Comparative Analysis of Cytotoxic Effects of Bisphenol A and Bisphenol S on Human Renal HEK293 Cells
Received: 30-Jan-2018 / Accepted Date: 12-Feb-2018 / Published Date: 20-Feb-2018
Abstract
Bisphenol A (BPA) and S (BPS) are synthetic compounds of immense commercial importance and are present in various industrial products, especially in packaging for food and beverages. Both compounds were shown to mediate estrogenic activities. In various studies, BPA was demonstrated to induce cytotoxic effects in the cardiovascular system as well as in the kidney. Similar to BPA, BPS was also found to mediate cardiotoxic activities. However, potential nephrotoxic effects of BPS as shown for BPA were not investigated so far. In this study, we set out to characterize the influence of BPS and BPA on human embryonic kidney cells (HEK293) in a comparative manner. Gene expression was analyzed by real-time PCR and Western blotting and cell viability was determined by a calcein acetoxymethyl assay. Here, we showed that low concentration (0.1-10 μM) of BPA and BPS had no significant influence on viability or gene expression in HEK293. In contrast to BPS, high amounts of BPA (1000 μM) decreased HEK293 viability as well as expression of protein kinase B, podoplanin and nuclear factor 'kappa-lightchain- enhancer' of activated B-cells p65 subunit RelA. Additionally, this was associated with an intense reduction of the B-cell lymphoma 2 and Bcl-2-associated death promoter (Bcl-2/BAD) ratio in renal cells. Moreover, pharmacological inhibition of RelA, but not of protein kinase B, was shown to reduce cell viability in HEK293. In sum, our data indicate that BPA’s cytotoxic impact on HEK293 viability and gene expression is stronger than that of BPS in vitro.
Keywords: Bisphenol; HEK293 ; Renal; Cell viability; Kidney
Introduction
Bisphenols, such as bisphenol A (BPA) and S (BPS) play an important industrial role for the production of various objects of daily use, especially packaging for food and beverages. Their ubiquitous presence consequently leads to a high risk of customer exposure, e.g. by food contaminations [1,2]. BPA was shown to be an estrogenic endocrine disrupting chemical potentially affecting various biological effects [3]. Moreover, BPA was demonstrated to induce different cytotoxic effects, especially in the context of cardiovascular and renal pathologies [1,4]. These results suggest that BPA plays an important pathophysiological relevant role in the cardiovascular system as well as in the kidney. As depicted for BPA, BPS was also indicated to exhibit estrogenic-like characteristics [5]. Moreover, a study done in female rats showed that BPS mediate cardiotoxic activities, similar to those described for BPA [6]. In contrast to BPA, potential effects of BPS in the kidney are until now widely unexplored.
Here, we set out to characterize the influence of BPS in human kidney cells in comparison to the effects of BPA in this experimental setting. On the one hand, we analyzed the impact of both compounds on the generation of the nuclear factor 'kappa-light-chain-enhancer' of activated B-cells (NFκB) p65 subunit RelA, a protein of central importance for gene expression control [7,8]. On the other hand we characterized the effects of BPS and BPA on the expression of factors associated to renal cell survival and viability, such as protein kinase B (Akt), podoplanin (PDPN), B-cell lymphoma 2 (Bcl-2) and Bcl-2- associated death promoter (BAD) [9-11]. Since kidney cell viability plays a crucial role for maintenance of renal function [12], we also analyzed the impact of BPS and BPA on HEK293 cell viability in this study.
Methods
Cell culture
Human embryonic kidney cells (HEK293 ) were cultured at 37°C with 5% CO2 in a humidified incubator using Dulbecco's Modified Eagle medium (DMEM; Biochrom GmbH, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS; Biochrom GmbH, Berlin, Germany) and 1% penicillin/streptomycin (Biochrom GmbH, Berlin, Germany). HEK293 were treated with BPA or BPS (BIOZOL GmbH, Eching, Germany), respectively, at different concentrations (0.1 μM-1000 μM) in FBS-free DMEM for 24 hrs. Control cells were incubated with the solvent alone (ethanol, 99.9%; Fisher Scientific GmbH, Schwerte, Germany) in equal amounts. Pharmacological inhibition of Akt was mediated via incubation of HEK293 with 10 μM triciribine (Selleck Chemicals, Houston, TX, USA) as done before [13]. NFκB and its subunit RelA was blocked by 10 μM BAY 11-7082 (Adipogen AG, Liestal, Switzerland) as described earlier [7]. Before experimentation, cells were starved overnight in FBS-free DMEM.
