Clinical Efficacy of a Unani Poly Herbal Formulation on Abnormal Vaginal Discharge
Received: 22-Feb-2018 / Accepted Date: 28-Feb-2018 / Published Date: 08-Mar-2018 DOI: 10.4172/2573-4555.1000269
Introduction
Vaginal discharge is a common and often distressing complaint. Causes of vaginal discharge includes physiological, infective and non-infective [1]. Normal physiological discharge changes with the menstrual cycle. It is thick and sticky for most of the cycle, but becomes clearer, wetter, and stretchy for a short period around the time of ovulation [2]. Abnormal vaginal discharge is characterized by a change of colour, consistency, volume, or odour, and may be associated with symptoms such as itch, soreness, dysuria, pelvic pain, or intermenstrual or post-coital bleeding [2]. Abnormal vaginal discharge is most commonly caused by infection; less commonly, abnormal vaginal discharge can have a non-infective cause. Infective causes includes Candida and bacterial vaginosis (the most common cause of discharge), Trichomoniasis, a sexually transmitted infection caused by the protozoan Trichomoniasis vaginalis (TV), Endocervical infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae may cause vaginal discharge or other symptoms such as; dysuria, post coital/intermenstrual bleeding, deep dyspareunia, pelvic pain and tenderness (if there is ascending pelvic infection), or reactive arthritis, Herpes simplex may rarely be associated with discharge [3]. Noninfective causes are retained foreign body such as a tampon, condom, or vaginal sponge, Inflammation due to allergy or irritation caused by substances such as deodorants, lubricants, and disinfectants, Tumours of the vulva, vagina, cervix, and endometrium, Atrophic vaginitis in post-menopausal women, Cervical ectopy or polyps [2].
Pelvic inflammatory disease (PID) is the clinical syndrome associated with ascending infection of the female genital tract. It is a major source of gynaecological morbidity [4]. PID is perhaps the most important avoidable cause of female tubal factor infertility, and its association with other chronic sequelae is well documented [5]. It is clinically presented with history of abnormal vaginal discharge, fever and adnexal tenderness [6-8]. Although incidence rates may have declined over the years, PID remains a major source of short- and long-term morbidity in women. There is no evidence to suggest that there has been any reduction in the serious reproductive complications traditionally associated with PID, which include infertility, ectopic pregnancy, and chronic pelvic pain. Treatment goals encompass not only the amelioration of the acute inflammatory condition but also the prevention or lessening of the risk for long-term reproductive sequelae. Hence, an early and accurate diagnosis of pelvic inflammatory disease (PID) is of paramount importance for the effective management of the acute illness and for the prevention of long-term sequelae.
Current modern treatment depends on the cause and generally involves use of antibiotic therapy. Despite of the claim of modern medicine with regard to the presence of anti-bacterial anti parasitic medicines that there is a definite treatment of PID, the above mentioned incidence of different organisms in causing pelvic inflammatory disease is still prevailing. Every antibacterial drug in modern medicine produces more or less adverse effects in the human body. In present era, everyone tends to become more health conscious and seeks the safer side in respect to treatment. Natural, herbal or traditional medicine is now being seen with an eye of great interest and hope. Unani medicine is one of them. This system not only provides the drugs information in abundance but also claims that the drugs are having least adverse effects.
The clinical study presented in this article evaluated the efficacy and tolerability of capsule Dabidulward on vaginal discharge associated with pelvic inflammatory disease. Cap Dabidulward is a poly herbal Unani formulation manufactured by the Dehlvi Naturals. It is aan aqueous extract of classical Unani compound formulation, Majoon dabidulward. It is documented for its efficacy in the management of warm-e-rahm (PID) in different Unani Pharmacopoeias which is in use by the Unani physician since ages. All the individual herbs of Capsule Dabidulward possesses anti-inflammatory, emmenogogue, anti-spasmodic, astringent, antiseptic, anti-microbial as well as antioxidant properties which are well known in Unani and modern pharmacology. These actions of individual drugs are known for their efficacy in gynaecological ailments. Similarly the compound formulation ‘‘Majoon Dabidulward’’ is also prescribed for visceral inflammations such as metritis, hepatitis, ileitis [9-11].
Materials and Methods
Study design
The study was designed as Randomized, Single-Blind and Placebo Controlled Clinical Trial. The clinical study was conducted in the Outpatient department of Amraze Niswan (Gynecology) at the Ayurvedic & Unani Tibbia College, Govt. of NCT Delhi, Karol Bagh, New Delhi, during the year 2012-2013. This study was started after the approval Board of Research Studies (BRS) and IEC.
