Clinical Differences between Methicillin-Resistant and -Susceptible Staphylococcus aureus Bacteremia in Adult Patients at a Tertiary Hospital in Japan
Received: 22-May-2020 / Accepted Date: 11-Jun-2020 / Published Date: 18-Jun-2020 DOI: 10.4172/2332-0877.1000427
Abstract
To determine clinical differences in features of methicillin-susceptible and resistant Staphylococcus aureus (MSSA and MRSA, respectively) bacteremia, 15 adult patients with MRSA bacteremia were compared with 30 adult patients with MSSA bacteremia who were hospitalized during 2015 – 2018.
Compared with MSSA bacteremia patients, MRSA bacteremia patients had a higher age (mean age, 82.0 years and 72.5 years, respectively) and were more likely to have diabetes mellitus significantly (p=0.04). Liver and kidney functions were also significantly decreased in MRSA bacteremia patients compared with MSSA bacteremia patients (p=0.037, p=0.001 and p=0,015, respectively). Moreover, MRSA bacteremia patients showed a much higher mortality rate than MSSA bacteremia patients (60% and 20%, respectively; odds ratio: 2.66, 95% CI; 1.806-4.288, p=0.007).
These data suggest that MRSA bacteremia is more lethal than MSSA bacteremia in adults. Thus, caution should be taken when Staphylococcus aureus is isolated from the blood of patients who are elderly, diabetic, or have liver and kidney dysfunction because MRSA can be more possible pathogens rather than MSSA.
Keywords: Age; Methicillin-resistant Staphylococcus aureus (MRSA); Methicillin-susceptible Staphylococcus aureus (MSSA); Liver dysfunction; Kidney dysfunction
Introduction
Staphylococcus aureus is a common pathogen that is susceptible to antibiotics usually, including penicillins. However, methicillin-resistant Staphylococcus aureus (MRSA) has recently become representative for community-and hospital-acquired infections, and a multinational surveillance study suggested a high prevalence of MRSA in many countries [1,2]. Various MRSA clones have been suggested to spread among the community and/or hospitals, as well as among countries. In Japan, MRSA bacteremia is a serious issue, which occurs frequently, exhibits resistance to antibiotics, and is related with high mortality [3].
Up to 20% of all individuals with bacteremia in United States hospitals and approximately 31% of those in intensive care units of Spanishhospitals have MRSA bacteremia [4,5]. Even with appropriate antibiotic therapy, reported mortality and morbidity rates related with MRSA bacteremia are higher than those for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, and reported mortality rate of MRSA bacteremia ranges 30% –40% [6-9].
In this investigation, we studied and compared clinical features of MRSA and MSSA bacteremia in adult patients.
Materials and Methods
Diagnostic and Patients
This study enrolled 45 adult patients (20 years or older) with Staphylococcus aureus bacteremia who were admitted to Tohoku Medical and Pharmaceutical University between November 2015 and December 2018. Bacteremia was defined as follows: one or more positive blood cultures from patients with clinical signs of infection, e.g., chills, fever, and sweats with or without local signs and symptoms. Age, male/female, underlying disease, clinical features, and laboratory data were evaluated. If MRSA had been isolated from the same patient on several occasions within the 3-year period, only the first episode of MRSA bacteremia was reviewed. This study was approved by the Committee for Clinical Scientific Research of Tohoku Medical and Pharmaceutical University Hospital in Oct 09, 2015 as No. ID2015-2-011 and the patients whose specimens were used provided written informed consent.
Identification of bacteria
Blood samples were cultured in BacT/Alert bottles (SysmexbioMérieux, Kobe, Japan). Rapid identification of the pathogens and antimicrobial susceptibility test of positive samples were performed using the MicroScanWalkAway 96-plus system (Siemens, Munich, Germany). All Staphylococcus aureus isolates were identified by Gram staining, colony morphological analysis, and catalase and coagulase tests. Isolates were identified as MRSA if the minimum inhibitory concentration (MIC) of oxacillin was ± 4 μg/mL.
Blood samples were cultured in BacT/Alert bottles (SysmexbioMérieux, Kobe, Japan). Rapid identification of the pathogens and antimicrobial susceptibility test of positive samples were performed using the MicroScanWalkAway 96-plus system (Siemens, Munich, Germany). All Staphylococcus aureus isolates were identified by Gram staining, colony morphological analysis, and catalase and coagulase tests. Isolates were identified as MRSA if the minimum inhibitory concentration (MIC) of oxacillin was ≥ 4 μg/mL.