Real-time polymerase chain reaction (PCR): Real-Time PCR was done as described previously [14]. Total ribonucleic acid (RNA) was isolated via the “Universal RNA purification kit” (Roboklon GmbH, Berlin, Germany). Reverse transcription of total RNA (1 μg per sample) was done using a “High-Capacity cDNA Reverse Transcription Kit” (Life Technologies GmbH, Darmstadt, Germany). A CFX96TM Real-Time System (Bio-Rad Laboratories GmbH, Munich, Germany) was used. The real-time PCR conditions: 50°C, 2 min; 95°C, 20 sec; 45 cycles 95°C, 3 sec; 60°C, 30 sec. For analyses of the mRNA expression of Bcl-2 and BAD, “TaqMan® Gene Expression Assays” (Thermo Fisher Scientific, Waltham, MA, USA) were used following the manufacturer’s protocol.
Western blot analyses: Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analyses of protein expression were performed as described previously [15,16]. Expression was determined in cell lysates of HEK293 using specific antibodies against RelA (Aviva Systems Biology, Corp., San Diego, CA, USA), Akt (Merck Chemicals GmbH, Schwalbach, Germany), PDPN (Sigma-Aldrich Chemie GmbH, Munich, Germany), and GAPDH (Calbiochem, Darmstadt, Germany). Quantification of Western blot results was performed by Gel-Pro AnalyzerTM software version 4.0.00.001 (Media Cybernetics, Bethesda, MD, USA) as described earlier [17,18].
Calcein acetoxymethyl (AM) cell viability assay
Calcein AM cell viability assay (Trevigen Inc. Gaithersburg, MD, USA) was performed as described earlier [9,19] and following manufacturer’s protocol. In brief, 1 x 104 HEK293 per well were seeded in 96-well plates. Then, cells were incubated with BPA, BPS, triciribine and/or BAY 11-7082 for 24 hrs. After that, samples were incubated with calcein AM solution for 30 min and fluorescence was determined at 490 nm excitation and 520 nm emission using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Statistics
All obtained data were analyzed via Student’s t-test or one-way analysis of variance (ANOVA), as appropriate using “GraphPad Prism” version 6.00 (GraphPad Software, Inc., La Jolla, CA, USA). Results were expressed as mean ± SEM and a probability value (p) ≤ 0.05 was deemed significant.
Results
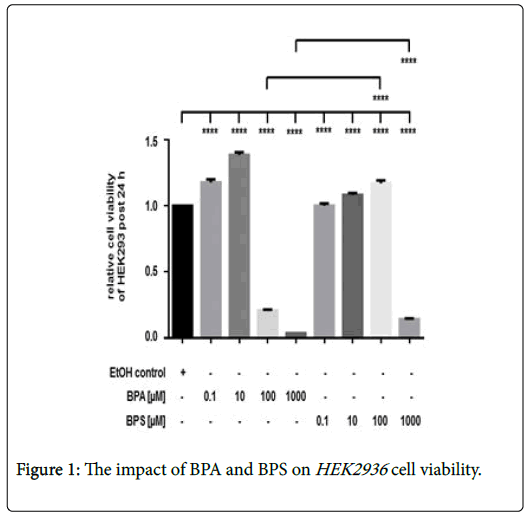
The effect of BPA and BPS on renal cell viability
Incubation of HEK293 with 0.1-10 μM BPA was associated with an increased cell viability after 24 hrs (Figure 1). In contrast to lower amount, treatment of cells with 100 μM or 1000 μM BPA, respectively, led to a significant and dose-dependent reduction of renal cell viability. Stimulation of HEK293 with low 0.1 μM and 10 μM BPS led to a slightly increased renal cell viability as also found for low BPA doses (Figure 1). In contrast to the cytotoxic effect of 100 mM BPA, incubation of renal cells with 100 μM BPS increased cell viability after 24 hrs, whereas, treatment of HEK293 with 1000 μM BPS also reduced cell viability. However, the inhibitory effect of 1000 μM BPA was significantly stronger than that of 1000 μM BPS in HEK293 (Figure 1). Depicted is the cell viability of HEK293 post 24 hrs. Cell viability was determined by measurement of calcein AM fluorescence at 490 nm excitation and 520 nm emissions. Compared were cells incubated with the solvent ethanol (equal amount; EtOH control) and HEK293 treated with 0 μM-1000 μM of BPA or BPS, respectively. Shown is the mean ± SEM of at least 3 independent experiments (****) p ≤ 0.0001.