Patient selection
In total about 70 subjects with abnormal vaginal discharge associated with PID as per screening criteria were enrolled for the study. All the subjects fulfilling the screening criteria were then subjected to inclusion and exclusion criterias. Out of 70, 20 subjects did not fulfil the inclusion criteria laid in the protocol and thus were excluded from the study. Of these remaining 50, 2 were Mantoux positive, 1 was VDRL positive, 5 subjects reported recurrent PID, 1 patient did not give consent for participation and 1 patient was excluded on the basis of ultrasonography showing presence of TO Abscess. Remaining 40 patients were subjected for clinical study after randomization. All the screened subjects with confirmed clinical diagnosis as per Revised CDC criteria, fulfilling all the conditions of inclusion criteria, were selected for the study and allocated into Control and Test group by randomization. Written informed consent was sought from every patient before inclusion in the study. During the enrolment procedure, complete history including general physical and systemic examination including the pelvic examination was carried out and recorded on a case report proforma. Table 1 shows the different inclusion and exclusion criteria’s of the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Table 1: Inclusion and exclusion criteria’s of the study.
Efficacy assessment
Abnormal vaginal discharge was assessed by a grading system [12].
Investigations
Certain investigations were carried out aiming at important objectives of the study i.e. to exclude the other patients as part of exclusion criteria and to establish the safety of the test drug (Cap. Dabidulward; a poly herbal Unani formulation). The baseline clinical laboratory investigations such as haemoglobin percentage, total leucocytes count, differential leucocytes count, erythrocyte sedimentation rate, VDRL and Random blood sugar were done to exclude general diseases. Ultrasonography and Papinicoulaou smear were done to exclude the pelvic pathology and malignancy. To assess the safety of drugs, blood urea, serum creatinine, SGOT, SGPT, and Alkaline phosphatase were done before and after trial. At every follow up of 7 days during three weeks of study period, abnormal vaginal discharge according to its grades was recorded in the case record form.
Intervention
The selected patients (n=40) were randomly allocated into two groups, Test group (n=24), capsule dabidulward and Control group (n=16), placebo. The treatment period in both, Test and control groups, was determined as 21 days. Test group patients were given 2 Capsules of the cap. Dabidulward 500 mg twice daily with plain water for 21 days after menses and control group patients were given 2 capsules of placebo (millet flour) twice daily with plain water for a period of 21 days after menses. For the duration of the study, patients were asked to practice abstinence and were advised to maintain personal and local hygiene.
Test drug
Test drug was prepared and provided by Dehlvi Naturals, 125-F.I.E., Patparganj Ind. Area, New Delhi-110092, Mfg. Lic. No. DL-301 A& U, and prepared strictly according to the GMP norms. Each 500 mg, Cap. Dabidulward contains dried aqueous extract of the contents of Majoon dabidulward which are Sunbul ut tib (Nardostachys jatamansi) 13 mg, Mastagi (Pistacia lentiscus) 13 mg, Zaffran (Crocus sativus) 13 mg, Tabasheer (Bambusa arundinacea) 13 mg, Darchini (Cinamomum zeylanicum) 13 mg, Izkhar makki (Andropogen schoenanthus) 13 mg, Asarun(Valerina wallchi) 13 mg, Qust shirin (Saussurea lappa) 13 mg, Ghafis (Gentiana olivieri) 13 mg, Tukhm kasoos (Cuscuta reflexa) 13 mg, Mujaith (Rubia cordifolia) 13 mg, Luk (coccus lacca) 13 mg, Tukhm kasni (Cichorium intybus) 13 mg, Tukhm karafs(Apium graveolens) 13 mg, Zarawund tawil (Aristolochia indica) 13 mg, Hab e balsan (Commiphora opobalsamum) 13 mg, Uood garqi (Aquilaria agallocha) 13 mg, Qaranfal (Eugenia coryphyllata) 13 mg, Dana illaishi khurd (Elettaria cardomum) 13 mg, Gul e surkh (Rosa damascena) 253 mg.
Outcome
The outcome measure was to assess the effectiveness of trial drug on abnormal vaginal discharge associated with PID. The patients each in the test and the control Group were assessed on day one before starting the treatment and after administration of the test drug or the placebo control for 21 days with follow up at every 7th day. The patient was considered cured when there is complete cessation of vaginal discharge, improved when there was decreased in vaginal discharge where as not cured, when there was no apparent response or worsening of vaginal discharge after treatment.
Statistical Analysis
The pre-treatment and post-treatment values in each group separately (within group differences) were statistically analyzed by using Mann-Whitney Test for Intra Group and Kruskal-Wallis Test with Post Dunn`s Multiple Comparisons Test for Inter Group comparisons.