Data collection and statistical analysis
Clinical and demographic data that were normally distributed were subjected to analysis of variance, with Fisher’s exact test for multiple comparisons. In addition, those that were non-normally distributed were analyzed by non-parametric statistics, such as the Mann – Whitney U-rank test. The results were corrected using the Bonferroni method when further analysis was necessary. Spearman ’ s rank correlation was performed to examine relationships among various parameters. Survival of MRSA and MSSA bacteremia patients was analyzed by Kaplan–Meier curves.All data are expressed as mean ± SD. p-values <0.05 denoted statistical significance. All analyses were carried out using Statview software (Abacus Concepts, Cary, NC, USA).
Results
Patients and complications
At first, we examined demographic and baseline characteristics of adults with MRSA (n=15) and MSSA (n=30) bacteremia (Table 1). In both groups, the majority of patients who were admitted and required critical care were elderly, as shown by the high mean age. We did not found significant differences in sex or most underlying diseases between the MRSA and MSSA groups. However, MRSA bacteremia patients were significantly more likely to have diabetes mellitus than MSSA bacteremia patients (p=0.04).
| MRSA (n = 15) | MSSA (n = 30) | p-value | |
|---|---|---|---|
| Age(y.o.) | 82.0 ± 8.4 | 72.5 ± 18.5 | p=0.008** |
| Male/Female | |||
| Male | 12 (80.0%) | 21 (70.0%) | p=0.47 |
| Female | 3 (20.0%) | 9 (30.0%) | |
| Wards | |||
| Respiratory | 1 (6.7%) | 2 (6.7%) | p=0.52 |
| Cardiology | 6 (40.0%) | 5 (16.7%) | p=0.08 |
| Gastrointestine | 1 (6.7%) | 7 (23.3%) | p=0.08 |
| Surgery | 0 | 2 (6.7%) | p=0.30 |
| Neurology | 0 | 4 (13.3%) | p=0.13 |
| Collagen diseases | 1 (6.7%) | 1 (3.3%) | p=0.60 |
| Orthopedics | 0 | 2 (6.7%) | p=0.30 |
| Diabetes mellutus | 2 (13.3%) | 0 | p=0.04* |
| General medicine | 2 (13.3%) | 2 (6.7%) | p=0.52 |
| Nephrology | 0 | 1 (3.3%) | p=0.47 |
| Rehabilitation | 0 | 1 (3.3%) | p=0.47 |
| Emergency medicine | 2 (13.3%) | 3 (10.0%) | p=0.73 |
Table 1: Clinical characteristics of adult MRSA and MSSA bacteremia patients.
Laboratory data
As shown in (Table 2), some laboratory data of MRSA bacteremia patients were significantly worse than those of MSSA bacteremia patients. Inflammatory data (e.g., white blood cell count, C-reactive protein level, and procalcitonin level) and nutritional status (e.g., blood albumin concentration) were almost similar between the MRSA and MSSA groups. However, liver dysfunction data (i.e., Alanine transaminase: ALT, p=0.037) and kidney dysfunction data (i.e., blood urea nitrogen; BUN and creatinine, p=0.001 and p=0,015, respectively) were significantly increased in MRSA bacteremia patients compared with MSSA patients.
| MRSA (n=15) | MSSA (n=30) | p-value | |
|---|---|---|---|
| WBC (×103/μl) | 13.0 ± 6.9 | 10.1 ± 7.4 | p=0.36 |
| Platelet (×103/μl) | 172 ± 146.1 | 168 ± 98.0 | p=0.46 |
| T-Bil (mg/dL) | 1.15 ± 0.7 | 0.6 ± 0.5 | p=0.060 |
| AST (U/L) | 52.0 ± 595.6 | 38.5 ± 423.6 | p=0.059 |
| ALT (U/L) | 41.0 ± 198.7 | 31.0 ± 134.5 | p=0.037* |
| LDH (U/L) | 347.0 ± 445.7 | 291.5 ± 495.4 | p=0.304 |
| BUN (mg/dL) | 63.0 ± 29.0 | 31.0 ± 22.6 | p=0.001** |
| Cr (mg/dL) | 1.96 ± 1.17 | 1.10 ± 0.24 | p=0.015* |
| Albumin (g/dL) | 2.7 ± 0.7 | 3.0 ± 0.9 | p=0.21 |
| CRP (mg/dL) | 16.1 ± 12.7 | 15.9 ± 11.9 | p=0.95 |
| PCT (mg/dL) | 5.6 ± 25.6 | 1.0 ± 20.8 | p=0.15 |
Table 2: Laboratory data of adult MRSA and MSSA bacteremia patients.