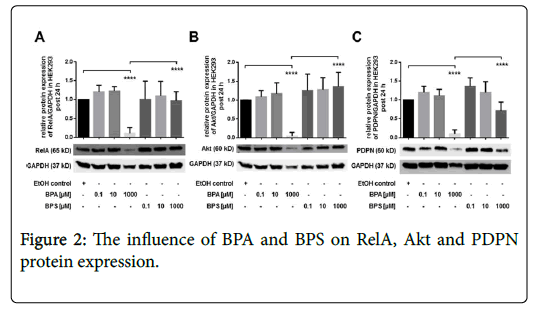
The impact of BPA and BPS on protein expression in HEK293 cells
In this part of the study, we characterized the effect of BPA and BPS on protein expression of RelA, Akt and PDPN in human renal cells post 24 hrs (Figures 2A-2C). Incubation of HEK293 cells with 0.1-10 μM BPA had no significant influence on protein expression of RelA (Figure 2A), Akt (Figure 2B) and PDPN (Figure 2C). Similar to BPA, stimulation of renal cells with 0.1 μM as well as 10 μM BPS exhibited no significant impact on protein expression of RelA, Akt, or PDPN, respectively (Figures 2A-2C). By contrast, treatment of HEK293 with 1000 μM BPA led to a substantial reduction of RelA, Akt and PDPN, whereas, 1000 μM BPS had no significant impact on expression of these proteins post 24 hrs (Figures 2A-2C) shown is the modulation of protein expression of (A) RelA (B) Akt and (C) PDPN in HEK293 24 hrs after treatment of cells with 0.1-1000 μM BPA or BPS, respectively. Controls were incubated with the solvent ethanol (EtOH control). GAPDH expression was used for normalization of protein expression. Depicted is the mean ± SEM of at least 3 independent experiments (****) p ≤ 0.0001.
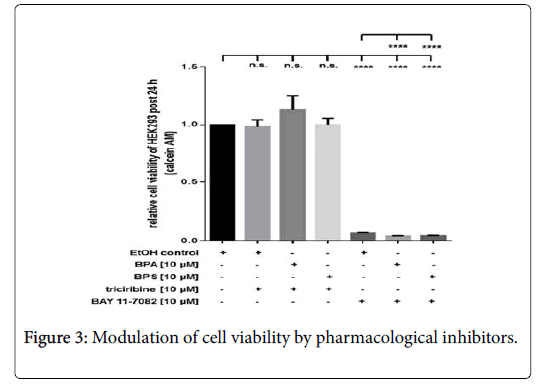
Inhibition of RelA, but not of Akt affects HEK293 cell viability
Compared to ethanol-treated controls (EtOH), pharmacological inhibition of Akt via triciribine (10 μM) had no inhibitory effect on cell viability of resting cells or HEK293 stimulated with 10 μM BPA or BPS, respectively (Figure 3). In contrast to triciribine, incubation of cells with 10 μM BAY 11-7082 led to a substantial reduction of HEK293 cell viability post 24 h (Figure 3). Additional application of 10 μM BPA or BPS, respectively, led to a further significant reduction of cell viability at this time point (Figure 3). Depicted it the HEK293 viability by measurement of calcein AM fluorescence at 490 nm excitation and 520 nm emission post 24 hrs. Cells were incubated with pharmacological inhibitors Akt (triciribine, 10 μM) or RelA-associated NFκB activity (BAY 11-7082, 10 μM), respectively. Additionally, cells were treated with or without BPA (10 μM) and BPS (10 μM). Controls were incubated with the solvent ethanol (equal amount; EtOH control). Shown is the mean ± SEM of at least 3 independent experiments (****) p ≤ 0.0001, (n.s.) no significant difference.
