Comparison of Anthropometric and Body Composition Outcomes between Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy: A Narrative Review
Received: 07-Jun-2022 / Manuscript No. JOWT-22-66051 / Editor assigned: 08-Jun-2022 / PreQC No. JOWT-22-66051(PQ) / Reviewed: 22-Jun-2022 / QC No. JOWT-22-66051 / Revised: 27-Jun-2022 / Manuscript No. JOWT-22-66051(R) / Published Date: 04-Jul-2022 DOI: 10.4172/2165-7904.1000501
Abstract
Bariatric surgery is a well-accepted treatment option for sustained weight loss and improvement in comorbid conditions; however, it is unclear which procedure (laparoscopic Roux-en-Y gastric bypass [LRYGB] or sleeve gastrectomy [LSG]) has the greatest effect on anthropometric and body composition outcomes. It is important to ascertain which bariatric procedure is most effective at maximizing excess weight loss and determining what body compartment is most affected so that individualized nutrition and physical activity interventions can be implemented. This article is a review of the literature published from 2016 to 2021 that investigates the effect of LRYGB compared to LSG on anthropometric and body composition parameters. Although the results indicate that clinical outcomes are comparable for LRYGB and LSG, further long-term research are warranted.
Keywords
Bariatric surgery; Body composition; Roux-en-Y gastric bypass; Sleeve gastrectomy
Introduction
The obesity epidemic continues to be a significant public health concern in the United States (US). According to the Centers for Disease Control and Prevention, approximately 42.5% of US adults aged 20 and above were obese, and 9.2% were severely obese in 2017-2018 [1,2]. Obesity is correlated with an increased probability of developing co-morbidities such as cardiovascular disease, type 2 diabetes (T2DM), and certain cancers [2]. In addition, the cumulative medical costs for a person with obesity were $260.6 billion in 2016 and these individuals paid $2,505 more for medical care than their healthyweight counterparts [3].
People with obesity that have been unsuccessful with traditional behavioral and medical management may be candidates for bariatric surgery. The National Institutes of Health (NIH) consensus statement indicates that adults who have a body mass index (BMI) of 35 to 40 kg/ m2 with co-morbidities or greater than 40 kg/m2 without co-morbidities are potential candidates for bariatric surgery [4]. The American Society for Metabolic and Bariatric Surgery (ASMBS) reports 256,000 bariatric surgeries were performed in 2019, reflecting less than 1% of the US population currently eligible for these surgeries based on the NIH criteria [5]. The most common procedures were the laparoscopic sleeve gastrectomy (59.4% LSG) and laparoscopic Roux-en-Y gastric bypass (17.8% LRYGB) [5]. Bariatric surgery is considered the most effective treatment modality for long-lasting weight loss success, resolution of metabolic co-morbidities, and improved mortality [6,7]. In a study that examined data from systematic reviews and metaanalyses, McGrice et al. [8] estimated that excess weight loss (% EWL) at 10-years following bariatric surgery was 53.6% for RYGB and 47.2% for SG. Moreover, when bariatric surgery was compared with lifestyle intervention, Vitiello et al. [9] found that individuals undergoing surgical intervention maintained an excess BMI loss (% EBMIL) of 69.1% compared to 14.6% EBMIL in patients that received lifestyle intervention alone (i.e., dietitian supervised diet program and exercise regimen).
The primary endpoint of prior literature that investigated the effectiveness of LRYGB versus LSG has focused more on anthropometric outcomes, such as weight loss. The Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) was a large multicenter, randomized controlled trial (RCT) conducted in 2007-2011 that explored the effect of LSG (n = 107) and LRYGB (n = 110) on weight loss, change in co-morbidities, adverse events, and some quality of life indices for 3,971 patients with clinically severe obesity [10]. Peterli et al. [10] found that patients with a LSG experienced a % EBMIL of 61.1% while the LRYGB group lost 68.3% at five years postoperative; however, the results were not significantly different after adjustment (P = 0.22, 95% CI: -14.30 to – 0.06). In a systematic review and meta-analysis that compared mid-and longterm weight loss between LSG and LRYGB, eight of the 14 studies (n = 2,486) evaluated mid-term weight loss (3-5 years), and six (n = 1,642) assessed long-term weight loss (≥ 5 years) between the two bariatric procedures [11]. Findings indicate that there was not a significant difference in mid-term weight loss between LRYGB and LSG (P = 0.88, 95% CI -0.38 to -0.33), but long-term weight loss success was better in the LRYGB group (P = 0.005, 95% CI: -0.25 to 0.05) [11]. In contrast, a recent systematic review and meta-analysis based on 18 studies, Han et al. [12] found comparable excess weight loss results between LSG and LRYGB (P = 0.36, 95% CI: -0.52 to 0.19) [12]. These studies highlight the inconsistencies in results on whether the LRYGB or LSG is more effective in improving anthropometric metrics [11-13].
Bariatric surgery is a well-accepted treatment option for sustained weight loss and improvement in comorbid conditions [14,15]; however, it is unclear which bariatric procedure has the greatest effect on anthropometric and body composition outcomes. It is important to determine which procedure (i.e. LRYGB or LSG) is most effective at amplifying excess weight loss and determine what body compartment is most affected so that targeted, patient-centered nutrition and physical activity strategies can be implemented. While reviews have done a comparative analysis of LRYGB and LSG in relation to anthropometrics and resolution of co-morbidities [11,12] we are not aware of any that compare the effect of these two bariatric procedures on anthropometric and body composition outcomes. This narrative review will explore the effect of LRYGB compared to LSG in adults with clinically severe obesity on anthropometric and body composition outcomes (i.e., weight loss, BMI, fat mass [FM], fat-free mass [FFM], lean mass [LM], and percent body fat [% BF]).
