Research Article Open Access
Comparative Adsorption Studies of Cd(II) on EDTA and Acid Treated Activated Carbons from Aqueous Solutions
Magda A Akl1*, Ali M Abou-Elanwar2, Magda D Badri2 and Abdel-fattah M Youssef1
1Chemistry Department, Faculty of Science, Mansorua University, Egypt
2National Research Centre, Dokki, Giza, Egypt
- *Corresponding Author:
- Magda A Akl
Chemistry Department, Faculty of Science
Mansorua University, Egypt
Tel: +20502217833
E-mail: magdaakl@yahoo.com
Received date: September 05, 2015 Accepted date: October 20, 2015 Published date: October 27, 2015
Citation: Akl MA, Abou-Elanwar AM, Badri MD, Youssef AFM (2015) Comparative Adsorption Studies of Cd(II) on EDTA and Acid Treated Activated Carbons from Aqueous Solutions. J Anal Bioanal Tech 6:288. doi:10.4172/2155-9872.1000288
Copyright: © 2015 Akl MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
This study plans to functionalize activated carbon with oxygen and nitrogen containing groups to improve removal of Cd(II) ions from aqueous solutions. Activated carbon (AC) was treated with nitric acid giving MC-HNO3 that was pursued by modifying with EDTA to form MC-EDTA. AC, MC-HNO3 and MC-EDTA were analyzed using FTIR, SEM, TEM, Boehm titration, point of zero charge and N2 adsorption-desorption analysis. The batch technique sorption studies of Cd(II) onto sorbents were conducted and the factors controlling the adsorption process of Cd(II) were tested. Langmuir and Freundlich models were used to analyze the isotherm data. The equilibrium data fitted well Langmuir isotherm for all adsorbents. Pseudo-first order, pseudo-second order, intraparticle diffusion and the Boyd equations were used to analyze the kinetic data. The rate constants, equilibrium capacities and related correlation coefficients (R2) for each kinetic model were evaluated and discussed. The second order model is the best fit model for the three sorbents. Though intraparticle diffusion has essential role in rate-controlling step in the adsorption process of Cd(II) onto the investigated sorbents, film diffusion is also governing this process. The thermodynamic parameters ΔG°, ΔH° and ΔS° for the sorption processes of Cd(II) onto the adsorbents were estimated, and spontaneity of adsorption was deduced from the negative sign of ΔG°. Desorption study highlighted the cost saving due to the easiness of regeneration for both MC-HNO3 and MC-EDTA.
Keywords
Water; Activated carbon; Adsorption; Cd(II); Nitric acid; EDTA
Introduction
Heavy metal pollution is a critical environmental issue due to its harmful impacts and accumulation throughout the food chain and therefore in the human body. Cadmium is considered as one of the highly harmful heavy metals pollutants whereas Cd(II) is listed as the 7th most hazardous substance by the Agency for Toxic Substances and Disease Registry [1]. A number of chronic and acute diseases caused by Cd(II) exposure, such as itai-itai disease, renal damage, emphysema, hypertension and testicular atrophy [2]. Cd(II) toxicity is likely related to its high tendency to form bonds with thiol functional groups in some enzymes which lead to the displacement of biologically vital metals [3]. Cd(II) enter the water bodies through diverse ways comprising erosion of natural deposits, metal refinery discharges, and electronic waste runoff [1,4,5]. To keep water healthy, the Cd (II) should be preserved below certain limits. The permissible limit for Cd(II), provided by Environmental Protection Agency (EPA), is 0.005 mg/l and the current guideline value of potable water defined by the World Health Organization (WHO) is 0.003 mg/l [4,6] therefore Cd(II) concentration should not pass these limits to keep water safe. Purification of Cd(II) ions from waste water can be accomplished by applying different conventional techniques that are generally recognized as inefficient and/or expensive [7]. Adsorption by activated carbon is known as one of the extremely efficient methods for the removal of heavy metals [8]. Although the adsorption efficacy of a carbon is essentially related to the pore structure and surface area of the carbon, also the nature and density of surface functional groups have of important role in the adsorption of ionic or polar species [9]. The present work targets to functionalize the surface of activated carbon by oxidation with nitric acid and treatment with EDTA as a chelating agent for the improvement of heavy metals removal from water. The influence of parameters e.g., solution pH, sorbent dose and metal concentration, adsorption isotherms, kinetics modes and thermodynamic parameters of Cd(II) adsorption have been studied and discussed. The experimental equilibrium adsorption data are analyzed by both Freundlich and Langmuir isotherm models. The desorption studies of sorbents were also examined.
Materials and Methods
Materials and apparatus
All activated carbon (Ubichem Ltd., UK) used in the study was in the granular form. The analytical grade reagents and EDTA disodium salt were purchased from Sigma-Aldrich. 1.7909 g of CdCl2.2H2O was dissolved in 1 liter of acidified bi-distilled to prepare 1000 ppm Cd(II) solution that used to prepare the other diluted concentrations. Buffer solution pH=5.5 was adjusted using CH3COOH and NaOH. Glacial acetic acid, nitric acid (65%), hydrochloric acid (36%) were brought from Merck and used without any purification. The concentration of the Cd(II) was analyzed using flame atomic absorption (Analyst 300 Perkin Elmer FAAS). An Analyst 300 Perkin Elmer (FAAS) was used for the quantitative determination. A pH meter (Hi 931401, HANNA Portugal) was used for pH measurements. The adsorbents were weighed using analytical balance. Shaking Water Bath (NE5, Nickel- Electro Ltd., UK) used for adsorption and desorption experiments.
