Clinical Evaluation of Neonatal Malaria in a Sub-urban Healthcare Facility in South-East Nigeria
Received: 13-Nov-2018 / Accepted Date: 18-Feb-2019 / Published Date: 26-Feb-2019 DOI: 10.4172/2572-4983.1000180
Abstract
Background: Malaria is a major killer in children under-5 years of age. Its incidence in neonates is commonly mis-diagnosed as sepsis and thus under-reported. Sometimes, a high index of suspicion for malaria in the neonate is necessary to make a diagnosis. Documentation of the clinical features of malaria in neonates and predictors of parasite density would aid in prompt diagnosis and management.
Objective: This study evaluated the clinical features of malaria in newborns in the first 28 days of life. It secondarily sought to determine factors that predict degree of malaria parasitemia in these newborns.
Methods: This longitudinal cross-sectional and descriptive study was conducted over a 7-month period. Four hundred and thirty neonates delivered and admitted in the Federal Teaching Hospital Abakaliki (FETHA) that met the inclusion criteria were consecutively enrolled after obtaining consent from their parent(s)/caregiver. Data on socio-demographics of the mother and neonates were obtained using the interviewer administered questionnaire. Blood samples were collected from neonates for malaria parasitemia using microscopy.
Results: Of the 430-newborn admitted during the study period, nineteen (4.4%) had neonatal malaria. Eighteen (94.7%) of these occurred within the first week of life. There was a cluster of malaria parasite density between 100-200 p/µl with a mean of 142.74 ± 70.96 p/µl. Neonates of mothers that received antimalarials during pregnancy [OR 1.556 (95% CI 0.318-7.608)], newborns of mothers with fever in pregnancy [OR 1.361 (95% CI 0.318-7.608)], newborns with birth weight ≥2.5 kg [OR 1.7 (95% CI=0.586-5.074)] and neonates within the first week of life [OR 3.319 (95% 0.394-27.982)] were more likely to have higher density of malaria parasitemia compared to newborns in the corresponding category. Malaria in surveyed neonates was noted to be mostly asymptomatic, but difficulty of breathing was the commonest symptomatic presentation among surveyed newborns.
Conclusion: Our study showed that malaria in neonates is mostly asymptomatic but could present as fever, breathing difficulty, yellowness of the eyes and lethargy. No specific clinical symptoms, maternal fever or use of anti-malaria in pregnancy, birth weight or post-natal age of neonates were significantly predictive of malaria parasite density in newborns.
Keywords: Neonates; Clinical features; Malaria; Parasite density; Abakaliki
Introduction
Malaria is one of the most common infectious disease worldwide and remains a significant disease burden in sub-Saharan Africa, especially among children under the age of five and pregnant women [1,2]. It contributes significantly to perinatal disease burden and neonatal mortality [2]. Malaria in neonates was thought to be rare in the past but recent reports suggest otherwise [3,4]. Globally, about 1-3 million children die each year from malaria with majority of these deaths occurring in Africa where malaria is responsible for one out of every five deaths in children under-five-years while in Nigeria it accounts for over 300,000 death per year [5]. The prevalence of malaria in neonates in sub-Saharan Africa varies from 0-46% [6,7]. This is attributable to increased resistance of Plasmodium falciparum to antimalarial drugs resulting in maternal parasitemia, increased virulence resulting from altered antigenic determinants and increased reporting of cases [8]. It has also been suggested that mothers on regular malaria chemoprophylaxis have low malaria antibody titers and so might transfer little protective antibodies to their newborns [8]. Neonates can be exposed to Plasmodium falciparum during pregnancy leading to transmission of malaria in utero [9]. This in utero transmission can result in the delivery of low birth weight and premature neonates [10].
Clinical malaria in the newborn commonly manifests after the first week of life but has been reported on the first day of life with symptoms and signs [6,11]. Clinical signs of neonatal malaria such as fever, poor feeding, lethargy, anemia, hepato-splenomegaly, jaundice, irritability, breathing difficulty and drowsiness may be indistinguishable from those of neonatal sepsis. It has been suggested that screening for malaria parasites be included as part of routine investigation in neonates with fever [12,13]. Sometimes, a high index of suspicion for malaria in the neonate is necessary to make quick diagnosis and initiate prompt management, in order to reduce neonatal morbidity, duration of hospital stay and mortality [12]. This will also reduce the inappropriate use of antibiotics since the clinical presentation of neonatal malaria can be very similar to that of neonatal sepsis. This study therefore sets out to assess the clinical features of newborns presenting with malaria within the first 28 days of life and the density of parasitemia in symptomatic malaria infection.