Literature Search Strategy
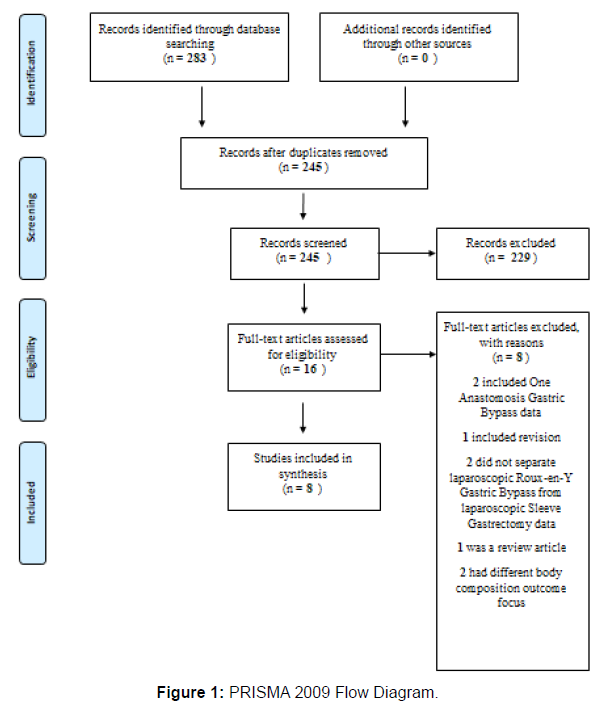
The CINAHL, Web of Science, and PubMed databases were searched to answer the following population, intervention, control, and outcome (PICO) question: Among adult patients undergoing bariatric surgery, what is the impact of Roux-en-Y gastric bypass compared to sleeve gastrectomy on anthropometric and body composition measurements? The search terms included key terms and medical subject headings of (gastric bypass surgery, RYGB, LRYGB, Rouxen- Y gastric bypass) AND (gastric sleeve surgery, LSG, SG) AND (body composition or body fat or fat-free mass or lean body mass) AND (weight or BMI or body mass index). Articles were restricted to primary research articles published in the English language, included adults who were at least 18 years of age, and published between 2016 and 2021. References were searched for additional studies that met the criteria for inclusion. The final articles were evaluated based on the exclusion criteria that consisted of non-English language, publications before 2016, patients who were under 18 years of age, did not compare LRYGB to LSG, review articles, and non-human studies. Although 283 records were found during the database search, following title and abstract screening only 16 remained and were evaluated for eligibility. Eight original research studies met the criteria for further review and analysis as shown in Figure 1 PRISMA Flow Diagram [12]. The lead author in consultation with co-authors provided a quality rating for each study as shown in the Table of Related Literature (Table 1) based on the Academy of Nutrition and Dietetics Evidence Analysis Library (EAL) Quality Criteria Checklist: Human Subjects [16].
Study Characteristics
The sample sizes of the eight reviewed articles consisted of 918 study participants ranging from 39 to 295 participants per study [17- 24]. The studies were conducted in Brazil [24], Switzerland [17,23], United States [19], Spain [21], Iran [18], Singapore [20], and Germany [22]. The postoperative follow-up appointments occurred at six months in two studies [18,24], 12 months in five studies [19-23], 24 months in three studies [20,21,23], 36 months in two studies [20,21], and after five or more years in two studies [17,21]. ASMBS categorizes bariatric research into short-term (< 3 years), mid-term (> 3 and < 5 years), or long-term (≥5 years) studies based on the duration of the study after bariatric surgery [25]. The majority of the studies (Venancio et al. [24], Kavanagh et al. [19], Golzarand et al. [18], Otto et al. [22], Kim et al. [20], and Schneider et al. [23]) included in this review were short-term studies that ranged from six months to three years in duration. In contrast, Martinaitis et al. [21] was a medium-term study, while Buhler et al. [17] was a long-term study. The study designs were primarily nonrandomized, cross-sectional, or cohort studies [17-22,24]. There was one study conducted by Schneider et al. [23] that utilized data from the multicenter RCT known as the SM-BOSS Study [13].
Bariatric Data Extraction and Reporting
Weight loss is one of the primary outcome measures routinely described after bariatric surgery and is often used as a barometer of surgical success [26]. Although absolute or total weight loss (TWL) may be clinically meaningful, it may not be the best anthropometric measure for comparisons across studies [25]. At this time, there is no universal standard for reporting weight loss [25]. In 2015, the ASMBS outlined recommendations for a uniform process of reporting outcomes in metabolic and bariatric surgery to improve consistency of data collection and allow investigators to compare results across programs and medical literature more easily [25]. The ASMBS guidelines advocate for the use of bariatric surgery outcomes such as mean baseline weight and BMI as close to the time of surgical intervention as medically feasible [25]. Changes in BMI, percent of total weight loss (% TWL), % EWL, and % EBMIL should also be reported [25]. The ASMBS did not address body composition outcomes in the bariatricrelated reporting guidance [25]. The Table of Related Literature (Table 1) describes recent studies that addressed our research question and included the recommended standardized outcomes reporting data, if available, and body composition Table 2 defines each of the ASMBS recommended standardized reporting outcomes.