Results
The Socio-demographic (age, literacy status, mizaj (temperament), socioeconomic status, sexual behavior, parity, use of contraceptives) characteristics of the test and control groups are shown in Table 2. It was found that the parameters were statistically not significant. (P > 0.05) Thus, the groups were homogenous before intervention (Table 2).
| GROUP | Mean Age (yrs) | literacy Status | Mizaj | SES | S.Behv. | Parity | Contraceptive | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Illeterate | Primary | Hr.Sec | Sr.Sec | Graduate | Damvi | Balghami | Safravi | Saudavi | Higher | Middle | Lower | Heterosex. | Homosex. | Bisex. | Nulli. | Primi. | Multi. | Oralpill | IUD | Barrier | Tub.ligation | None | ||
| CONTROL | 3 | 5 | 5 | 3 | 0 | 0 | 16 | 0 | 0 | 1 | 8 | 7 | 16 | 0 | 0 | 3 | 3 | 10 | 1 | 3 | 7 | 1 | 4 | |
| TEST | 11 | 6 | 5 | 1 | 1 | 0 | 24 | 0 | 0 | 0 | 15 | 9 | 24 | 0 | 0 | 3 | 2 | 19 | 1 | 2 | 10 | 1 | 10 | |
Table 2: Socio-demographic data of the study subjects (n=40).
Efficacy of test drug and control on abnormal vaginal discharge
The data was statistically analysed using Mann-Whitney Test for Intra Group and Kruskal-Wallis Test with Post Dunn`s Multiple Comparisons Test for Inter Group comparisons. The median rating score after treatment in the test group when compared with median rating score before treatment in control and median rating score after treatment in control was found to be extremely significantly reduced (P<0.001) (Table 3) 21 days study was divided into three visits of follow up which were made at 7th, 14th and 21st day. At every visit, the patients were asked about the improvement or worsening in their symptoms and subjected to examination to assess clinical findings. Concomitant treatment was not allowed during the protocol period. After 21 days, 38 subjects completed the study and 2 were drop outs.
| GROUP | ASSESSMENT DAY | |||
|---|---|---|---|---|
| TEST (n=22) | 0 Day | 7 Day | 14 Day | 21 Day |
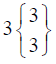 |
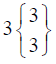 |
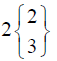 |
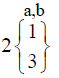 |
|
| CONTROL (n=16) | 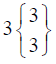 |
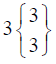 |
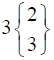 |
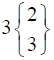 |
Table 3: Efficacy of test drug and control on abnormal vaginal discharge (n=38) (Median rating with range in brackets).
Statistical Test Used: Mann-Whitney Test for Intra Group and Kruskal-Wallis Test with Post Dunn`s Multiple Comparisons Test for Inter Group comparisons.
P at 14th day (T/C) <0.01(HS) (Mann-Whitney Test)
P at 21st day (T/C) <0.0001(ES) (Mann-Whitney Test)
α. p<0.001 with respect to 0 day test
β. p<0.0001 with respect to 21 day control
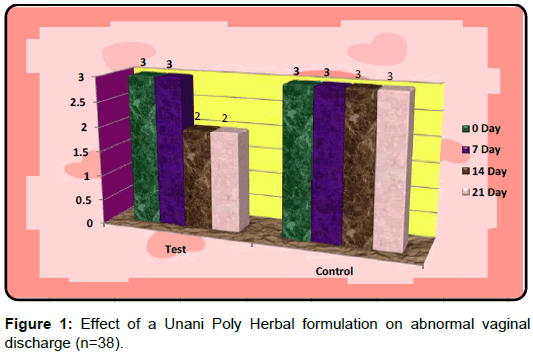
In control group, the median score for vaginal discharge was 3 (3- 3) at baseline, 3 (3-3) on 7th day, 3 (2-3) on 14th day and 3 (2-3) at the end of the treatment. While in test group, the median score for vaginal discharge was 3 (3-3) at baseline which remains the same 3 (3-3) on 7th day, 2 (2-3) on 14th day and decreased to 2 (1-3) at the termination of the treatment.
Kruskal-Wallis Test with Post Dunn`s multiple comparisons Test was applied for intergroup analysis of test group and was found extremely significant p<0.001 for 0 day v/s 21 day. Mann-Whitney test was applied to see the difference in between the test and control group, which was found highly significant on 14th day (p<0.01) and extremely significant on 21st day (p<0.001) (Table 3).
Therapeutic outcome
In the test Group, all the of 22 patients of abnormal vaginal discharge were cured completely and in the control Group out of 16 none showed any response.