Functionalization of activated carbon
The functionalization of activated carbon was processed based in a previous work [10]. 50 g of activated carbon (0.8-1.25 mm) was stirred in 1000 ml round bottom flask containing 500 ml concentrated nitric acid for 4 h then the AC was filtered off and washed several times with water until the pH of filtrate became 5.5. The AC was air dried overnight at room temperature and then in oven for 24 h at 110°C. This oxidized AC was labeled as MC-HNO3. 20 g of MC-HNO3 was refluxed in 1000 ml round bottom flask containing 500 ml 0.15 M EDTA disodium salt at 80°C for 4 h. Then filtered and washed with hot distilled water for many times to get rid of excess nonreacted EDTA and the carbon was dried for 24 h at 110°C and the product was labeled as MC- EDTA.
Characterization of the adsorbents
Fourier transform infrared spectrophotometer (FTIR), Jasco, Model 6100- Japan, was used to investigate the functional groups existed on the sorbents. The oxygen-containing groups were estimated using Boehm titration [11-13]. Also point of zero charge pHPZC and surface pH were measured [10,14]. Surface Area and Pore Size Analyzer (Quantachrome-Nova 2000 Series) was applied at 77K to investigate N2 adsorption onto the adsorbents and to measure the BET surface areas (SBET) [15]. The αs method was applied to analyze the N2 adsorption isotherms to calculate: Sα (total surface area), Sαn (non-microporous surface area) and the volume of micropores (Vαm ) [16]. Also the average pore radius 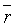 (nm) and the total pore volume (VT) were estimated [10]. Scanning electron microscope JSE-T20 (JEOL, Japan) at 40 kW and high resolution transmission electron microscope (JOEL JEM2010 HR-TEM) at 200 kV were used to analyze the morphological features of the adsorbents.
(nm) and the total pore volume (VT) were estimated [10]. Scanning electron microscope JSE-T20 (JEOL, Japan) at 40 kW and high resolution transmission electron microscope (JOEL JEM2010 HR-TEM) at 200 kV were used to analyze the morphological features of the adsorbents.
Adsorption experiments
Batch adsorption studies of Cd(II) ions onto AC, MC-HNO2 and MC-EDTA were performed using 100 ml to 1 L conical flasks and shaker water bath at 150 rpm. The influence of pH solution on Cd(II) adsorption was investigated by shaking 0.1 g sorbent with 25 ml of 150 ppm Cd(II) solutions for 24 h at 298.15K from 2 to 8. The pH of Cd(II) ion solution was fitted using 0.1M HCl or 0.1M NaOH. The isotherm studies were executed by shaking 0.1 g of adsorbents with 25 ml solution of various Cd(II) concentrations from 10 to 300 ppm for 24 h at 298.15K. Kinetics studies were performed by agitating 0.5 g of the adsorbents in 500 ml containing 100 ppm Cd(II) solutions at certain time periods from 5 min to 48 h then samples were withdrawn from the solution by fast filtration. The influence of sorbent doses was examined by equilibrating 25 ml of 350 ppm metal solutions and different adsorbents doses ranging from 0.05 g to 0.3 g. The effect of the temperature was investigated by shaking 0.1 g of sorbents with 25 ml solution at certain concentration of Cd(II) ranging from 10 to 300 ppm at 298.15, 308.15 and 318.15K.
The adsorption capacities were evaluated as follow:
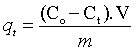 (1)
(1)
Where, qt (mg adsorbate/g adsorbent) is the adsorption capacity at certain time t; Co(mg/L) is the initial concentration of Cd(II) ion ; Ct is the remaining concentration of Cd(II) after adsorption for certain time t (mg/L); V(L) is volume of Cd(II) solution and m(g) is mass of the sorbents. The removal % of Cd(II) ions from solution is estimated as follows:
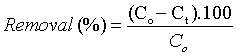 (2)
(2)
The desorption percentages of Cd(II) from adsorbents were examined by equilibrating 0.1 g of the carbons into 50 ml solution whose concentration is )80 ppm Cd(II) with pH=5.6). After equilibrium, the total adsorbed Cd (II) concentration was calculated, and then the solution was filtrated using a membrane to recover the sorbents. These sorbents were dried for 6 h at 80°C and then placed into 50 ml distilled water. The pH of the dispersed solutions was tuned from 1.5 to 5.5 using suitable amounts of HCl. After equilibrium, Cd (II) concentration was analyzed. These adsorption/desorption procedures were performed for three times, to further ascertain the desorption capability of adsorbents. The Cd(II) desorption % was evaluated by the following equation:
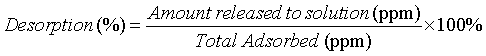 (3)
(3)
Results and Discussion
Characterization of the adsorbents
FT-IR: Figure 1 illustrated alike FT-IR spectra for the three sorbents. The wide bands at (3251-3430 cm-1) appeared for all sorbents were due to stretching model of O-H of hexagonal group. All sorbents possessed shoulders at (2845-2955 cm-1) that may be assigned to the C-H Aliphatic [17]. The signals appeared around 1700 cm-1 were attributed to stretching vibrations (C=O) of carboxyl groups, ketones lactones or aldehydes [18]. The clear peak appeared at about 1645 cm-1 for the samples may be due to C=O moieties in quinine. Peaks ranging from 1000-1200 cm-1 is problematic due to numerous overlapping broad bands and hence they were very hard to be described as simple motion of precise chemical bonds or functional [19]. Many researchers attributed this interfering band to phenolic and epi-oxide structures and ether stretching vibrations presented in several structural backgrounds [19-21].