Anthropometric Measurements
Comparison of % TWL, % EWL, and BMI between LRYGB and LSG
Percent EWL is excess weight lost by the patient expressed as a percent of total excess weight [25]. It is a valuable metric to compare data for individuals who have varying amounts of excess weight to lose and different baseline weights [25]. LRYGB and LSG in all eight studies were effective procedures for promoting sustained % EWL over six months and up to five years [17-22,24,27]. Golzarand et al. [18], Kavanagh et al. [19], Kim et al. [20], and Otto et al. [22], all investigated the effectiveness of bariatric surgery on anthropometric and body composition and found no significant differences in % EWL for patients who had a LRYGB compared to LSG at six months and one year postoperative after BMI adjustments. In a secondary analysis of data samples from adult patients (N = 295) with clinically severe obesity that underwent bariatric surgery, Kim et al. [20] found that % EWL was higher in the LRYGB group compared to the LSG group at one (77.5±30.4% vs. 71.8±30.5%), two (77.3±37.2% vs. 66.8±46.6%) and three years (67.7±32.5% vs. 64.3±37.8%) postoperative; however, the results were not significant (P > 0.05) [20]. This finding by Kim et al. [20] is consistent with studies by Kavanagh et al. [19] (N = 63) and Otto et al. [22] (N = 173) that also found that LRYGB resulted in greater % EWL compared to LSG at one year, but differs from Golzarand et al. [18] (N = 43) that found LSG resulted in better % EWL at six months postoperative. Study participants in the LRYGB group in Golzarand et al. [18] weighed more than individuals in the LSG group, which required data adjustment for baseline weight. Evidence suggests that a higher baseline weight and BMI are associated with greater % EWL outcomes in those undergoing a LRYGB compared to LSG [28]. The % EWL results by Kavanagh et al. [19], Otto et al. [22], and Golzarand et al. [18] were not statistically significant; therefore, further research is necessary to determine if LRYGB is truly more efficacious at promoting % EWL compared to LSG. According to the ASMBS, % EWL is expected to be approximately 60% at six months and 77% at 12 months postoperative [29]. In this review, % EWL for the study participants in Kim et al. [20] was comparable to the expected weight loss after LRYGB and LSG at one year postoperative (77.5±30.4% vs. 71.8±30.5%) while studies by Kavanagh et al.19 (53.4% vs. 47.2%, P = 0.165) and Otto et al.22 (62.9% vs. 52.3±15.0%, P = 0.86 after BMI adjustment) at one year and Golzarand et al. [18] (52.3±13.4% vs. 66.4±23.8%, P > 0.05) at six months postoperative fell below the expected % EWL outcome [19,20,22]. A majority of the study participants in Kim et al. [20] (87%) and Otto et al. [22] (73%) underwent LRYGB compared to 47% of study participants in Kavanagh et al. [19] and 51% in Golzarand et al. [18]. Also, the LRYGB group had Roux-en-Y and biliopancreatic limbs that ranged from 100 to 150 cm and 50 to100 cm, respectively [18- 20,22]. Differences in bariatric operative techniques, including gastric pouch size and alimentary limb measurements, could also influence the amount of excess weight lost postoperative by causing variability in the study participants' food consumption and absorptive capacity [30].
Recent studies have cast doubt on whether % EWL is the best approach to evaluate weight loss outcomes after bariatric surgery [31,32]. Corcelles et al. [32] conducted a 12-month retrospective study investigating the best outcome measure to assess weight loss postbariatric surgery in 2,420 US patients. The findings indicated that % TWL loss was superior to % EWL since it was not influenced as much by preoperative BMI [32]. % TWL is defined as the difference in initial weight and postoperative weight as a proportion of initial weight [25]. In this review, three out of eight studies assessed weight loss based on % TWL [17,20,22]. Study participants in Kim et al. [20] had a % TWL of 26.1±7.7% in the LRYGB group compared to 26.3±9.8% in the LSG group (P > 0.05) at one year post-operative; conversely, people included in Otto et al. [22] experienced a % TWL of 31.7±8.4% in the LRYGB group compared to 30.48±6.7% in the LSG group (P > 0.4). Kim et al. [20] also found that % TWL remained stable at two years postoperative (LRGB 25.7±9.5 vs. LSG 26.9±17.3%, P > 0.5), but there was some weight regain noted at three years postoperative (LRYGB 23.7±10.1% vs. LSG 23.9±11.1, P > 0.05); however, the results were not statistically significant. While Kim et al. [20] and Otto et al. [22] demonstrated successful weight loss in the short term, Buhler et al. [17] found that both the LRYGB and the LSG were effective at long-term weight loss after five or more years postoperative. None of the % TWL outcomes in Kim et al., [20] Otto et al., [22] or Buhler et al. [17] were statistically significant between LRYGB compared to LSG.