Discussion
According to classical Unani text, Sailan-Ur-Raham (Abnormal Vaginal Discharge) is the most striking and the commonest symptom of all types of warm-e-raham (PID). This discharge is derived either from the uterus itself or from other organs of the body. This discharge is mostly balghami (phlegmatic) and sticky and sometimes watery in consistency [13]. Excess of vaginal discharge is abnormal and its presence signifies falling of abnormal humours on uterus. The type of humour involved in causation of abnormal vaginal discharge can be recognised, if a woman places a clean pad and when it gets filled with the discharge, it is removed and air dried. If the colour of the pad remains the same, it is of balghami (phlegmatic) humour and if it becomes reddish, it is of damvi (sanguine) matter. Presence of yellowish colour on drying signifies involvement of safravi (choleric) matter, while that of blackish colour signifies involvement of saudavi (melancholic) matter [13]. It involves mucous coat of uterus and is from a type of chronic inflammation, which disturbs its quwat-e-ghaziya (nutritive faculty) [14].
This study demonstrates that the test drug was effective in the management of abnormal vaginal discharge. The laboratory investigations were within normal range before and after treatment showing that the drug was safe. Till date, none of the studies in the Unani system of medicine had evaluated or documented the efficacy and safety of the test drug in the management of abnormal vaginal discharge associated with PID. Thus, it is difficult to correlate the finding with other clinical studies but it validates the claim made by the Unani Scholars. According to the Unani Scholars, warm-eraham (PID) is caused by Ufunat (infection) and it needs dafe tafun (antimicrobial) and mohallil (anti-inflammatory) drugs to relieve infections and associated symptoms. The observations of the study show that the test drug has significant analgesic, anti spasmodic and anti-inflammatory activity as compared to the control group where the symptom worsened. All the individual drugs of capsule dabidulward are known to possess the above mentioned activities [15-20]. Mastagi (Pistacia lentiscus) Resin produced statistically significant inhibition of edema at all doses when compared to the control groups in rats. A 100% inhibition of inflammation was observed at 800 mg/kg i.p. [21]. Zaffran (Crocus sativus), the effects of aqueous and ethanolic maceration extract of Crocus sativus L. stigmas (CSS) and petals have been studied in mice. The anti-inflammatory effects of the extracts may be due to their content of flavonoids, tannins, anthocyanins, alkaloids and saponins. Only the stigma extracts showed weak to moderate effect against acute inflammation. In chronic inflammation, both aqueous and ethanolic stigma extracts, as well as ethanolic petal extract, exerted anti-inflammatory effects. In higher doses, the aqueous and ethanolic extracts of stigma showed significant activity against the acute inflammation. It was concluded that saffron stigma and petal aqueous and ethanolic maceration extracts shows acute and/or chronic anti-inflammatory activity [22-24]. Tabasheer (Bambusa arundinacea) also proved to be a potent anti-inflammatory drug in experimental studies [25]. Hab-e-balsan (Commiphora opobalsamum) [26], Mujaith (Rubia cordifolia) also produced significant analgesic and anti-inflammatory activity [27]. Kasni (Cichorium intybus) has been found to have anti-bacterial activity [28].
Effect of test drug and control on abnormal vaginal discharge
100 % (16 of 16 in control and 22 of 22 in test group) subjects presented with vaginal at baseline of study. The percentage relief observed for vaginal discharge in test group was 66.6% and no improvement at all in the control group. The improvement in vaginal discharge in test group can be attributed to astringent, desiccant, anti-septic, anti-inflammatory properties of the drugs of Capsule Dabidulward [15-20,29]. Hence, it is assumed that the properties of the test drugs have caused relief in abnormal vaginal discharge.
Conclusion
The PID is considered as a potential risk factor for sexually transmitted diseases. It is a major public health problem. In addition, the abnormal vaginal discharge has substantial impact on many aspects of quality of life, including reproductive ability, sexual functioning, mental health and the ability to work and perform routine physical activities. Therefore, it must be treated with due care. This study proves that Unani poly herbal formulation Cap. Dabidulward was found to be safe and effective in the management of abnormal vaginal discharge. The study also validated the claim of the Unani physicians in the treatment of warm-e-rahm (PID). The test drug is cheaper, easily available and well tolerated by the patients without having any side effects (Figure 1).
Acknowledgement
The authors are indebted to Mr. Mohsin Dehlvi, Proprietor of Dehlvi Naturals, who sponsored the trial drug for this study. We are also thankful to Dr. Ahmad Yasin, Principal & Medical Superintendent, A & U Tibbia College & Hospital, for his kind support during the study.