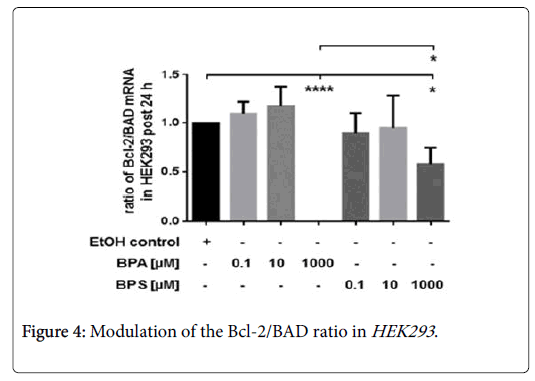
The impact of BPA and BPS on the Bcl-2/BAD ratio
Incubation of HEK293 with low concentration (0.1-10 μM) of BPA and BPS had no influence on the Bcl-2/BAD ratio post 24 hrs (Figure 4).
Compared to controls, treatment of renal cells with 1000 μM BPA or BPS, respectively, significantly reduced the ratio of Bcl-2/BAD. However, the inhibitory effect of 1000 μM BPA on the Bcl-2/BAD ratio was significantly stronger than that of 1000 μM BPS in HEK293 post 24 hrs (Figure 4). Here, we determined the impact of 0.1 μM-1000 μM BPA or BPS, respectively, on mRNA expression anti-apoptotic Bcl-2 and pro-apoptotic BAD in renal cells after 24 hrs. Depicted is the Bcl-2/BAD ratio. Compared were HEK293 treated with 10 μM BPA or 10 μM BPS, respectively. Control cells were incubated with the solvent ethanol (equal amount; EtOH control). Shown is the mean ± SEM of at least 3 independent experiments (*) p ≤ 0.05, (****) p ≤ 0.0001.
Discussion
BPA and BPS were demonstrated to mediate estrogenic-like characteristics [5]. Increased BPA concentrations were found to associate with an increased risk for the development of cardiovascular, hepatic as well as renal pathologies, such as coronary artery disease, fatty liver disease and chronic kidney disease [20-22]. In this regard, it was indicated that BPA induce various cytotoxic, genotoxic, and carcinogenic effects in vitro and in vivo [23-26]. Similar to BPA, it was shown that BPS mediated cardiotoxic as well as hepatotoxic effects too [6,22,27]. However, literature regarding the nephrotoxic potential of BPS, especially in comparison to other bisphenols, such as BPA are sparse. In this study, we performed a comparative analysis of BPAs and BPSs effects on cell viability and gene expression of human renal HEK293 cells. Here, we demonstrated for the first time that BPS affects HEK293 viability and expression of renal factors in a concentrationdependent manner. Moreover, we showed that BPA’s nephrotoxic impact on renal cell viability and gene expression was stronger than that of BPS in vitro.
In our experiments, we showed that BPA as well as BPS reduced cell viability in a concentration-dependent manner. Similar to BPS, low doses of BPA had no effects on HEK293 viability, whereas, cell treatment with high concentrations reduced cell viability post 24 hrs. A significant reduction of HEK293 viability was mediated by treatment of cells with 100 μM and 1000 μM BPA. In line with this, other groups also demonstrated BPA to modulate renal cell viability in a concentration-dependent manner in vitro [28-30]. In 2011, Kuo et al. showed that low BPA doses had no significant influence on canine renal tubular cell viability, whereas, high BPA concentrations (300 μM-500 μM) led to reduced cell viability post 24 hrs [29]. Substantiating this, Michalowicz and colleagues also depicted that incubation of human peripheral blood mononuclear cells with low BPA doses (0 μM-220 μM) induced no toxic effect, whereas, 220 μM-440 μM BPA significantly diminished cell viability [30].