Surface acidity and Boehm titration: Boehm titration results are shown in Table 1. The total number of acidic functional groups was increased significantly after treatment with nitric acid and EDTA. The total number of basic functional groups were found to be smaller than that for acidic ones and these numbers were agreed the surface pH values and the pHpzc. The MC-EDTA indicted minor increment in the total acidic groups and big increment of basic groups in compared with MC-HNO3.
| Adsorbent | Carboxyli(mmol/g) | Lactonic(mmol/g) | Phenolic(mmol/g) | TotalAcidic group (mmol/g) | BasicGroups(mmol/g) | Moisture content% | Ashcontent % | SurfacepH | Point ofzero charge |
|---|---|---|---|---|---|---|---|---|---|
| AC | 0.078 | 0.0225 | 0.0456 | 0.1461 | 0.125 | 10 | 9 | 6.5 | 7 |
| MC-HNO3 | 0.585 | 0.0476 | 0.2523 | 0.8849 | 0.084 | �?�?�? | 6.38 | 3.5 | 3.5 |
| MC-EDTA | 0.4038 | 0.489 | 0.0966 | 0.9894 | 0.26 | �?�?�? | 3.7 | 7.2 | 7.45 |
Table 1: Surface properties of the adsorbents.
Nitrogen adsorption-desorption data: The nitrogen adsorptiondesorption isotherms for the AC, MC-HNO3 and MC-EDTA are shown in Figure 2. The porous features of the AC, MC-HNO3 and MCEDTA estimated from the exploration of N2 adsorption isotherms are indicated in Table 2 [22]. The 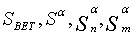 surface areas and pore volumes
surface areas and pore volumes 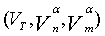 for the activated carbon were declined after treatment with nitric acid and EDTA with widening of the Average pore radius
for the activated carbon were declined after treatment with nitric acid and EDTA with widening of the Average pore radius 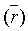 . This could interpreted by the difficult accessibility of nitrogen molecules into the internal adsorption sites because of the increased functional groups existed on MC-HNO3 and MC-EDTA at the pore entrance leading to electrostatic repelling of surface probe molecules (N2). Also perhaps by the destruction of some walls with narrow pores that occurred during the oxidation of the AC [23,24]. Additional treatment of MC-HNO3 with EDTA blocked the pore entrance with further functional groups resulting in extra electrostatic repelling and thinner pores thus causing to additional decrement in surface areas as well as the average pore radius with enlarging in micro
. This could interpreted by the difficult accessibility of nitrogen molecules into the internal adsorption sites because of the increased functional groups existed on MC-HNO3 and MC-EDTA at the pore entrance leading to electrostatic repelling of surface probe molecules (N2). Also perhaps by the destruction of some walls with narrow pores that occurred during the oxidation of the AC [23,24]. Additional treatment of MC-HNO3 with EDTA blocked the pore entrance with further functional groups resulting in extra electrostatic repelling and thinner pores thus causing to additional decrement in surface areas as well as the average pore radius with enlarging in micro 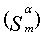 .
.
| SBET(m2/g) | Sá(m2/g) | 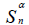 (m2/g) (m2/g) |
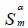 (m2/g) (m2/g) |
VT(ml/g) | 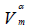 (ml/g) (ml/g) |
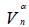 (ml/g) (ml/g) |
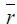 (nm) (nm) |
|
|---|---|---|---|---|---|---|---|---|
| AC | 2114.6 | 1765.68 | 1181.25 | 584.43 | 1.7515 | 0.35 | 1.40 | 1.657 |
| MC-HNO3 | 934.4 | 720.944 | 576.332 | 144.611 | 0.87575 | 0.20 | 0.67 | 1.874 |
| MC-EDTA | 587.8 | 516.874 | 287.196 | 229.678 | 0.5084 | 0.16 | 0.35 | 1.730 |
Table 2: Nitrogen adsorption-desorption data.
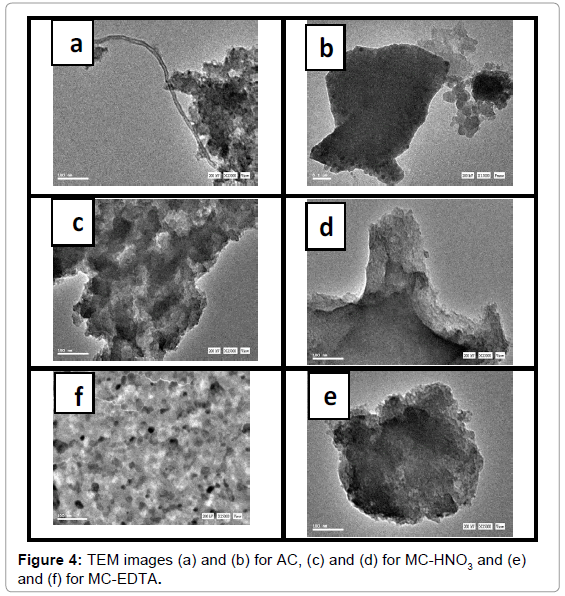
SEM and TEM: Obvious changes in the morphology of the adsorbents were noticed from SEM as shown in Figure 3. MC-HNO3 illustrated a broader pores than that of AC which ensuring the eroding action of nitric acid and collapsing of large grains into small ones and might form a very large micropores as a result of the destruction in the adjacent micropores due oxidation by nitric acid. MC-EDTA showed alike micrographs with that of MC-HNO3 but with smaller number of wider pores. Some slight alterations in the surface are noticed from TEM images as shown in Figure 4 and porous structure of MC-HNO3 and MC-EDTA are demonstrated in comparing with AC.