Patients’ outcome
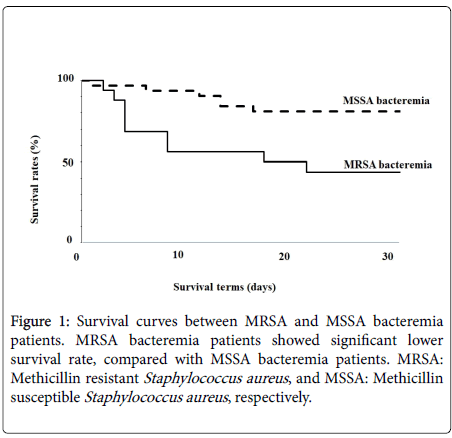
Furthermore, we compared survival rates between MRSA and MSSA bacteremia patients. The 30-day non-survival rates after Staphylococcus aureus was isolated from the blood were 60% (9/15 patients) in the MRSA group and 20% (6/30 patients) in the MSSA group (Figure 1).
Mortality was significantly higher with MRSA isolation compared with MSSA isolation (odds ratio: 2.64, 95% CI; 1.806-4.288, p=0.007).
Discussion
Bacteremia caused by Staphylococcus aureus , especially MRSA bacteremia, is one of the most important problems in infectious disease, resulting in substantial high morbidity and mortality [3,6-10]. Thus, clinical characteristics of MRSA bacteremia should be assessed accurately to select appropriate management and treatments.
We identified 45 adult patients with bacteremia caused by Staphylococcus aureus from 2015 to 2018, among which 15 MRSA bacteremia patients were analyzed and compared with 30 MSSA bacteremia patients. MRSA bacteremia patients showed higher mean age and worse laboratory data than MSSA bacteremia patients. Moreover, many MRSA bacteremia patients had diabetes mellitus and liver and renal failure, which were similar to findings in previous reports [8,11,12]. We previously reported that non-survived MRSA bacteremia patients had cardiovascular disease, kidney dysfunction, and poor nutritional status, and complicationswere caused by lower respiratory tract infections, intravascular devices, and surgical site infections [3]. These data suggested the risk of MRSA bacteremia and the importance of prophylaxis and care of such patients and devices, although we did not find a significant focus of MRSA bacteremia compared with MSSA bacteremia (data not shown).
In addition, we found survival rates were significantly lower in MRSA bacteremia patients compared with MSSA bacteremia patients. These data suggested the difficulty of treating MRSA bacteremia compared with MSSA bacteremia. Although toxicity of MRSA was similar or milder than that of MSSA, anti-MRSA drugs, including vancomycin (VCM), teicoplanin, arbekacin, and daptomycin, required more detailed adjustment of doses and therapeutic drug monitoring to ensure adequate efficiency and low toxicity, as opposed to antibiotics for MSSA, such as penicillins and other beta-lactams [3,13-18]. Additionally, Anti-MRSA drugs may be less effective and/or have more side effects than anti-MSSA drugs. Therefore, the administration of these drugs might also partially explain the higher mortality observed in MRSA bacteremia compared with MSSA bacteremia. We previously found survival was significantly greater among patients who were the subject of infection control team (ICT) consultation compared with those who were not among MRSA bacteremia cases [3]. Thus, we have recommended treatments and care for not only severe or complicated patients, but also mild to moderate MRSA bacteremia patients. However, those who received ICT consultation were given a range of anti-MRSA drugs more frequently. These results suggested that ICT consultation and the choice of anti-MRSA and other drugs are important for reducing the mortality of MRSA bacteremia patients.
In this study, there was not a significant difference in the trough level of VCM between ICT-positive and -negative patients or between survived and non-survived patients with MRSA bacteremia (data not shown). However, accurate drug loading methods and treatment duration were recently reported in Japanese guidelines of antimicrobial/diagnostic stewardship activity to be as critical [16,17] as exact and non-delayed diagnosis of MRSA bacteremia using appropriate diagnostic methods, including blood culture bottle, PCR, and time-of-flight mass spectrometer (TOF-MS) [19-23].