In contrast to BPA, treatment of cells with a higher BPS dose of 100 μM had no inhibitory impact on HEK293 . However, incubation of cells for 24 hrs with 1000 μM BPS reduced cell viability, indicating concentration-dependent influence of BPS on renal cell viability too. Nevertheless, the inhibitory effect of 1000 μM BPA was significantly stronger than that of BPS in this setting. This in in line with the results of other groups [27,31]. In 2016, Zhang and colleagues also demonstrated that treatment of murine renal cells with 0 μM-300 μM BPS induced no cytotoxic effects, whereas, high BPS doses of 500 μM-1000 μM diminished renal cell viability post 12 hrs in a concentration-dependent manner [31]. Comparable results were obtained in another experimental setting in chicken embryonic hepatocytes [27]. Incubation of these cells for 36 hrs with 0 μM-10 μM BPA had no influence on viability. Stimulation of cells with higher doses of BPA (100 μM-300 μM) concentration-dependently decreased cell viability [27]. In contrast to BPA, no cytotoxic effects were found in hepatocytes treated with 0 μM-300 μM BPS [27].
Renal cell viability is orchestrated via an interplay of various factors modulating the balance of pro-survival vs. pro-apoptotic processes, such as Akt, PDPN or members of the Bcl-2 family [9,32]. On the one hand, Akt and PDPN plays an important role in regulating prosurvival signaling and structural integrity in renal cells [9,19,33]. On the other hand, NFκB-including its subunit RelA is of major importance for cell viability and apoptosis control, amongst others via modulating gene expression of pro-survival and pro-apoptotic Bcl-2 family members [7,32,34,35].
We found that low doses of BPA (0.1 μM-10 μM) had no influence, whereas 1000 μM BPA led to a nearly complete inhibition of Akt, PDPN, and RelA protein expression in HEK293 post 24 hrs. In line with our finding, Zhao and colleagues also depicted that low amounts of BPA (0 μM to 10 μM) had no significant impact on Akt expression in primary isolated murine ovaries ex vivo [36]. Further substantiating our data, Vahdati Hassani et al. showed in 2017 that treatment of Wistar rats with a high dose of BPA of 0.5 mg/kg for 30 d led to a reduced Akt protein expression in the liver of these animals [26]. The impact of BPS on Akt, PDPN, and RelA expression was so far unexplored, especially in the kidney. In this context, we showed for the first time that, in contrast to BPA, incubation of renal cells with low or high concentrations of BPS did not affect RelA or Akt expression in our setting. Moreover, expression of PDPN was moderately diminished by 1000 μM BPS. However, the inhibitory impact of BPA was significantly stronger than the effect of BPS in HEK293 . This indicates that BPS and BPS differentially affects expression of Akt, RelA and PDPN in HEK293 . Substantiating our observations, Boucher et al. recently demonstrated that treatment of human subcutaneous preadipocytes with BPA or BPS, respectively led to distinct modulations of the gene expression pattern [37]. In this context, they showed BPA to diminish the expression of NFκB-regulated factors, i.e. chemokine ligand 11 as well as mitogen-activated protein kinase pathways involved in intracellular signaling, whereas, BPS had no impact on these factors [37]. In another experimental setting, Peyre et al. also found BPS to be less cytotoxic that BPA [22]. In their study, the authors showed that 100 μM BPA reduced the expression of metabolism-related factors, such as fatty acid synthase. This was associated with an increased hepatotoxic potential of BPA [22]. In contrast to BPA, BPS treatment was less hepatotoxic and had no significant impact on gene expression of these factors [22]. In sum, these findings are in line with our observations. Members of the Bcl-2 family were demonstrated to be centrally involved in the regulation of pro-apoptotic processes in the renal system, which in turn plays an important role in modulating renal cell viability [10,32,34]. In our study, we depicted for the first time that BPA and BPS differentially affected the expression Bcl-2 and BAD in HEK293 in a concentrationdependent manner. Low amounts of BPS and BPA had no effect on the ratio of Bcl-2/BAD. Cell treatment with a high BPS dose (1000 μM) moderately reduced expression of pro-survival Bcl-2 vs. pro-apoptotic BAD. In contrast to BPS, 1000 μM BPA incubation let to a full dramatic reduction of the Bcl-2/BAD ratio in renal cells post 24 hrs. Bcl-2 was shown to be a pro-survival factor, whereas, BAD was is a pro-apoptotic member of the Bcl-2 family [35,38]. In this context, a reduction of the Bcl-2/BAD ratio corresponds to a shift of HEK293 towards a pro-apoptotic phenotype and reduced renal cell viability. In our experimental setting, the reduced level of Bcl-2 vs. BAD corresponds to the rigorous reduction of HEK293 viability found in cells incubated with higher doses of BPA, compared to BPS treated HEK293 . Substantiating our findings, Bontempo et al. also found that treatment of leukemia NB4 cells with 60 μM BPA led to an increased activation of pro-apoptotic BAD and induced cell apoptosis [25]. In another experimental setting, Zhang et al. also demonstrated that treatment of goat sertoli cells with high dose BPA (500 μM) led to an increased ration of the pro-apoptotic Bcl-2 family member BAX vs. pro-survival Bcl-2 which was associated with reduced viability of these cells [39]. Regarding the impact of BPS on the expression of Bcl-2 members, Liu and colleagues recently showed that long-term treatment of zebrafish with high concentration of BPS only induced moderate changes of the ratio of pro-apoptotic vs. pro-survival Bcl-2 family member in zebrafish eyes [40]. Furthermore, they showed that this was associated to a moderate injury in the visual system of these animals [40]. These data are in line with our results and corresponds well to our observations made in HEK293.