BMI declined across all studies at six months [18,24], one year [20,22], and two years after bariatric surgery [20]. Buhler et al. [17] (N = 142) investigated the effect of LRYGB compared to LSG on body composition at five years or more after bariatric surgery and demonstrated sustained weight loss based on BMI and % TWL for both bariatric procedures; however, some recidivism occurred at three years in the study by Kim et al. [20] Although the baseline BMI for study participants in Buhler et al. [17] and Kim et al. [20] were comparable between treatment groups, the majority of the study participants in Kim et al. [20] underwent a LSG. Many were lost to follow-up by year three, which may explain the differences in results between the two studies [17,20]. Short-term, prospective studies conducted by Venancio et al. [24] (N = 39) and Golzarand et al. [18] (N = 43) found no significant differences in BMI between the LRYGB compared to the LSG at six months postoperative (P = 0.749 and P > 0.05, respectively). Also, Kim et al. [20] found no significant differences in BMI for LRYGB compared to LSG at one, two, or three years postoperative (P > 0.05). Similarly to absolute or total weight loss (TWL), absolute change in BMI may not be the best way to compare results across studies. Another way to assess differences in BMI is to calculate the % EBMIL. [25] Martinaitis et al. [21] (N = 121) examined long-term outcomes after LRYGB compared to LSG up to five years postoperative in patients with super obesity. The authors found that LRYGB was superior to LSG in regards to % EBMIL at one (65.2% vs. 46.7%, P = 0.002), two (65.8% vs. 44.9%, P = 0.004), three (64.4% vs. 30.5%, P = 0.001), and five years (55.6% vs. 17.6%, P = 0.016) after bariatric surgery. [21] A few potential reasons why Martinaitis et al. [21] found a difference in % EBMIL between the two bariatric procedures could be related to significant differences in baseline demographics with a higher initial BMI (P = 0.006) and weight (P = 0.003) in the LSG group coupled with declining followup compliance. Moreover, % EBMIL for people with super obesity was greater in the LRYGB at one year (58.7% vs. 40.9%, P = 0.015), two years (62.8%, vs. 43.0%, P = 0.033) three years (60.2% vs. 35.1%, P = 0.031), and five years (56.7% vs. 16.9%, P = 0.013) postoperative compared to their LSG counterparts [21]. The results by Martinaitis et al. [21] are consistent with Schneider et al. [23] (N = 43) who found % EBMIL was greater in the LRYGB compared to the LSG group (76.4±22.2% vs. 64.4±24.2%, P < 0.046) up to two years postoperative. There were no significant differences between the LRYGB and LSG groups at baseline for age, gender, BMI, or co-morbidities in the study conducted by Schneider et al. [23]. Additionally, Schneider et al. [23], found that study participants without DM had a higher % EBMIL than individuals with DM (74.9±25.8% vs. 55.8±23.9%, P = 0.52); however, the results were not statistically significant. Schneider et al. [23] was the only study in this review that examined the role of DM status on postoperative bariatric outcomes; therefore, more research in this area is warranted.
Body Composition
As people lose weight, their % BF and FM decline, but this is often accompanied by a loss in LM or FFM which can impact their overall health [33-36]. Zalesin et al. [36] used dual-energy x-ray absorptiometry (DEXA) to evaluate FM and LM in patients with clinically severe obesity after bariatric surgery and found a rapid decline in both body composition metrics despite study participants completing an exercise program. Loss of LM can result in a decrease in metabolic rate and subsequently lead to weight regain [33]. The comparison of body composition outcomes is another way to evaluate the effectiveness of LRYGB versus LSG. Table 1 contains body composition data (i.e. FFM, FM, LM, and % BF) for the eight studies included in this review. Comparison of FM, FFM, and LBM changes between LRYGB and LSG
| Author, Year, Study Design, Country, Funding Source |
Quality Grade (+, -, Ø) |
Study Purpose | Study Population (Demographics) |
Intervention and Setting | Outcome Data | Conclusions/ Results | Limitations of Findings |
|---|---|---|---|---|---|---|---|
| Venancio FA, Almeida LA, et al.24 Year: 2021 Study Design: Prospective Cross sectional Class Rating: D Country: Brazil Funding Source: Espirito Santo Research and Innovation Support grant and a partial scholarship |