Conclusion
In conclusion, the outcome of MRSA bacteremia was worse than that of MSSA bacteremia. Higher age, underlying diseases, and kidney and liver dysfunction may be important co-factors responsible for high mortality in MRSA bacteremia patients. Prediction of MRSA and more rapid diagnosis and administration of anti-MRSA drugs could contribute to the improvement of MRSA bacteremia in patients with these characteristics.
References
- Song JH, Hsueh PR, Chung DR (2011). Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J AntimicrobChemother 66:1061-1069.
- Yanagihara K, Matsumoto T, Aoki N (2019) Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for clinical microbiology in 2014: General view of the pathogens' antibacterial susceptibility. J Infect Chemother 25:657-668.
- Isobe M, Uejima E, Seki M (2012) Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother 18:841-848.
- Tiemersma EW, Bronzwaer SL, Lyytikäinen O, Degener JE, Schrijnemakers P, et al. (2004) European Antimicrobial Resistance Surveillance System Participants. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg Infect Dis 10:1627-1634.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39: 309-317.
- Laupland KB, Ross T, Gregson DB (2008)Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis 198: 336-343.
- Gómez J, GarcÃa-Vázquez E, Baños R, Canteras M, Ruiz J, et al. (2007) Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: the role of empiric antibiotic therapy. Eur J ClinMicrobiol Infect Dis 26: 239-245.
- Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, et al. (2003) Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36: 53-59.
- Liu C, Bayer A, Cosgrove SE (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52: 285-292.
- Hidayat LK, Hsu D, Quist R, Shriner KA, Wong-Beringer A (2006) High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 166:2138-2144.
- Silvestre J, Póvoa P, Coelho L, Almeida E, Moreira P, et al.(2009) Is C-reactive protein a good prognostic marker in septic patients? Intensive Care Med35:909-913.
- Seki M, Yabuno K, Miyawaki K, Miwa Y, Tomono K (2012) Loading regimen required to rapidly achieve therapeutic trough plasma concentration of teicoplanin and evaluation of clinical features. ClinPharmacol4: 71-75.
- Yabuno K, Seki M, Miyawaki K, Miwa Y, Tomono K (2013) High-dose, short-interval daptomycin regimen was safe and well tolerated in three patients with chronic renal failure. ClinPharmacol5: 161-166.
- Matsumoto K, Takesue Y, Ohmagari N (2013) Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother19: 365-380.
- Matsumoto K,Taesue Y, Ohmagari N (2016) Revised practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Japanese J of Chemotherapy (in Japanese)1:1-120.
- Kamioka Y, Suzuki H, Seki M, Ouchi R, Kashiwagura S, et al. (2016)After deriving the initial dose setting of vancomycin with a conventional computer software and a nomogram based on the revised japanese 2016 therapeutic drug monitoring guidelines for antimicrobial agents, Pharm and Phramacol9:88488.
- Okada K, Kimura T, Mikamo H (2014) Clinical practice guidelines for therapeutic drug monitoring of arbekacin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother 20:1-5.
- HariuM, Watanabe Y, Oikawa N, Seki M (2017) Usefulness of matrix-assisted laser desorption ionization time-of-flight mass spectrometry to identify pathogens, including polymicrobial samples, directly from blood culture broths. Infect Drug Resist 10:115-120.
- Hariu M, Watanabe Y, Oikawa N, Manaka T, Seki M (2018) Evaluation of blood culture broths with lysis buffer to directly identify specific pathogens by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry methods. Infect Drug Resist 11:1573-1579.
- Watanabe Y, Oikawa N, Hariu M, Seki M (2019) Evaluation of agar culture plates to efficiently identify small colony variants of methicillin-resistant Staphylococcus aureus. Infect Drug Resist 12:1743-1748.
- Seki M, Takahashi H, Yamamoto N (2015) Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist 14:113-118.
- Takahashi H, Seki M, Yamamoto N (2015) Validation of a phage-open reading frame typing kit for rapid identification of methicillin-resistant Staphylococcus aureus (MRSA) transmission in a tertiary hospital. Infect Drug Resist 14:107-111.
Citation: Watanabe Y, Takano K, Maya H, Kamioka Y, Shimada D, et al. (2020) Clinical Differences between Methicillin-Resistant and - Susceptible Staphylococcus aureus Bacteremia in Adult Patients at a Tertiary Hospital in Japan. J Infect Dis Ther 8: 427. DOI: 10.4172/2332-0877.1000427
Copyright: © 2020 Watanabe Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3030
- [From(publication date): 0-2020 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2165
- PDF downloads: 865