NFκB as well as the members of the Bcl-2 family are of great significance for regulating cell viability and apoptosis in general [7,32,34,35]. In this context, expression of Bcl-2 family members, such as the pro-survival factor Bcl-2 was shown to be regulated by NFκB [41,42]. Here, we demonstrated that treatment of HEK293 with 1000 μM BPA led to reduced renal cell viability, which was associated with diminished expression of the NFκB subunit RelA and the Bcl-2/BAD ratio. These data indicate an important role of NFκB for modulating renal cell viability post BPA treatment. Therefore, we here shed further light on this issue. In our experiments, we found that inhibition of RelA-associated NFκB activity by BAY 11-7082 nearly completely diminished HEK293 viability in resting as well as in BPA or BPStreated cells, suggesting a significant role of NFκB in this context. In line with our data, Garcia et al. also depicted that inhibition of NFκB by BAY 11-7082 increased cell apoptosis of murine leukemia cells [43]. Moreover, the importance of NFκB for the control of kidney cell viability was further substantiated by findings of Dieguez-Acuña and colleagues [44]. They depicted that NFκB activity determines the sensitivity of kidney epithelial cells to apoptosis and that this play a central role in the pathogenesis of toxin-induced renal injury and failure [44]. Therefore, these data suggests RelA-associated NFκB activity to play a major role in renal cell viability control, which may be mediated via its important function in gene expression regulation.
Conclusion
In this study, we performed a comparative analysis of the impact of BPA vs. BPS on renal cell viability and gene expression in human HEK293 cells. We showed for the first time that both bisphenols exhibited differential effects on these biological processes. We demonstrated BPA to reduce renal cell viability and expression of Akt, RelA, PDPN and the Bcl-2/BAD ratio in a concentration-dependent manner, indicating a high nephrotoxic potential of this compound. Moreover, we found that the cytotoxic potential of BPS in HEK293 was substantially lower than that of BPA. Compared to BPA, BPS exhibited a much lower cell viability-reducing potential on HEK293 , and just at the highest tested dose. In this context, we demonstrated for the first time that BPS did not affect the generation of Akt and RelA in HEK293 and exhibited only a moderate inhibitory effect on the expression of PDPN and the Bcl-2/BAD ratio.
In summary, these data indicate that BPS may be less cytotoxic than BPA in renal cells. However, further studies are necessary to validate these first experimental in vitro results, especially in the context of adequate in vivo settings.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgement
We thank Dr. Anja Brehm for substantial methodological and technical support. Moreover, we thank Dr. Andreas Zakrzewicz for its mental support and its helpful advice in the context of academic matters in general.
References
- Schecter A, Malik N, Haffner D, Smith S, Harris TR, et al. (2010) Bisphenol A (BPA) in US food. Environ Sci Technol 44: 9425-9430.
- Chen Y, Shu L, Qiu Z, Lee DY, Settle SJ, et al. (2016) Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLoS Genet 12: e1006223.
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA (2012) The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 354: 74-84.
- Olea-Herrero N, Arenas MI, Munoz-Moreno C, Moreno-Gomez-Toledano R, Gonzalez-Santander M, et al. (2014) Bisphenol-A induces podocytopathy with proteinuria in mice. J Cell Physiol 229: 2057-2066.