Adsorption and desorption studies
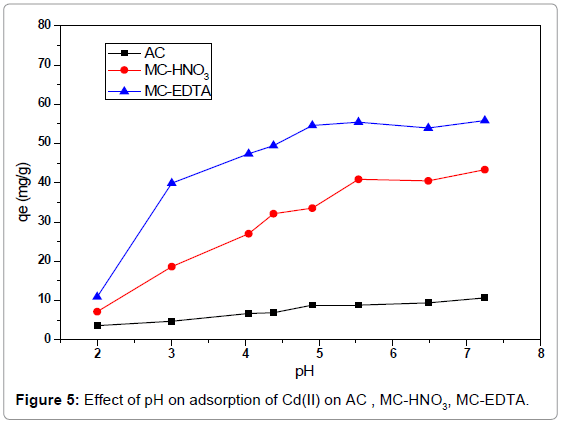
Effect of the solution pH: The pH of the solution governs the charge of the surface charge, ionization extent of the functional groups for the sorbents as well as the form of the metal ions in the aqueous solution. Therefore pH of the solution seems to be the most important parameter affecting the sorption process. The pH of the Cd(II) solutions was altered from 2 to 7.4 which is below the pH necessary for precipitation. It was apparent from Figure 5 that the uptake rate is rapid below pH 5.6 but above this pH the increasing in the uptake became slow and the adsorption capacity is near the maximum one. At lower pH, the adsorption quantity of Cd(II) was quite low that is assigned to the repelling between positive Cd(II) ions and positive function groups on the adsorbents surface. However, with raising the pH, the adsorbents surfaces accepted further negative charges from the ionization of the function groups, that could improve the electrostatic attractions of Cd(II) ions with negatively charged adsorbents surfaces so enhance sorption process. It is noticed that the adsorption capacities for MC-EDTA and MC-HNO3 were larger than that of AC over the studied pH range which indicate the adsorption capacity is increased by the introduction of function groups on the surface of the activated carbon after treatment with HNO3 and EDTA. A pH=5.6 was selected as the peak for further experiments.
Effect of initial concentrations: The equilibrium isotherms for AC, MC-HNO3and MC-EDTA are shown in Figure 6 indicated that the adsorption capacities improved with raising the initial Cd(II) concentrations. This is due to the increment in the driving force produced from the concentration gradient [25,26]. This figure showed that the treated AC seemed to be more efficient under higher and lower Cd(II) concentration whereas the maximum adsorption capacity increased from 42 mg/g in case of AC to 82 mg/g for MC- HNO3 (1.95 times as AC)and 89 mg/g for MC-EDTA(2.12 times as AC). It was observed, the surface treatments with nitric and EDTA had created new sites for binding with Cd(II) ions, which was responsible for enhancing the adsorption.
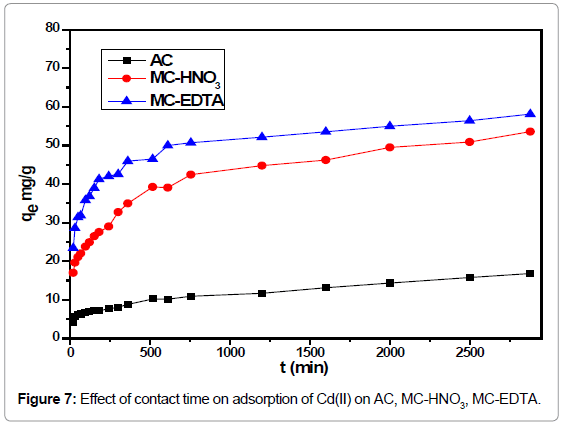
Effect of contact time: The influence of contact time was investigated for the adsorbents are shown in Figure 7. It was observed that the adsorption of Cd(II) ions increased rapidly until the first 6 hr. The rapid uptake at the initial period may attribute to the sufficient presence of active sites on the surface of the sorbents. After 10 hr, the adsorption was steady therefore it could be selected as the equilibrium time for Cd(II) adsorption.
Effect of adsorbent dose: Figure 8 showed the effect of sorbent dose on the adsorption process. With raising the sorbent dosage from 0.05 g/25 ml to 0.25 g/25 ml, the % of Cd(II) ions uptake improved from 30.2 to 34 for AC, from 51.7% to around 95.1% for MC-HNO3 while increased from 54% to 100% in case of MC-EDTA. It was noticed that the adsorption per unit mass declined by raising the sorbent concentration. The reduction in adsorption density with raising in the adsorbent dosage is mostly because of the unsaturated of adsorption sites during the adsorption process [27,28]. This behavior could also resulted from the aggregation due the particle interaction and these aggregation might resulted in decrement in total surface area of the carbons and an increment in diffusional path length [28].