Ø | To explore outcomes for patients with obesity who underwent LRYGB compared to LSG at 6-months postoperative. | N = a convenience sample of 39 adults who underwent bariatric surgery. Aged 18 to 60 years old. BMI > 40 kg/m2 or > 35 kg/m2 plus co-morbidities and psychological testing. Exclusion criteria included pregnancy, pacemaker users, and subjects with metal implants. Demographics Mean Age (years) LRYGB: 41.2±7.8 LSG: 42.9±5.3 Sex (n M/F) LRYGB: 5/20 LSG:2/12 Anthropometric Mean BMI (kg/m2) LRYGB: 43.7±5.9 LSG: 42.3±6.8 % Co-morbidities (LRYGB/LSG) DM: 52 / 21 HTN: 68 / 50 Dyslipidemia: 56 / 14 Attrition Rate: 18.8% |
Intervention: Subjects underwent bariatric surgery of either n = 25 LRYGB or n = 14 LSG Surgical Technique: N/A Anthropometric and Body Composition Body weight and height were measured using a scale and stadiometer, respectively. Biodynamics 450 BIA (Biodynamics Co., Shoreline, WA, USA) was used to assess body composition. Setting: Bariatric and Metabolic Surgery Program of the Espirito Santo University Hospital |
Anthropometrics Weight kg (6 months): N/A Median BMI kg/m2 (6 months) LRYGB: 31.6 (28.5-34.9) LSG: 32.6 (30.8-34.8) (Overall, there was no significant difference in BMI between LRYGB compared to LSG at 6 months, P = 0.749) Body Composition Median % FM (6 months) LRYGB: 39.6 (35.9-43.3) LSG: 43.2 (38.0-44.7) (Overall, there was no significant difference in FM% between LRYGB compared to SG at 6 months, P = 0.156) Median FFM kg (6 months) LRYGB: 48.4 (43.8-56.2) LSG: 48.9 (43.8-51.3) (Overall, there was no significant difference in FFM between LRYGB compared to LSG at 6 months, P = 0.803) |
Significant reductions occurred in BMI, % FM, and FFM at 6 months after bariatric surgery (P < 0.001). Anthropometric and body composition between LSG and LRYGB were comparable. | Strengths
|
| Buhler J, Rast S, et al.17 Year: 2021 Study Design: Non-randomized Prospective Cross sectional Study Class Rating: D Country: Basel, Switzerland Funding Source: Uniscientia Foundation and Peterli J is a consultant for Johnson & Johnson |
Ø | To assess the effects of LRYGB compared to LSG on body composition and bone mineral density after at least 5 years postoperative. | N = 142 adults who underwent bariatric surgery. Demographics Median Age (years) LRYGB: 45 (37-52.75) LSG: 46 (33.5-54.25) Sex (n M/F) LRYGB: 27/43 LSG: 26/46 Anthropometrics Median Weight (kg) LRYGB: 124.55 (109.37-141.50) LSG: 127.10 (109.07-148.25) Median BMI (kg/m2) LRYGB: 41.90 (39.15-46.03) LSG: 44.35 (41.03-49.80) Body Composition Median FM (kg) LRYGB: 49.20 (44.90-56.65) LSG: 51.45 (45.62-58.67), P = 0.109 Median % Body Fat LRYGB: 44.50 (39 LSG: 43.65 (39.25-46.58), P = 0.043 Median LM (kg) LRYGB: 63.40 (55.90-74.40) LSG: 61.80 (56.62- 78.90), P = 0.117 Co-morbidities (%) (LRYGB/LSG) DM: 34.3 / 18.1 HTN: 65.7 / 62.5 Dyslipidemia: 80 / 77.8 Cardiopathy: 5.6 / 5.7 Kidney disease: 8.6 / 2.8 Thyroid disease: 5.6 / 1.4 Attrition Rate: 0% |
Intervention: Subjects underwent bariatric surgery of either n = 70 LRYGB or n = 72 LSG Surgical Technique: N/A Anthropometric and Body Composition: Baseline health data were received from the patient medical records. Hologic QDR 4500 DEXA scanner (Hologic Inc; Bedford, MA, USA) was used to measure body composition. Setting: Department of Endocrinology and Nutrition in St. Claraspital Basel and St. Clara Research Ltd in Basel, Switzerland |
Anthropometrics Median Weight kg (≥ 5 years) LRYGB: 90.00 (76.25-106.80) LSG: 96 (79.07-110.55) % TWL (≥ 5 years) RYGB: 26.3% LSG: 24.1% (P = 0.243) Median BMI kg/m2 (≥ 5 years) LRYGB: 31.20 (28.20-34.15) LSG: 33.05 (28.70-39.20) Body Composition Median FM kg (≥ 5 years) LRYGB: 34.8 (28.85-41.40) LSG: 38.95 (31.52-46.3), P = 0.445 Median % Body Fat (≥ 5 years) LRYGB: 40.80 (36.55-45.35) LSG: 44.10 (38.35-47.70), P = 0.414 Median LM kg (≥ 5 years) LRYGB: 45.30 (41.45-59.10) LSG: 47.85 (40.62-55.85), P = 0.014 (LM loss was higher in the LSG group (P = 0.059) while LRYGB had higher loss of total weight (P value not reported) and FM (P value not reported) for individual at the median age.) |
Overall, both procedures have comparable results for anthropometric and body composition indices at ≥ 5 years postoperative except for absolute LM. LRYGB group had a slightly lower LM than the LSG group. | Strengths
|
| Golzarand M, Toolabi K, et al.18 Year: 2019 Study Design: Prospective Cross sectional Study Class Rating: D Country: Tehran, Iran Funding Source: None |