Methodology
This study is the second part of a dissertation work carried out in the Neonatal special care unit of the Federal Teaching Hospital, Abakaliki (FETHA).
Study location
Abakaliki is the largest and capital city of Ebonyi State, in the South Eastern Nigeria. Abakaliki is inhabited and populated primarily by Igbo ethnic group. The capital city has a population of about 151,723 according to the 2006 population census [14]. Its inhabitants are of different educational attainment. About 75% of the population of Ebonyi state are rural dwellers with subsistence farming as the major occupation, low literacy levels and high poverty rates [15,16]. Ebonyi is bounded to the North by Benue State, to the West by the Enugu State, to the East by the Cross-River State and to the South by Abia State, with a land area of about 5,935 km2. It lies approximately within longitudes 70 30’and 80 30’E and latitudes 50 40’ and 60 45’N. There are many primary and secondary health facilities in the state with the Federal Teaching Hospital, Abakaliki (FETHA) being the only tertiary hospital and a major referral health facility providing both general and specialist care to the state and its environs.
Study site
The Newborn Special Care Unit of FETHA caters for babies from the first day of life to the twenty-eighth day of life. It has two sections, one for inborn and the other for babies referred from outside the hospital. Averages of 50 babies are admitted in both wards per month with a total of 591 admitted in a period of one year. Services are rendered all through the day. The labor ward of FETHA has a delivery room with six couches and a fourth stage room with twelve beds, where babies and their mothers are kept immediately after delivery till about two hours before transfer to the postnatal ward. The total number of babies delivered in the year 2014 was 2,416 giving an average of 200 deliveries per month.
Study population
Participants were newborn babies (term and preterm) between the ages of zero to twenty-eight days of life delivered in the labor ward and/or admitted into the inborn and out born wards of the newborn special care unit of the Federal Teaching Hospital, Abakaliki. Inclusion criteria include term and preterm newborns delivered and/or admitted into the newborn special care unit of the Federal Teaching Hospital, Abakaliki, irrespective of indication for admission. Newborn babies delivered in the Federal Teaching Hospital, Abakaliki, and/or admitted in the newborn special care unit whose parents refused consent, those already on treatment for malaria or have been treated for malaria and newborn babies who have received blood transfusion before enrollment were all excluded from the study.
Study design
This was a longitudinal cross-sectional and descriptive study. Consecutive newborns delivered in the Federal Teaching Hospital and those admitted into the inborn and out born ward of the Newborn Special Care Unit of the Federal Teaching Hospital within the study period of seven months that satisfied the inclusion criteria were enrolled. Informed consent was obtained from the caregiver. The number of newborns enrolled was calculated based on an assumed prevalence rate of 50%, a 5% precision degree and a 10% default rate to accommodate possible sample loss due to attrition.
Ethical approval and consent
Ethical approval was obtained from the Ethics and Research Committee of the Federal Teaching Hospital, Abakaliki with reference number FETHA/REC/VOL1/2015241. Approval from the parent(s)/ caregiver was obtained via the consent letter. For those unable to read, the form was explained verbally by a research assistant in a language that they could understand. Those who consented to the study and their babies were recruited. Confidentiality was maintained. Patient identity was coded and all relevant information concerning the study was kept in sole custody of the researchers.
Data collection
Data collection was done by trained research assistants who are registrars in the department of Paediatric. The assistants were trained on administration of the study tool. Parent(s) or caregiver(s) of babies who met the inclusion criteria were approached by the research assistants after the delivery or admission of the neonates. A proforma was used to obtain information on demographic variables such as maternal age, marital status, parent’s occupation, and parent’s highest educational qualification. Prenatal history on the number of deliveries, fever in pregnancy and maternal knowledge of intermittent preventive therapy (IPT) on malaria was also obtained. Further information obtained includes; the baby’s age in days, sex, birth weight and symptoms the child presented with, if any.