Equilibrium adsorption studies: The equilibrium adsorption isotherms are very useful for studying and designing of sorption modules. Langmuir, Sips, Freundlich and Redlich-Peterson models are commonly applied to describe the adsorption of solutes onto solid adsorbents [29]. Langmuir and Freundlich models describe the relation between the concentration of the adsorbate solution at equilibrium and the adsorption capacity at a certain temperature. Langmuir and Freundlich models are used to interpret the adsorption isotherms using regression analysis. The Langmuir equation (linear formula) is indicated as follows:
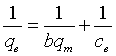 (4)
(4)
Where, qe (mg/g) is the amount adsorbed of Cd(II) per unit mass of adsorbent, Ce is the equilibrium liquid phase concentration of Cd(II), b (l/mg) is Langmuir equilibrium constant, qm(mg/g) is the monolayer adsorption capacity. Both b and qm are estimated by plotting Ce/qe against Ce as shown in Figure 9a. Langmuir isotherm is frequently evaluated by a separation factor, RL, which expressed as follows:
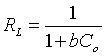 (5)
(5)
Where, Co in that situation is the maximum initial concentration of Cd(II). The type of the isotherm and the nature of the adsorption process could be described according to the value of the separation factor. The adsorption can be irreversible RL=0, unfavorable if RL>1, linear when RL=1 or favorable when 0<RL<1 [30]. In all the studied case, the RL values were existed between 0<RL<1 which ensured favorability of the adsorption for Cd (II) ions. From the RL values the favorability of the adsorption ordered as follow: MC-EDTA>MC-HNO3>AC. Freundlich model is completely empirical and it well interprets the adsorption process on heterogeneous surfaces [31]. Freundlich model (linear formula) is expressed as follow:
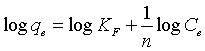 (6)
(6)
Where KF (l/g) is Freundlich constant whereas n is Freundlich exponent. These constants are evaluated by plotting log qe against log Ce as shown in Figure 9b. The isotherm constants for the adsorption of Cd(II) ions onto the adsorbents are illustrated in Table 3. It was observed that the Langmuir adsorption isotherm well fitted the experimental data for AC (r2>0.9857), MC-HNO3 (r2>0.9764) and MCEDTA (r2>0.9941).
| Langmuir constants | Freundlich constants | |||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbents | qm(mg/g) | b (L/mg) | R2 | RL | KF | 1/n | R2 | |
| AC | 102.77 | 0.0010 | 0.9857 | 0.526316 | 0.8520 | 0.5755 | 0.9208 | |
| MC-HNO3 | 85.84 | 0.0207 | 0.9764 | 0.050942 | 19.3335 | 0.2193 | 0.9550 | |
| MC-EDTA | 91.91 | 0.0376 | 0.9941 | 0.028703 | 32.8746 | 0.1540 | 0.8720 | |
Table 3: Langmuir and Freundlich isotherm constants for Cd(II) adsorption on AC ,MC-HNO3 and MC-EDTA.
Adsorption kinetics
Pseudo-first-order and pseudo-second-order: Pseudo-first-order and pseudo-second-order were used to explore the adsorption kinetics of Cd (II) on the surface of the carbons. The pseudo-first-order model is commonly used to analyze kinetic data [32]. Its linear form is given as follows:
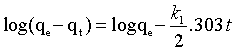 (7)
(7)
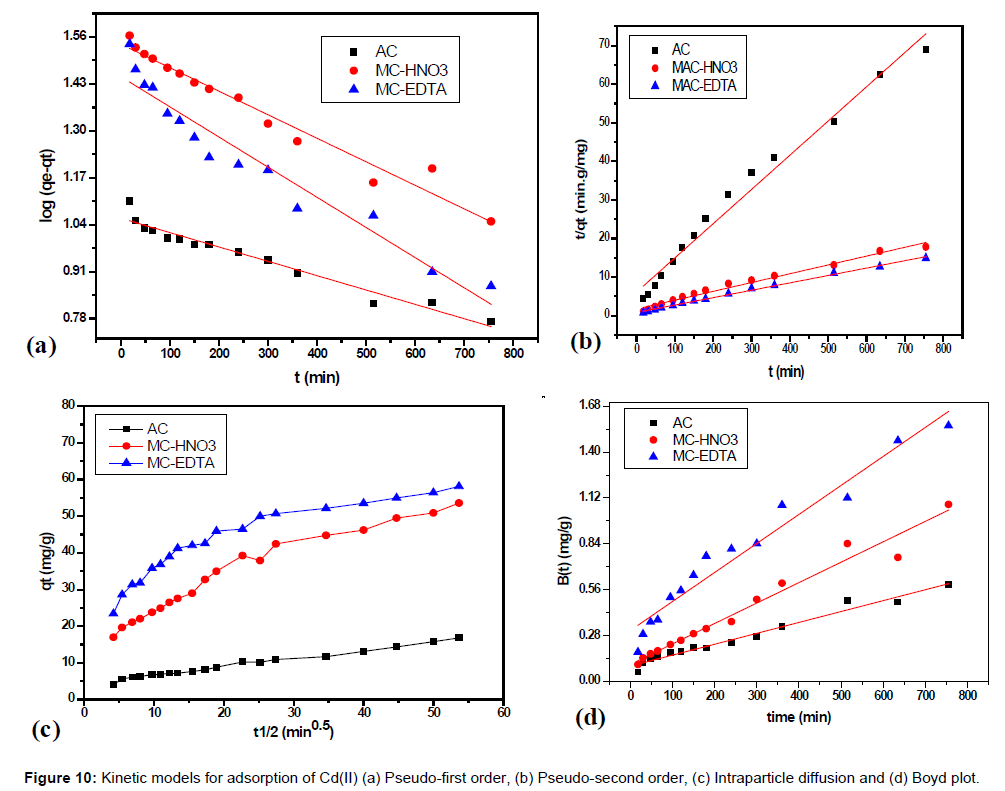
Where, qe and qt (mg/g) are the adsorption capacities of Cd(II) at equilibrium and at certain time t and k1 (min-1) is the equilibrium rate constant which was estimated from the slopes by plotting ln(qe-qt) against t (as shown in Figure 10a).