Ø | To examine outcomes in body composition, diet, and substrate oxidation after RYGB compared to SG at 6 months postoperative. | N = 43 adult patients who underwent bariatric surgery in January 2017 to September 2017. Met guidelines for bariatric surgery - BMI > 40 kg/m2 or ≥ 35 kg/m2 plus co-morbidities and had to be at least 19 years of age. Exclusion criteria consisted of prior bariatric surgery, thyroid condition, drug/alcohol abuse, pregnancy/lactation, and any heart, lung, kidney, or psychiatric diseases. Demographics Mean age (years) LRYGB: 40.6±6.8 LSG: 40.3±12.7 Sex (n M/F) LRYGB: 1 / 21 LSG: 0/21 Anthropometrics Mean Weight (kg) LRYGB: 117±18 LSG: 101±9, P < 0.05 Mean BMI (kg/m2) LRYGB: 45.9±4.6 LSG: 39.5±4.2, P < 0.05 % Co-morbidities (LRYGB/LSG) DM: 36.4 / 23.8 HTN: 22.7 / 14.3 Dyslipidemia: 31.8 / 38.1 Fatty liver: 81.8 / 66.7 OSA: 60.2 / 58.7 Attrition rate: 0% |
Intervention: Subjects underwent bariatric surgery of either a n =22 LRYGB or n = 21 LSG Surgical Technique: LRYGB: 25-30 mL pouch and 150 cm Roux-en-Y limb LSG: used a 32-Fr bougie Anthropometric and Body Composition InBody 770 multi-frequency BIA was used to assess body composition. Weight (baseline and at follow-up) and height were measured using a scale and stadiometer, respectively. Diet and Exercise Counseling Patients received nutrition advice from a dietitian and were encouraged begin an exercise program (5 to 6 hours per week) that included strength training. No protein supplements were given during the study. Setting: Erfan hospital in Tehran, Iran |
Anthropometric Mean Weight kg (6 months) LRYGB: 89.6±12.8 LSG: 78.4±9.8, P > 0.05 Mean % TWL (6 months) LRYGB: 22.3±4.3 LSG: 23.7±5.6 Mean % EWL (6 months) LRYGB: 52.3±13.4 LSG: 66.4±23.8, P > 0.05 (No significant difference after adjustment for pre-op weight.) Mean BMI kg/m2 (6 months) LRYGB:35.0±3.6 LSG: 30.3±4.0, P > 0.05 Mean % BMI Decrease (6 months) LRYGB: -22.2±4.3 LSG: -23.7±5.4 for Body Composition Mean FM kg (6 months) LRYGB: 41.0±8.1 LSG: 32.8±8.1, P > 0.05 (Mean FM decrease was -32.3±7.0% for LRYGB group and -37.1±8.7% for LSG group at 6 months postoperative.) Mean FFM kg (6 months) LRYGB: 48.0±4.9 LSG: 45.9±4.1, P > 0.05 (Mean FFM decrease was -11.7±3.9% for LRYGB group and -11.8±4.3% for the LSG group at 6 months postoperative.) Physical activity MET-h per week (6 months) LRYGB: 196±313 to 867±956 LSG: 392±457 to 1003±1121, P = 0.83 |
There was not a statistically significant difference in weight, BMI, FM, or FFM after 6 months for LRYGB compared to LSG. | Strengths
|
| Kim G, Tan CS, et al.20 Year: 2019 Study Design: Retrospective Cohort Study/Secondary Analysis Class Rating: B Country: Singapore Funding Source: None |
Ø | To compare the effects of SG and RYGB on body composition in a multi-ethnic Asian population at 3 years postoperative. | N = Secondary analysis of data for 295 adult patients who underwent bariatric surgery in August 2008 to December 2015. Demographics Age (years) LRYGB: 39.9±12.3 LSG: 39.5±11.2 Sex (n M/F) LRYGB: 15/24 LSG: 107/149 % Ethnicity (LRYGB/LSG) Malay n = 114: 30.8 / 39.8 Chinese n = 81: 35.9 / 26.2 Indian n = 74: 28.2 / 24.6 Other n = 26: 5.1 / 9.4 Anthropometrics Weight (kg) LRYGB: 110.7±24.9 LSG: 117.6±24.2 BMI (kg/m2) LRYGB: 40.6±7.2 LSG: 43±7.6 Body Composition FM (kg) LRYGB: 44.6±12.7 LSG: 49.3±13.2 FFM (kg) LRYGB: 66±18.3 LSG: 67.8±18 % Body fat LRYGB: 41±8 LSG : 42.2±8.4 Attrition rate: 0% |
Intervention: Subjects underwent bariatric surgery of either a n =256 LRYGB or n = 39 LSG Surgical Technique: LRYGB: 100-cm Roux-en-Y limb and 100-cm BP limb LSG: used a 38-Fr bougie Anthropometric and Body Composition GAIA 359 PLUS BIA (Jawon Medical, Korea) was used to assess body composition measurements. Body weight was measured at baseline and follow-ups. Setting: bariatric unit of a tertiary university hospital in Singapore |
Anthropometrics Mean Weight kg (1 year) LRYGB: 79.7±18 LSG: 82.4±17.7 Mean Weight kg (2 years) LRYGB: 82.4±20.3 LSG: 83.2±22.6 Mean Weight kg (3 years) LRYGB: 86.8±16.2 LSG: 85.7±19.8, P > 0.05 % TWL (1 year) LRYGB: 26.1±7.7 LSG: 26.3±9.8 % TWL (2 year) LRYGB: 25.7±9.5 LSG: 26.9±17.3 % TWL (3 year) LRYGB: 23.7±10.1 LSG: 23.9±11.1 (No significant difference in % TWL between the LRYGB vs. LSG groups (P > 0.05) Mean % EWL (1 year) LRYGB: 77.5±30.4 LSG: 71.8±30.5 Mean % EWL (2 years) LRYGB: 77.3±37.2 LSG: 66.8±46.6 Mean % EWL (3 years) LRYGB: 67.7±32.5 LSG: 64.3±37.8, P > 0.05 Mean BMI kg/m2 (1 year) LRYGB: 29±4.7 LSG: 30.2±5.6 Mean BMI kg/m2 (2 years) LRYGB: 29.3±5.8 LSG: 30.9±8.7 Mean BMI kg/m2 (3 years) LRYGB: 30.4±5.8 LSG: 32±6.9, P > 0.05 Body Composition Mean FM kg (1 year) LRYGB: 23.2±11.5 LSG: 27.7±12.6 Mean FM kg (2 years) LRYGB: 28.2±11.9 LSG: 27.4±15 Mean FM kg (3 years) LRYGB: 24.1±13 LSG: 29.5±14.9, P > 0.05 Mean FFM kg (1 year) LRYGB: 52.6±12 LSG: 52.7±12.6 Mean FFM kg (2 years) LRYGB: 51.2±11.7 LSG: 54.6±13.2 Mean FFM kg (3 years) LRYGB: 64.5±9.1 LSG: 54.7±12.9 P = 0.047 (The LRYGB group had better FFM preservation than the LSG group; however, this disappeared after multivariate analysis.) Mean % Body fat (1 year) LRYGB: 30±11.5 LSG: 33.9±11.6 Mean % Body fat (2 years) LRYGB: 35.2±12.5 LSG: 32.7±12.1 Mean % Body fat (3 years) LRYGB: 26±10 LSG: 34.1±11.6, P > 0.05 Follow-Up Rates % (LRYGB/LSG) 1 year:54.3/53.3 2 years: 40/31.3 3 years: 26.7/27.8 |