References
- Mitchell H (2004) Vaginal discharge causes, diagnosis and treatment. BMJ 328: 1306-1308
- Clinical Knowledge Summary: Vaginal discharge (2013). Last revised May 2013.
- Health Protection Agency. Management of Abnormal Vaginal Discharge in Women. Quick Reference Guide for Primary Care. For Consultation and Local Adaptation. Last updated Sept 2014.
- Department of Health: Summary and Conclusion of COM’s Expert Advisory Group. London: Department of Health, 1998.
- McCormack WM (1994) Pelvic inflammatory disease. N Engl J Med 330: 115-119.
- Hager WD, Eschenback DA, Spece MR, Sweet RL (1983) Criteria for diagnosis and grading of salpingitis. Obst Gynecol 61: 113-114.
- Jacobson L, Westrom I (1969). Objectivized diagnosis of pelvic inflammatory disease. Diagnosis and prognostic value of routine laparoscopy. Am J Obstet Gynecol 105: 1088-1092.
- Centers for Disease Control and Prevention (2002) Sexually transmitted diseases treatment guidelines 2002. MMWR Morb Mortal Wkly Rep 51: 1-77.
- Hakeem Zil-ur-Rahman (1991) Kitab-ul-Murakabat AMU Edition pp: 162-163.
- Anonymous (2006) NFUM, part 1, CCRUM, Dept. of AYUSH, Ministry of health & Family Welfare, Government of India p: 124.
- Padubidri VG, Daftary SN, Howkins B (2008) Shaw‘s Textbook of Gynaecology 14th ed. New Delhi: Elsevier 7: 293-294.
- Ibn Habal Bugdadi (2007) Kitab-Ul-Mukhtarat-Fil-Tibb’’, Urdu Translation, CCRUM 4: 42-44.
- Ibn Nafees (YNM) ‘’Maolijat-E-Nafeesi’’, Maulvi Abid Hussain, Munshi Naval Kishore, Lucknow.
- Nadkarni A K, Nadkarni KM (1994). ‘‘Indian Materia Medica’’, Vol. 1, Popular Parkashan, Bombay.
- Kabeeruddin H (2000) ‘‘Makhzan-ul-Mufradat Almaroof-ba-Khawas-ul-Advia’’, Vol.1, Faisal Publications, Deoband.
- Ahmad THN (2010) Taj-ul-Mufradat Khwasa-ul-Advia’, Idara Kitab-ul-Shifa, pp: 121-607.
- Mohammed KS (1995) Standardisation of Single Drugs of Unani Medicine, 3: 1-52.
- Mahmoudi M, Ebrahimzadeh MA, Nabavi SF, Hafezi S, Nabavi SM, et al. (2010). Antiinflammatory and antioxidant activities of gum mastic. Eur Rev Med Pharmacol Sci 14: 765-769.
- Hosseinzadeh H, Ramezani M, Salmani GA (2000) Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 73: 379-385.
- Saxena R S, Gupta B, Saxena K K, Singh R C, Prasad D N (1984) Study of anti-inflammatory activity, Indian medicinal plant. J Ethnopharmacol 11: 319-330.
- Ma S, Zhou S, Shu B, Zhou J (1998) Pharmacological studies on Crocus glycosides I. Effects on anti-inflammatory and immune function. Zhongcaoyao 29: 536-539.
- Muniappan M, Sundararaj T (2003) Antiinflammatory and antiulcer activities of Bambusa arundinacea. J Ethnopharmacol 88: 161-167.
- Abbas FA, Al-Massarany SM, Khan S, Al-Howiriny TA, Mossa JS, et al. (2007). Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat Prod Res 21: 383-391.
- Chander shekar T, Bahuguna YM, Vijender S (2010) Anti inflammatory activity of ethanolic stem extract of Rubia cordifolia Linn. in rats. Int Jour Res Ayur Pharm 1: 126-130.
- Petrovic J, Stanojkovic A, Comic LJ, Curcic S (2004) Antibacterial activity of Cichorium intybus’. Fitoterapia 75: 737-739.
- Ibn Baitar (1248AD). The Book of Medicinal and Nutritional Terms. CCRUM: 116-117.
Citation: Naaz F, Rahman RU, Kausar F (2018) Clinical Efficacy of a Unani Poly Herbal Formulation on Abnormal Vaginal Discharge. J Tradit Med Clin Natur 7:269. DOI: 10.4172/2573-4555.1000269
Copyright: © 2018 Naaz F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7955
- [From(publication date): 0-2018 - Nov 21, 2025]
- Breakdown by view type
- HTML page views: 6883
- PDF downloads: 1072