The pseudo-second-order kinetic model is defined as follows:
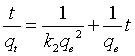 (8)
(8)
Where, k2 (g/mg min) is the equilibrium rate constant whereas qe is evaluated by plotting of t/qt against t (Figure 10b) [33]. The correlation coefficients for both kinetic models are shown in Table 4. For AC, MCHNO3 and MC-EDTA: The second order model provides the best fit for practical kinetic data because the value of the calculated qe agreed very well with the practical data and r2 is higher than 0.992 for all carbons.
| qe, exp (mg/g) | AC | MC-HNO3 | MC-EDTA | |
| 16.85 | 53.6 | 58.1 | ||
| First-orderkinetic equation | q1 (mg/g) | 11.4256 | 34.659 | 28.17 |
| k1 (min-1)× 10-3 | 0.91 | 1.5 | 1.92 | |
| R12 | 0.95011 | 0.96403 | 0.93294 | |
| Second-orderkinetic equation | q2 (mg/g) | 17.31 | 55.13 | 58.96 |
| k2[g/(mg min)]×10-3 | 3.01 | 1.02 | 2.03 | |
| R22 | 0.962 | 0.989 | 0.9974 | |
| Intraparticlediffusion equation | kint[mg/(g min1/2)] | 0.22248 | 0.48327 | 0.32747 |
| C | 3 | 20 | 34 | |
| Rint2 | 0.99059 | 0.93791 | 0.89998 | |
| Boyd equation | Intercept | 0.0952 | 0.1059 | 0.3121 |
| R2 | 0.9720 | 0.9678 | 0.9528 |
Table 4: Kinetic parameters for the adsorption of for Cd(II) adsorption on AC ,MC-HNO3 and MC-EDTA
Intraparticle diffusion equation and Boyd equation: The intraparticle diffusion and Boyd models were used to identify diffusion mechanisms [34-36]. Intraparticle diffusion equation is expressed as follows:
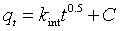 (9)
(9)
where kint (g/mg min1/2) is the intraparticle rate constant for Cd(II) adsorption, C is the intercept and can be estimated by plotting qt against t1/2 (Figure 10c) [37]. Multi-linearity existed in such graphs [38,39], illustrating that two or more stages occurred. The first stage is sharper part and caused by the external surface or instantaneous adsorption. The second part is the gradual sorption stage, while intraparticle diffusion is rate-determined and from it kint is estimated. The third part is the last equilibrium step while intraparticle diffusion starts to become steady due to extremely small solute concentrations of aqueous solutions.
Also Boyd model was used to investigate the kinetic data [40] to find whether adsorption undergoes to an external diffusion or intraparticle diffusion mechanism, which is defined as follows:
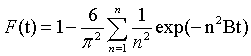 (10)
(10)
Where F is the fractional of equilibrium at different periods (t), and B (t) is mathematical function of F. while n is integer that describes the infinite series solution and F is the fractional attainment of equilibrium at time t and is given as follows:
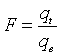 (11)
(11)
where qt and qe is the adsorbed amount of Cd(II) ion at certain time (t) and equilibrium. Reichenberg [41] gave the next approximations:
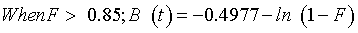 (12)
(12)
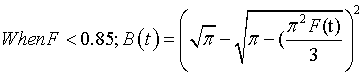 (13)
(13)
Figure 10c showed that the intraparticle diffusion plot did not passed through the origin point which indicated that both film diffusion (boundary layer diffusion) and intraparticle diffusion affect the ratedetermining step of Cd(II) adsorption process for all the studied adsorbents. Also the same result could be deduced from the Boyd plots (Figure 10d) because the plots did not pass with the origin point.
Thermodynamic studies: The different values of the Langmuir constants b (l/mol) at different temperature used to calculate the change in the free energy ÄGᴏ for Cd(II) adsorption using the following expression:
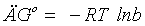 (14)
(14)
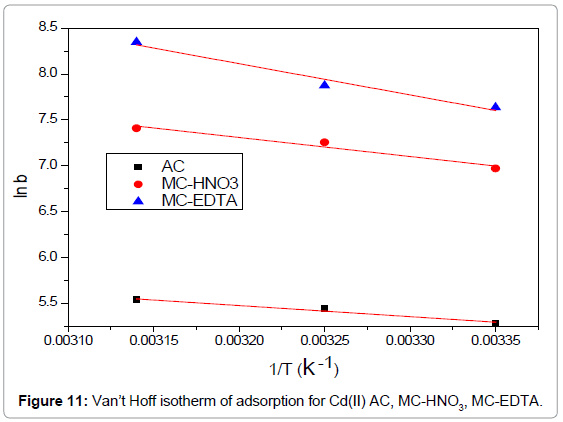
Where R is general gas constant (8.314 J/mol/K), T is the temperature in K0. The Standard enthalpy (ÄHᴏ) and entropy (ÄSᴏ) for Cd(II) adsorption were calculated from Van't Hoff equation:
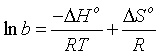 (15)
(15)
Figure 11 illustrated the relations between ln b against 1/T were linear and from the slopes the enthalpies  were evaluated while the entropies
were evaluated while the entropies 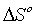 were estimated from the intercept. Spontaneity of Cd(II) adsorption was deduced from the negative sign of ΔG° for all the adsorbents as shown in Table 5. The amount of the adsorbed Cd(II) at equilibrium was raised with raising the temperature for all cases. The adsorption processes were found to be endothermic due to the positive values of enthalpies
were estimated from the intercept. Spontaneity of Cd(II) adsorption was deduced from the negative sign of ΔG° for all the adsorbents as shown in Table 5. The amount of the adsorbed Cd(II) at equilibrium was raised with raising the temperature for all cases. The adsorption processes were found to be endothermic due to the positive values of enthalpies 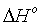 for all cases. The affinity of Cd(II) adsorption by the carbons were predicted from positive signs of
for all cases. The affinity of Cd(II) adsorption by the carbons were predicted from positive signs of 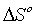 also proposed some structural variations in the activated carbons and Cd(II).