Anthropometric and body composition results were comparable for LRYGB compared to LSG after multivariate analysis. | Strengths
|
| Otto M, Elrefai M, et al.22 Year: 2016 Study Design: Prospective Cohort Study Class Rating: B Country: Germany Funding Source: None |
Ø | To compare the effects of LSG and LRYGB on body composition post adjustment for BMI at 1 year. | N = 173 adult patients who underwent bariatric surgery in January 2007 to February 2012. Met guidelines for bariatric surgery - BMI > 40 kg/m2 or ≥ 35 kg/m2 plus co-morbidities. Demographics Age: N/A Sex (n M/F) LRYGB: 34/93 LSG: 17/29 Anthropometrics Mean Weight (kg) LRYGB: 129.8±22 LSG: 163.9±29.4 Mean BMI (kg/m2) LRYGB: 45.6±5.7 LSG: 55.9±7.8 Body Composition Mean LM (kg) LRYGB: 71.4±15.9 LSG: 83.5±20.5 Mean % Body Fat LRYGB: 46±7.5 LSG: 49.2±7.7 Attrition rate: 26.6% (only included if subjects showed up at all follow-up appointments.) |
Intervention: Subjects underwent bariatric surgery of either a n =127 LRYGB or n = 46 LSG Patients with a BMI > 60 kg/m2 or previous small bowel surgeries were offered the LSG. Surgical Technique LRYGB: 150-cm Roux-en-Y limb and 50-cm BP limb LSG: used a 42-Fr bougie Anthropometric and Body Composition Nutriguard-M BIA (Data input GmbH, Darmstadt, Germany) was used to assess weight and body composition measurements. Diet and Exercise Patients received nutrition counseling by a bariatric specialist at each outpatient visit. Encouraged participants to begin an active exercise regimen and consume 1.5 g protein per kg IBW. Protein supplements were recommended for patients that were deemed to be protein deficient. Setting: Germany |
Anthropometrics Mean Weight kg (1 year) LYRGB: 90.6±18.3 LSG: 112.4±23.5 % TWL (1 year) LRYGB: 31.7±8.4% LSG: 30.48±7.6, P > 0.4 Mean % EWL (1 year) LRYGB: 62.9±18 LSG: 52.3±15.0, P = 0.0024 Mean BMI kg/m2 (1 year) LRYGB: 31.4±5.4 LSG: 38.2±6.6 Body Composition Mean LM kg (1 year) LRYGB: 61.7±12 LSG: 68.8±13.7, P = 0.33 Mean % Body Fat (1 year) LRYGB: 30.5±9.7 LSG: 37.1±9.2, P = 0.01 (After BMI adjustment, % EWL (P = 0.86), LM (P = 0.92), and % Body Fat (P = 0.16) were not statistically significant.) |
RYGB and LSG have comparable results for % EWL and body composition measures at 1-year post-bariatric surgery after adjustment for baseline BMI. % TWL did not require adjustments for variability in BMI between the two groups; therefore, it may be a better tool than % EWL. | Strengths
|
| Schneider J, Peterli R, et al.23 Year: 2016 Study Design: Randomized Controlled Trial Class Rating: A Country: Basel, Switzerland Funding Source: Swiss National Science Foundation Grant |
+ | To evaluate differences in LRYGB compared to LSG on body composition and energy metabolism at 1 to 2 years postoperative. | N = 42 adult patients who participated in the prospective RCT known as the SM-BOSS study which had an inclusion criterion of: Met guidelines for bariatric surgery - BMI > 40 or > 35 kg/m2 with one or more co-morbidities, between the ages of 18 and 65 years, and failed conservative management over 2 years. Subjects were excluded if they had severe symptomatic GERD, adhesions of the bowel, inflammatory bowel disease, large hiatal hernias, or any other contraindications to abdominal surgery. Demographics Mean Age (years) LRYGB: 40.3±10.9 LSG: 41.2±10.4 Sex (n M/F) LRYGB: 3/16 LSG: 3/20 Anthropometric Mean Weight (kg) LRYGB: 125.8±22.7 LSG: 120.1±19.2 Mean BMI (kg/m2) LRYGB: 44.4±6.3 LSG: 43.4±5.9 Body Composition Mean FM (kg) LRYGB: 56.4±12.1 LSG: 50.7±8.6 % FM per kg LRYGB: 45.1±4.3 LSG: 43.7±4.4 Mean LM (kg) LRYGB: 65.9±13.3 LSG: 62.9±10.2 % LM per kg LRYGB: 52.9±44 LSG: 54.2±4.3 % Comorbidities (LRYGB/LSG) DM:42 / 57 HTN: 11 / 30 Dyslipidemia: 53 / 43 OSA: 37 / 57 GERD: 84.2 / 87 Attrition Rate: 0% |
Intervention: Subjects underwent bariatric surgery of either a n =19 LRYGB or n = 23 LSG by the same bariatric surgeon. Surgical Technique LRYGB: 150-cm Roux-en-Y limb and 50-cm BP limb LSG: sized using a 35-Fr bougie Anthropometric and Body Composition Hologic QDR 4500 DEXA (Hologic, Inc., Bedford MA, USA) were used to assess body composition measurements. Diet Counseling Patients received nutrition counseling by a nutritionist regarding a balanced diet. Setting: Interdisciplinary Center of Nutritional and Metabolic Disease in St. Claraspital Basel in Switzerland |