- Le Fol V, Ait-Aissa S, Sonavane M, Porcher JM, Balaguer P, et al. (2017) In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf 142: 150-156.
- Gao X, Ma J, Chen Y, Wang HS (2015) Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: Evidence of female-specific proarrhythmic effects. Environ Health Perspect 123: 571-578.
- Leppert U, Gillespie A, Orphal M, Bohme K, Plum C, et al. (2017) The impact of alpha-Lipoic acid on cell viability and expression of nephrin and ZNF580 in normal human podocytes. Eur J Pharmacol 810: 1-8.
- Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, et al. (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine) 536 phosphorylation. Diabetes 60: 1907-1916.
- Eisenreich A, Langer S, Herlan L, Kreutz R (2016) Regulation of podoplanin expression by microRNA-29b associates with its antiapoptotic effect in angiotensin II-induced injury of human podocytes. J Hypertens 34: 323-331.
- Wu H, Cao L, Li F, Lian P, Zhao J (2015) Multiple biomarkers of the cytotoxicity induced by BDE-47 in human embryonic kidney cells. Chemosphere 126: 32-39.
- Pastore D, Della-Morte D, Coppola A, Capuani B, Lombardo MF, et al. (2015) SGK-1 protects kidney cells against apoptosis induced by ceramide and TNF-alpha. Cell Death Dis 6: e1890.
- Lan A, Du J (2015) Potential role of Akt signaling in chronic kidney disease. Nephrol Dial Transplant 30: 385-394.
- Eisenreich A, Malz R, Pepke W, Ayral Y, Poller W, et al. (2009) Role of the phosphatidylinositol 3-kinase/protein kinase B pathway in regulating alternative splicing of tissue factor mRNA in human endothelial cells. Circ J 73: 1746-1752.
- Eisenreich A, Zakrzewicz A, Huber K, Thierbach H, Pepke W, et al. (2013) Regulation of pro-angiogenic tissue factor expression in hypoxia-induced human lung cancer cells. Oncol Rep 30: 462-470.
- Eisenreich A, Boltzen U, Poller W, Schultheiss HP, Rauch U (2008) Effects of the Cdc2-like kinase-family and DNA topoisomerase I on the alternative splicing of eNOS in TNF-alpha-stimulated human endothelial cells. Biol Chem 389: 1333-1338.
- Eisenreich A, Rauch U (2013) Regulation of the tissue factor isoform expression and thrombogenicity of HMEC-1 by miR-126 and miR-19a. Cell Biol: Res Ther 2.
- Eisenreich A, Bogdanov VY, Zakrzewicz A, Pries A, Antoniak S, et al. (2009) Cdc2-like kinases and DNA topoisomerase I regulate alternative splicing of tissue factor in human endothelial cells. Circ Res 104: 589-599.
- Eisenreich A, Boltzen U, Malz R, Schultheiss HP, Rauch U (2011) Overexpression of alternatively spliced tissue factor induces the pro-angiogenic properties of murine cardiomyocytic HL-1 cells. Circ J 75: 1235-1242.
- Langer S, Kreutz R, Eisenreich A (2016) Metformin modulates apoptosis and cell signaling of human podocytes under high glucose conditions. J Nephrol 29: 765-773.
- Hu J, Wang Y, Xiang X, Peng C, Gao R, et al. (2016) Serum bisphenol A as a predictor of chronic kidney disease progression in primary hypertension: a 6 year prospective study. J Hypertens 34: 332-337.
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, et al. (2012) Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125: 1482-1490.
- Peyre L, Rouimi P, de Sousa G, Helies-Toussaint C, Carre B, et al. (2014) Comparative study of bisphenol A and its analogue bisphenol S on human hepatic cells: A focus on their potential involvement in nonalcoholic fatty liver disease. Food Chem Toxicol 70: 9-18.
- Gassman NR (2017) Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen 58: 60-71.
- Gowder SJ (2013) Nephrotoxicity of bisphenol A (BPA): An updated review. Curr Mol Pharmacol 6: 163-172.
- Bontempo P, Mita L, Doto A, Miceli M, Nebbioso A, et al. (2009) Molecular analysis of the apoptotic effects of BPA in acute myeloid leukemia cells. J Transl Med 7: 48.