Sample collection
The researchers performed the sample collection after introducing self to the parent(s) or caregiver(s) of each participant. Materials such as gloves, methylated spirit, cotton wool, lancet (ACCU-CHEK, 28G, 0.4 mm) and micropipette were assembled on a tray. Thick and thin smear template was used. The researchers observed universal precautions. Each slide was cleaned with dry cotton wool to wipe off dirt from the surface and labeled with a marker bearing the child’s initials, a unique serial number with the date of the sample collection. Blood sample was collected from the lateral aspect of the middle finger or the heel after being thoroughly cleaned with a methylated spirit swab and pricked with a lancet. The calibrated micropipette was used to obtain blood from the punctured site and a drop of blood measuring 12 μl was dropped at the center of the slides.
In making the thick film, blood was dropped at the center of the slide guided by the template and another clean spreader slide was used to make the smear in four circles to avoid breaking the cytoplasm of the malaria parasite as the malaria parasite is said to be fragile. To make the thin film the micropipette was adjusted to drop 4 μl of blood at the other side of the slide; a spreader slide held at angle of 45 degrees towards the blood was used to push the blood smoothly and rapidly. Thereafter, the slides were allowed to air dry completely. Within one hour, the dried slides were transported in the slide box to the laboratory. The microscopist stained and read the slides with the researchers present in the whole process. The thin film was fixed with 100% methanol and allowed to dry. Three percent stock solution of Giemsa stain was used in staining both the thick and thin film. After staining, the slides were washed with buffered distilled water and allowed to air dry. This was followed by reading the slides under the microscope at x 100 oil immersion magnification. A thick film was said to be malaria parasite negative or positive after examination of a 100 high power fields. Parasite density was recorded as a ratio of parasites to white blood cells (WBC) in thick films. White blood cells were indirectly and relatively used in microscopy to estimate the density of malaria parasite infections using the following formula:
Parasite density (Parasite/microliter)=(Number of Parasite count × 8000)/WBC.
According to the World Health Organization standard, 8000 is a constant based on assumption that there was an average of 8000 white blood cell per microliter [17]. The parasite density and interpretations were performed by microscopists. Two microscopists were involved in the microscopy. A WHO trained and certified microscopist reviewed all the slides for quality assurance. Quality assurance was ensured in the preparation of the smear, ensuring that the buffered distilled water had a pH of 7.2 as most available water in the environment was acidic. Also, the slide was properly air dried to avoid the smear being washed off. The slide was flooded with appropriate volume of Giemsa stain. The time of staining was kept for ten minutes. The microscopists read the slides independent of each other and the findings were subsequently compared. Any conflicting slide was isolated for a recheck.
Data analysis
Data obtained were collated and analyzed with Epi info version 7.0.8.3 (CDC Georgia USA 2011). The level of significance was set at p-value less than 0.05. The cross tabulations of malaria parasitemia with independent variables in 2 x 2 tables were compared using the Pearson chi square test. The results were presented in charts, tables and figures.
Results
Characteristics of newborns and mothers and/or care-givers surveyed
Four hundred and thirty mother-baby pair were enrolled during the study period. Male-female ratio was approximately 1:1. Three hundred and forty-nine (81%) of the enrolled neonates were delivered at term while the remainder (19%) were preterm deliveries. All but four of the mothers were married (96%) and 80 (18%), 176 (41%) and 174 (41%) had primary education or less, secondary and tertiary education respectively. Forty-one percent of the mothers are in the low socioeconomic class, 26% in the middle and 33% belonged to the high socio-economic class. Majority of the respondents, 364 (85%) had knowledge of the intermittent preventive therapy for malaria in pregnancy with roughly 343 of these (94%) having received IPT in index pregnancy. About 6-in-10 used insecticide treated bed net while pregnant while 220/430 (51%) received anti-malaria treatment for symptomatic malaria in index pregnancy (Table 1).
| Characteristics | Variables | Number (n) | Percentage (%) |
|---|---|---|---|
| Newborns parameters | |||
| Age (n=430) | ≤ 7 days | 366 | 85 |
| 8- 28 years | 64 | 15 | |
| Gender (n=430) | Male | 214 | 50 |
| Female | 216 | 50 | |
| Gestational at birth (n=430) | Term | 349 | 81 |
| Pre-term | 81 | 19 | |
| Maternal parameters | |||
| Marital status (n=430) | Currently married | 411 | 96 |
| Currently single | 19 | 4 | |
| Educational attainment (n=430) | Primary or less | 80 | 18 |
| Secondary | 176 | 41 | |
| Tertiary | 174 | 41 | |
| Socio-economic class (n=430) | Lower | 174 | 41 |
| Middle | 112 | 26 | |
| Upper | 144 | 33 | |
| Knowledge of IPT (n=430) | Yes | 364 | 85 |
| No | 66 | 15 | |
| Use of IPT in pregnancy (n=430) | Yes | 343 | 80 |
| No | 87 | 20 | |
| Use of ITN in pregnancy (n=430) | Yes | 261 | 61 |
| No | 169 | 39 | |
| Use of anti-malaria in pregnancy (n=430) | Yes | 220 | 51 |
| No | 210 | 49 | |
IPT: Intermittent Preventive Therapy; ITN: Insecticide Treated Net.