also proposed some structural variations in the activated carbons and Cd(II).
| AC | MC-HNO3 | MC-EDTA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T (k) | 298.15 | 308.15 | 318.15 | 298.15 | 308.15 | 318.15 | 298.15 | 308.15 | 318.15 |
| ΔG0(KJ/mol) | -11.604 | -13.258 | -14.498 | -17.702 | -18.928 | -19.977 | -18.931 | -20.174 | -22.083 |
| ΔH0(KJ/mol) | 31.6024 | 16.2539 | 27.9456 | ||||||
| ΔS0(J/mol/k) | 145.133 | 113.98 | 156.877 | ||||||
Table 5: Thermodynamic parameters for the adsorption of Cd(II) on on AC,MC-HNO3 and MC-EDTA.
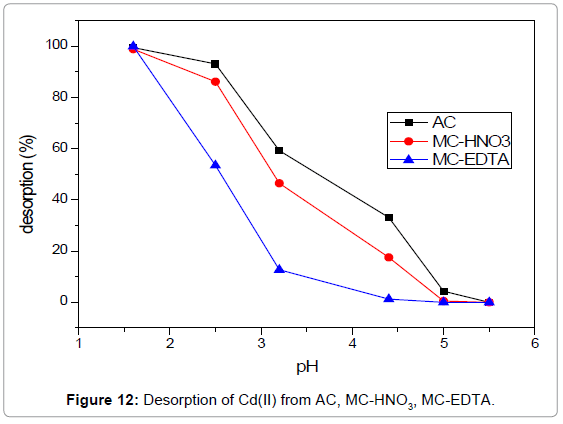
Desorption study: The cost saving of the adsorption systems that applied for water treatment is related to both the adsorption capacity and reversibility of the adsorption process. Therefore identical adsorbents should possess high adsorption capacity and easiness for desorption. Figure 12 illustrated the Cd (II) desorption % with regard to solutions at different pH values. It was found that the Cd (II) desorption % improved with decreasing the solution pH. The Cd(II) desorption % raised rapidly from pH=4.5 to pH=1.6 and peaked at pH=1.6 with values above 99.5% for all cases. These results indicated that the adsorbed Cd(II) by the carbons could be simply desorbed and can be applied currently in water decontamination from Cd(II) ions. Moreover, the recycling easiness illustrated that the adsorption mechanism is mainly controlled by ion exchange.
Conclusion
Treatment of AC with HNO3 and EDTA highly functionalized its surface with oxygen and nitrogen containing groups which accompanied with significant alteration in its textural and morphological features of the activated carbon. Cd (II) adsorption capacities were controlled significantly by pH, metal concentration, equilibration time, sorbent dose, temperature. Functionalization of AC improved the adsorption capacity of AC from 42 mg/g to 82 mg/g in case of MC-HNO3 and 89 mg/g for MC-EDTA. The adsorption isotherms of the adsorbents were well described by Langmuir model. Pseudo second-order well fitted the kinetic data AC, MC-HNO3 and MC-EDTA. Both external and intraparticle diffusions controlled the rate-determining step for Cd(II) adsorption process. The three sorbents adsorbed the Cd(II) spontaneously that was deduced from negative values of ΔG°. Desorption study illustrated the cost saving due to the easiness of regeneration for both MC-HNO3 and MC-EDTA.
Acknowledgements
The author(s) express a grateful acknowledgement to The Academy of Scientific Research and Technology (ASRT) of Egypt for the financial support. Grant name: Scientist for Next Generation grant, cycle 3, group B Grant code: ASRT/TRG/B/2010-16.
References
- ATSDR (2011) Priority List of Hazardous Substances.
- Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresource Technology 76: 63-65.
- Baes CF, Mesmer RE (1976) The hydrolysis of cations. Wiley, New York, USA.
- USEPA (2009) National Primary Drinking Water Regulations
- Cadmium C, Nickel N (2007) Monitored Natural Attenuation of Inorganic Contaminants in Ground Water.
- WHO (2011) Guidelines for drinking-water quality.
- Mahmoud ME, Hafez OF, Alrefaay A, Osman MM (2010) Performance evaluation of hybrid inorganic/organic adsorbents in removal and preconcentration of heavy metals from drinking and industrial waste water. Desalination 253: 9-15.