- Vahdati Hassani F, Mehri S, Abnous K, Birner-Gruenberger R, Hosseinzadeh H (2017) Protective effect of crocin on BPA-induced liver toxicity in rats through inhibition of oxidative stress and downregulation of MAPK and MAPKAP signaling pathway and miRNA-122 expression. Food Chem Toxicol 107: 395-405.
- Ma M, Crump D, Farmahin R, Kennedy SW (2015) Comparing the effects of tetrabromobisphenol-A, bisphenol A, and their potential replacement alternatives, TBBPA-bis(2,3-dibromopropyl ether) and bisphenol S, on cell viability and messenger ribonucleic acid expression in chicken embryonic hepatocytes. Environ Toxicol Chem 34: 391-401.
- Abdul Kafi M, Kim TH, Yagati AK, Kim H, Choi JW (2010) Nanoscale fabrication of a peptide layer in cell chip to detect effects of environmental toxins on HEK293 cells. Biotechnol Lett 32: 1797-1802.
- Kuo CC, Huang JK, Chou CT, Cheng JS, Tsai JY, et al. (2011) Effect of bisphenol A on Ca(2+) fluxes and viability in Madin-Darby canine renal tubular cells. Drug Chem Toxicol 34: 454-461.
- Michalowicz J, Mokra K, Bak A (2015) Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol In Vitro 29: 1464-1472.
- Zhang R, Liu R, Zong W (2016) Bisphenol S interacts with catalase and induces oxidative stress in mouse liver and renal cells. J Agric Food Chem 64: 6630-6640.
- Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80: 29-40.
- Canaud G, Bienaime F, Viau A, Treins C, Baron W, et al. (2013) AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288-1296.
- Bao H, Ge Y, Peng A, Gong R (2015) Fine-tuning of NFκB by glycogen synthase kinase 3beta directs the fate of glomerular podocytes upon injury. Kidney Int 87: 1176-1190.
- Del Principe MI, Del Poeta G, Venditti A, Buccisano F, Maurillo L, et al. (2005) Apoptosis and immaturity in acute myeloid leukemia. Hematology 10: 25-34.
- Zhao Q, Ma Y, Sun NX, Ye C, Zhang Q, et al. (2014) Exposure to bisphenol A at physiological concentrations observed in chinese children promotes primordial follicle growth through the PI3K/Akt pathway in an ovarian culture system. Toxicol In Vitro 28: 1424-1429.
- Boucher JG, Gagne R, Rowan-Carroll A, Boudreau A, Yauk CL, et al. (2016) Bisphenol A and bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS One 11: e0163318.
- Boltzen U, Eisenreich A, Antoniak S, Weithaeuser A, Fechner H, et al. (2012) Alternatively spliced tissue factor and full-length tissue factor protect cardiomyocytes against TNF-alpha-induced apoptosis. J Mol Cell Cardiol 52: 1056-1065.
- Zhang Y, Han L, Yang H, Pang J, Li P, et al. (2017) Bisphenol A affects cell viability involved in autophagy and apoptosis in goat testis sertoli cell. Environ Toxicol Pharmacol 55: 137-147.
- Liu W, Zhang X, Wei P, Tian H, Wang W, et al. (2017) Long-term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio). J Appl Toxicol 38: 248-258.
- Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, et al. (2003) NF- kappa B2/p100 induces Bcl-2 expression. Leukemia 17: 1349-1356.
- Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr (1999) NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 19: 5923-5929.
- Garcia MG, Alaniz L, Lopes EC, Blanco G, Hajos SE, et al. (2005) Inhibition of NF-kappaB activity by BAY 11-7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leuk Res 29: 1425-1434.
- Dieguez-Acuna FJ, Polk WW, Ellis ME, Simmonds PL, Kushleika JV, et al. (2004) Nuclear factor kappa B activity determines the sensitivity of kidney epithelial cells to apoptosis: implications for mercury-induced renal failure. Toxicol Sci 82: 114-123.
Citation: Podlich S, Plum C, Bohme K, Kreutz R, Leppert U, et al. (2018) Comparative Analysis of Cytotoxic Effects of Bisphenol A and Bisphenol S on Human Renal HEK293 Cells. Arch Sci 2: 114.
Copyright: © 2018 Eisenreich A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 7689
- [From(publication date): 0-2018 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 6542
- PDF downloads: 1147