Table 1: Characteristics of newborns and mothers/caregivers enrolled in the study.
Density of malaria parasitaemia in the neonates
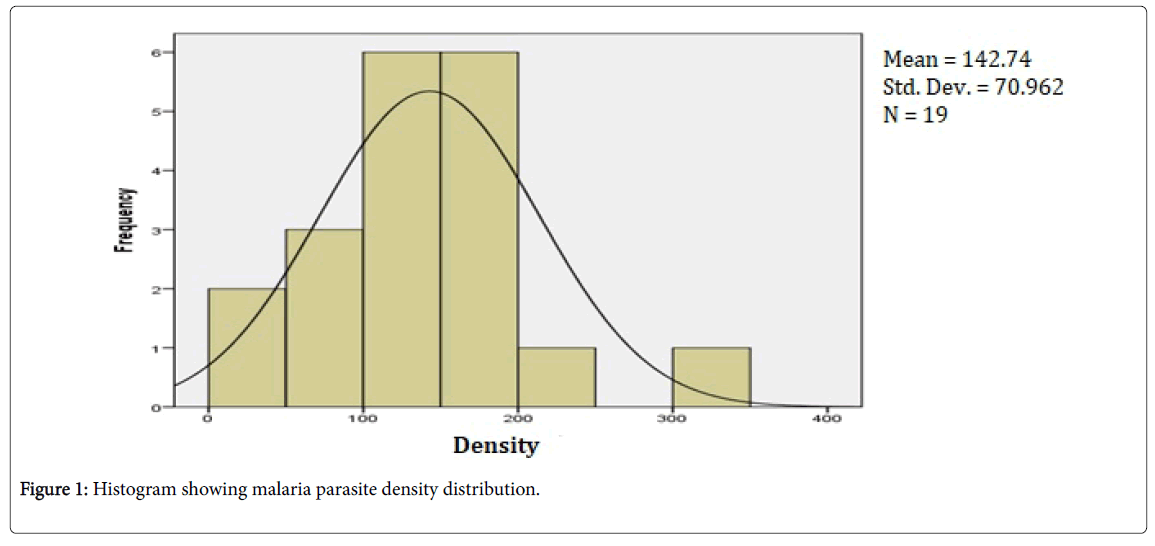
Malaria parasite density histogram is shown in Figure 1. There was a cluster of malaria parasite density between 100-200 p/μl with a mean of 142.74 ± 70.96 p/μl. There is one outlier with a very high parasite density above 300 p/μl while two neonates had a parasite density Table 2 shows no statistically significant difference between the malaria parasites densities of the term neonates compared to the preterm. Similarly, there was no significant difference in malaria parasite density based on gender and birth weight of the surveyed neonates.
| Characteristics | Variables | Malaria Parasite Density | χ2 | |
|---|---|---|---|---|
| < 200 p/µl | ≥ 200 p/µl | p-value | ||
| Gender (n=19) | Male | 7 (41.2) | 1 (50) | 0.057 |
| Female | 10 (58.8) | 1 (50) | 0.811 | |
| Gestational at birth (n=19) | Term | 14 (82.4) | 1 (50) | 1.127 |
| Pre-term | 3 (17.6) | 1 (50) | 0.288 | |
| Birth weight (n=19) | < 1.5 kg | 1 (5.26) | - | 2.012 |
| 1.5 to2 (10.5)1 (5.26)0.366 | ||||
| 2.5 to14 (73.7)1 (5.26) | ||||
| ≥ 4.0 kg | - | - | ||
Table 2: Relationship between malaria parasite density with some newborn parameters.
