear within the range 0.0001 mm to 0.0008 (including noise) resulting the growth mechanism inactive. Upper range may be considered for designing any switching indicator. We found (Figure 5a) the total growth in the presence of light satisfies the Lockhart equation (iii) and equation (v). Equation (xii) shows ΔLRis a photo morphogenic effect which is regulating JV and dependent on the Water uptake dynamic Pressure P=w/g.
Conclusion
We have investigated the plant growth mechanism in of plant at nano level in wet and dry condition of soil caused by drought as an effect of climate change. Effect of light is considered for triggering the change in water flow through the stem. We are able to detect and measure the Nano-dynamic irreversible and reversible changes in plant stem diameter due to variable water uptake dynamic pressure which is known as the effect of climatic change. Also, the total growth in the presence of light and rain fall satisfying Lockhart expression in general and concluding the photo morphogenic reversible change ΔLR is an effect of water transpiration rate ( JV). We conclude that the permanent growth is an effect of incomplete dynamic stress relaxation (ΔLI) and depends on reversible growth ( ΔLR ). Also conclude that we can identify the localized drought condition as climate change by precise observation of highly sensitive ΔLR . This dynamic reversible mutable response at the nanometer level is the cause of major phonological changes and quantified response (Table 1) is useful for micro biomechanical switching application and measurement of soil hydraulic conductivity, global warming effect on plant health, and plant reaction time.
Acknowledgements
Authors are grateful to the Director CSIR-CSIO for his guidance & overall inputs for the execution of the work.
References
- Schopfer P (2006) Biomechanics of plant growth.Am j botany 93: 1415-1425.
- Felekis D, Muntwyler S, Vogler H, Beyeler F, Grossniklaus U, et al. (2011) Quantifying growth mechanics of living, growing plant cells in situ using micro robotics. Micro Nano Lett 6: 311-316.
- Lockhart JA (1967) Physical nature of irreversible deformation of plant cells. Plant Physiol 42: 1545-1552.
- Lockhart JA (1965b) Cell extension. In: Bonner J, Varner JE (eds.) Plant biochem, Academic Press, New York, New York, USA, pp. 826-849.
- Ray PM, Green PB, Cleland R (1972) Role of turgor in plant cell growth. Nature 239: 163-164.
- Cosgrove DJ, Van Volkenburgh E, Cleland RE (1984) Stress relaxation of cell walls and the yield threshold for growth. Planta 162: 46-54.
- Lockhart JA (1965a)Â An analysis of irreversible plant cell growth. J Theoretical Biol 8: 264-275.
- Lockhart JA, Bretz C, Kenner R (1967)Â An analysis of cell-wall extension. Annals New York Acad Sci 144: 19-33.
- Farah SM, Arar A, Miller DE (1988) Water requirements and the irrigation management of pea, lentil, faba bean and chickpea crops. In:Summerfield RJ (ed.) World Crops: Cool season food legumes, pp. 271-278.
- Chappell, Matthew (2013) Implementation of wireless sensor networks for irrigation control in three container nurseries. HortTechnol 23: 747-753.
- Schopfer P (2006) Biomechanics of plant growth. Ame J Bot 93: 1415-1425.
Citation: Das S, Mittal SK (2015) Characterization of Climate Extremes on Plants and Soil Properties Using Nano-Dynamic Elastomechanic Response Analysis. J Earth Sci Clim Change 6: 278. DOI: 10.4172/2157-7617.1000278
Copyright: ©2015 Das S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15555
- [From(publication date): 6-2015 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 10869
- PDF downloads: 4686