- Tian Y, Yin P, Qu R, Wang C, Zheng H, et al. (2010) Removal of transition metal ions from aqueous solutions by adsorption using a novel hybrid material silica gel chemically modified by triethylenetetraminomethylenephosphonic acid. Chemical Engineering Journal 162: 573-579.
- Vasu AE (2008) Surface Modification of Activated Carbon for Enhancement of Nickel(II) Adsorption. E-Journal of Chemistry 5: 814-819.
- Youssef AM, Dawy MB, Akland MA, Abou-Elanwar AM (2013) EDTA Versus Nitric Acid Modified Activated Carbon For Adsorption Studies of Lead (II) From Aqueous Solutions. Journal of Applied Sciences Research 9: 16.
- Goertzen SL, Thériault KD, Oickle AM, Tarasuk AC, Andreas HA (2010) Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 48: 1252-1261.
- Boehm HP (1994) Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 32: 759-769.
- Boehm HP (1966) Chemical Identification of Surface Groups, in Advances in Catalysis. Eley HPDD, Paul BW (Eds), Academic Press. pp: 179-274.
- Rivera-Utrilla J, Bautista-Toledo I, Ferro-García MA, Moreno-Castilla C (2001) Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. Journal of Chemical Technology & Biotechnology 76: 1209-1215.
- Brunauer S, Emmett PH, Teller E (1938) Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society 60: 309-319.
- Selles-Perez MJ, Martin-Martinez JM (1991) Application of α and n plots to N2 adsorption isotherms of activated carbons. Journal of the Chemical Society, Faraday Transactions 87: 1237-1243.
- Kennedy LJ, Vijaya JJ, Sekaran G (2005) Electrical conductivity study of porous carbon composite derived from rice husk. Materials Chemistry and Physics 91: 471-476.
- Kennedy LJ, Vijaya JJ, Sekaran G (2004) Effect of Two-Stage Process on the Preparation and Characterization of Porous Carbon Composite from Rice Husk by Phosphoric Acid Activation. Industrial & Engineering Chemistry Research 43: 1832-1838.
- Painter P, Starsinic M, Coleman M (1985) Fourier Transform Infrared Spectroscopy. Academic Press, New York. pp: 169-189.
- Gómez-Serrano V, Acedo-Ramos M, López-Peinado AJ, Valenzuela-Calahorro C (1994) Oxidation of activated carbon by hydrogen peroxide. Study of surface functional groups by FT-IR. Fuel 73: 387-395.
- Fanning PE, Vannice MA (1993) A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 31: 721-730.
- Brunauer S, Deming LS, Deming WE, Teller E (1940) On a Theory of the van der Waals Adsorption of Gases. Journal of the American Chemical Society 62: 1723-1732.
- Salame II, Bagreev A, Bandosz TJ (1999) Revisiting the Effect of Surface Chemistry on Adsorption of Water on Activated Carbons. The Journal of Physical Chemistry B 103: 3877-3884.
- Choma J, Burakiewicz-Mortka W, Jaroniec M, Li Z, Klinik J (1999) Monitoring Changes in Surface and Structural Properties of Porous Carbons Modified by Different Oxidizing Agents. Journal of Colloid and Interface Science 214: 438-446.
- Ozacar M, Sengil IA (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96: 791-795.
- Sun G, Xu X (1997) Sunflower Stalks as Adsorbents for Color Removal from Textile Wastewater. Industrial & Engineering Chemistry Research 36: 808-812.
- Yu LJ, Shukla SS, Dorris KL, Shukla A, Margrave JL (2003) Adsorption of chromium from aqueous solutions by maple sawdust. J Hazard Mater 100: 53-63.
- Shukla A, Zhang YH, Dubey P, Margrave JL, Shukla SS (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95: 137-152.
- Ania CO, Parra JB, Pis JJ (2002) Influence of oxygen-containing functional groups on active carbon adsorption of selected organic compounds. Fuel Processing Technology 79: 265-271.
- Karagöz S, Tay T, Ucar S, Erdem M (2008) Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour Technol 99: 6214-6222.
- Ng C, Losso JN, Marshall WE, Rao RM (2002) Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin-water system. Bioresour Technol 85: 131-135.
- Hameed BH, Ahmad AL, Latiff KNA (2007) Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes and Pigments 75: 143-149.
- Franca AS, Oliveira LS, Ferreira ME (2009) Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 249: 267-272.
- Özacar M (2003) Equilibrium and kinetic modelling of adsorption of phosphorus on calcined alunite. Adsorption 9: 125-132.
- Wu FC, Tseng RL, Juang RS (2001) Adsorption of dyes and phenols from water on the activated carbons prepared from corncob wastes. Environ Technol 22: 205-213.
- Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92: 263-274.
- Altenor S, Carene B, Emmanuel E, Lambert J, Ehrhardt JJ, et al. (2009) Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J Hazard Mater 165: 1029-1039.
- Qi L, Xu Z (2004) Lead sorption from aqueous solutions on chitosan nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 251: 183-190.
- Yan G, Viraraghavan T (2003) Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res 37: 4486-4496.
- Boyd GE, Adamson AW, Myers LS (1947) The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. Journal of the American Chemical Society 69: 2836-2848.
- Reichenberg D (1953) Properties of Ion-Exchange Resins in Relation to their Structure. III. Kinetics of Exchange. Journal of the American Chemical Society 75: 589-597.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15633
- [From(publication date):
December-2015 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 10889
- PDF downloads : 4744