Table 3 shows the logistic regression analysis of malaria density in neonates and some maternal-newborn parameters. Neonates of mothers that received antimalarials in pregnancy were about 1.6 times more likely to have higher density of malaria parasite compared to those whose mothers did not take antimalarial in pregnancy [OR 1.556 (95% CI 0.318-7.608)]. Similarly, newborns of mothers with fever in pregnancy were roughly 1.4 times more likely to have higher malaria parasite density than neonates whose mother had no fever in index pregnancy [OR 1.361 (95% CI 0.318-7.608)]. Furthermore, newborns with birth weight ≥2.5 kg were about twice more likely to have higher malaria parasitaemia compared to those whose weight are less [OR 1.7 (95% CI=0.586-5.074)]. while neonates within the first week of life are about 3 times more likely to have higher density of malaria parasite compared to those beyond the first week of life. [OR 3.319 (95% 0.394-27.982)]. None of these however attained statistical significance.
| Predictors Variables | Reference | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|
| Maternal antimalarial | No | 1.556 | 0.318-7.608 | 0.585 |
| Maternal fever in pregnancy | No | 1.361 | 0.259-7.147 | 0.716 |
| Birth weight | 1.7250.586-5.0740.322 | |||
| Postnatal age | >7 days | 3.319 | 0.394-27.982 | 0.270 |
CI- confidence interval
Table 3: Logistic regression of predictors of malaria parasite density in the neonates.
Clinical presentation of malaria in neonates
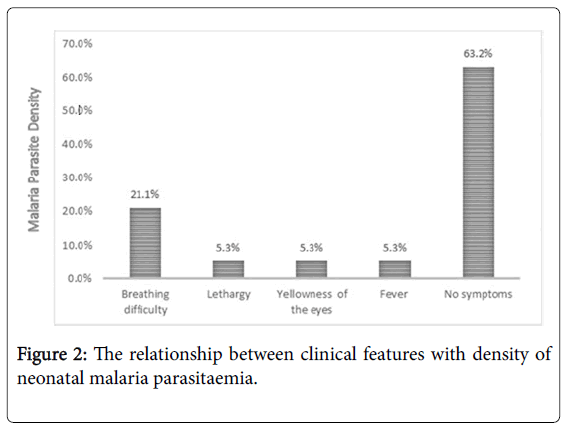
Among the nineteen neonates that were positive for malaria parasitaemia, four (21%) had breathing difficulty, one (5.3%) had fever, lethargy (5.3%) and yellowness of the eyes (5.3%). Majority, 12 (63.2%) of the babies did not manifest any symptom (Figure 2).
Discussion
The malaria parasite density was low in this study with a mean of 142.74 ± 70.96. This is similar to the findings by Ojukwu et al. [18] but differ from that of Obu and Ibe [19] in the Gambia. This might possibly be due to the high use of maternal antimalarial prophylaxis during pregnancy and the several antimalarial drugs taken by the mothers which may have led to parasite clearance. It could also be that the mothers residing in Ebonyi State which is an area of high transmission for malaria could have developed relative immunity to malaria thereby protecting the fetus. The low parasite density rate in this study suggests that placental barrier could still be effective. In corroboration with the study by Ouedraogo et al. [20] reporting on the effectiveness of placental barrier.
Our study found out that majority of the neonates with malaria parasitaemia were asymptomatic. This could be because the transplacental transmission of maternal immunity may have provided transient immunity to the neonates thereby delaying the onset of clinical manifestation in the newborn babies. Another possible reason could be that the incubation period for the malaria parasite had not been reached. The neonates who had malaria parasitaemia were within the first one week of life therefore the neonates could not have manifested with symptom(s) during the period of the study.
It is pertinent to know that breathing difficulty is the predominant symptom associated with malaria parasitaemia in the surveyed neonates. One neonate with malaria parasitaemia presented with fever. This is in contrast to the findings of Opare [21] and Nnaji et al. [22] where fever and poor suck were the major symptoms found. In our study however, majority of the neonates did not present with any symptoms or signs. This therefore, support the advocacy for the screening of every newborn with symptoms admitted in the neonatal unit for malaria parasitaemia.
Furthermore, it was noted in this study that there was no significant relationship between neonates having malaria parasitaemia and maternal fever, the child’s birth weight and age postpartum. This insignificant association may be due to the small sample size of neonates with diagnosed with malaria. Nevertheless, for every neonate whose mother had fever in pregnancy and treated with antimalarial is more likely to develop malaria more than once compared to the neonates delivered by mothers without fever in pregnancy. Neonates with birth weight ≥2.5 kg were more likely to develop more malaria parasitemia more than once than neonates
The limitation of this study included the possibility of a recall bias such that poor history recall by mothers could have affected the quality of information about the number of times and at what gestational age they had prenatal fever. Also, there was no documented evidence of malaria in mothers during pregnancy.
Conclusions
We conclude that the clinical features of malaria in neonates are mostly asymptomatic but could include fever, breathing difficulty, yellowness of the eyes and lethargy. It was also noted that the gender, gestational age and birth weight were not associated with malaria parasite density. Finally, we found that no specific clinical symptoms, maternal fever or use of anti-malaria in pregnancy, post-natal ages of neonates were significantly predictive of malaria parasite density in surveyed neonates.
References
- Hindi RD, Azimi PH (1980) Congenital Malaria due to Plasmodium falciparum. Pediatrics 66: 977-979.
- Mwaniki MK, Talbert AW, Mturi FN, Berkley JA, Kager P, et al. (2010) Congenital and Neonatal Malaria in a rural Kenyan district hospital: an eight-year analysis. Malar J 9: 313-314.
- Hashemzadeh A, Heydarian F (2005) Congenital malaria in a neonate. Arch of Iranian Med 8: 226-228.
- Wilson WR, Sande MA, Drew WL (2001) Current diagnosis and treatment in infectious diseases. Lange Med Books /Mc Grawtill, New York, US. p: 798.
- Enweronu-Laryea CC, Adjei GO, Mensah B, Duah N, Quashie NB (2013) Prevalence of congenital malaria in high risk Ghanian newborns: a case cross-sectional study. Malar J 12: 17-19.
- Uneke CJ (2007) Congenital plasmodium falciparum malaria in sub-Saharan Africa: a rarity or frequent occurrence. J Parasitol Res 101: 835-842.
- Mukhtar M (2007) The growing incidence of neonatal malaria: A situation review in developing countries. Nig J Med 16: 25-27.
- Oni GA, Oguntibeju OO (2006) Relationship between the malaria parasite density and children anaemia, brief communication. Int J Path 4: 1-3.
- Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, et al. (2012) Malaria prevention in Pregnancy, birth weight and neonatal mortality: a meta- analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 12: 942-949.
- Alam M, Ashraf T (2009) Congenital malaria due to Plasmodium falciparum. Parkistan Armed Forces Med J 4: 9-10.
- Runsewe-Abiodun IT, Ogunfowora OB, Fetuga MB (2006) Neonatal malaria in Nigeria- a 2-year review. BMC Pediatr 6: 19-21.
- Olarenwaju WI (1999) Malaria in the neonate: report of 2 cases. West Afr J Med 18: 139-140.
- Umeora OU, Egwuatu VE (2009) The role of unorthodox and traditional birth care in maternal mortality. Trop Doc 40: 13-17.
- Chukwu EO (2012) Estimates of rural poverty level and income distribution in Ebonyi State of Nigeria. NJAFE 8: 52-61.
- Adu-Gyasi D, Adams M, Amoako S, Mahama E, Nsoh M, et al. (2012) Estimating malaria parasite density: assumed white blood cell count of 10000/ul of blood is appropriate measure in central Ghana. Malar J 11: 238-243.
- Ojukwu JU, Ezeonu CT, Ogbu CN (2004) Severe malaria in neonates masquerading as septicaemia. Nig J Paediatr 31: 48-55.
- Obu HA, Ibe BC (2011) Neonatal malaria in the Gambia. Ann Med Health Sci Res 1: 45-54.
- Ouedraogo A, Tiono AB, Diarra A, Bougouma EC, Nebie I, et al. (2011) Transplacental Transmission of Plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J Trop Med 2012: 109705.
- Opare DA (2010) Congenital malaria in newborn twins. Ghana Med J 44: 76-78.
- Nnaji GA, Ezeagwuna DA, Olu EA (2011) Prevalence and pattern of cord blood malaria parasitaemia in a general practice setting in sub- Saharan Africa. Niger J Med 20: 83-89.
Citation: Nwokeji-Onwe L, Ikefuna AN, Okike CO, E eanosike OB, Iloh KK, et al. (2019) Clinical Evaluation of Neonatal Malaria in a Sub-urban Healthcare Facility in South-East Nigeria. Neonat Pediatr Med 5: 180. DOI: 10.4172/2572-4983.1000180
Copyright: © 2019 Nwokeji-Onwe L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3819
- [From(publication date): 0-2019 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 2959
- PDF downloads: 860