Anthropometric Mean Weight loss kg (1-2 years) LRYGB: 39±9.7 LSG: 32.1±12.7 Mean BMI kg/m2 (1-2 years) LRYGB: 30.7±6.3 LSG: 31.6±4.7 Mean % EBMIL (1-2 years) LRYGB: 76.4±22.2 LSG: 64.4±24.2, P < 0.046 Mean % EBMIL (1-2 years) DM: 55.8±23.9 No-DM: 74.9±25.8, P = 0.52) Body Composition Mean FM kg (1-2 years) LRYGB: 35.5±19.9 LSG: 33.1±10.6, P > 0.05 Mean % FM per kg LRYGB: 39±14.5 LSG: 37.2±9.4 (% FM decline was significantly different from baseline in the LSG group, P = 0.037.) Mean FM Loss kg (1-2 years) DM: 34.5±6.6 No DM: 34.5±17, P > 0.05 (Mean Decline in FM Was comparable for people with DM: - 18.2±10 vs. No-DM: -19±12.4, P = > 0.05) Mean LM kg (1-2 years) LRYGB: 48.8±11 LSG: 52.4±12.7, P > 0.05 (LM loss contributed to 45% of the total weight loss for the LRYGB group and 37% loss in the LSG group.) Mean LM kg (1-2 years) DM: 57.9±14.4 No DM: 48.7±10.5, P = 0.037 (Mean decline in LM was higher in people without DM vs. with DM -16.3±15.7 kg vs. -12.6±5.8kg, P =0.55) Mean % LM per kg LRYGB: 58±14.2 LSG: 59.9±9.2, P > 0.05 (% LM increased in both the LRYGB P = 0.14) and LSG (P = 0.07) groups postoperative.) |
Both bariatric procedures led to weight loss; however % EBMIL was greater in the LRYGB compared to the LSG group. FM and LM declined significantly from baseline (P < 0.001), but were not significantly between the two groups. | Strengths
|
Table 1: Comparison of Anthropometric and Body Composition Outcomes Between Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy Table of Related Literature.
Abbreviations: ANCOVA, analysis of covariance; BF, body fat; BIA, bioelectrical impedance analysis; BMI, body mass index; BP, biliopancreatic limb; cm, centimeter; DEXA, dual-energy x-ray absorptiometry; DM, diabetes mellitus; EBMIL, excess body mass index loss; EWL, excess weight loss; F, female; Fr, French; FFM, fat free mass; FM, fat mass; GERD, gastroesophageal reflux disease: HTN, hypertension; JD, joint disease; NIH, National Institutes of Health; kg, kilograms; kg/m2, kilograms per meter squared; LM, lean body mass; M, male; mL, milliliter, OG, orogastric; OSA, obstructive sleep apnea; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; SM-BOSS, Swiss Multicenter Bypass Or Sleeve Study; SO, super obese; TWL, total of weight loss; WHO, World Health Organization
| Bariatric Reporting Outcomes | Equation |
| Initial Weight | Weight measured as close to the time of bariatric surgery |
| Initial BMI | BMI assessed as close to the time of bariatric surgery |
| Change in BMI | Initial BMI – Post-op BMI |
| % TWL | [Initial Weight – Postop Weight] / [(Initial Weight)] x 100 |
| % EWL | [(Initial Weight) – (Postop Weight)] / [(Initial Weight) – (IBW)] |
| % EBMIL | [ΔBMI / (Initial BMI – 25)] x100 |
Table 2: ASMBS Recommendations for Standardized Bariatric Reporting Outcomes
Reference: Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. May-Jun 2015;11(3):489-506.
ASMBS, American Society for Metabolic and Bariatric Surgery; BMI, body mass index; % EBMIL, percent excess body weight loss; % EWL, percent excess weight loss; IBW, the weight that corresponds to a BMI of 25 kg/2; % TWL, percent total weight loss
Weight loss usually consists of FM and/or LM loss [37]. The terms FFM and LM are often used interchangeably; however, there are some distinct differences. The 2019 American Society for Parenteral and Enteral Nutrition Clinical Guidelines on The Validity of Body Composition Assessment in Clinical Populations, defines FFM to include lean tissue plus body cell mass while LM refers to all lean tissues in totality and excludes bone [38]. Prospective studies conducted by Venancio et al. [24] and Golzarand et al. [18] investigated outcomes for patients with clinically severe obesity who underwent either LSG or LRYGB and found no significant differences in FFM (P = 0.803 and P > 0.05, respectively) assessed using bioelectrical impedance analysis (BIA) at six months postoperative [18,24]. In contrast, Kim et al. [20] initially found that those who had a LRYGB (n = 256) had better preservation of their FFM than their LSG counterparts (n = 39) as evaluated by BIA at three years postoperative (P = 0.047); however, these findings were not statistically significant after multivariate analysis. Three studies assessed LM instead of FFM to determine if LSG or LRYGB was more effective [17,19,22]. Kavanagh et al. [19], Schneider et al. [23], and Otto et al [22]. found no significant differences in LM at one to two years postoperative for patients who had the LRYGB compared to the LSG; however, Buhler et al. [17] found that the LRYGB group had a slightly lower LM than the LSG group (45.30 vs. 47.85, P = 0.014) at a median of 6.7 years postoperative. Buhler et al. [17] assessed body composition data for 115 out of 142 study participants using DEXA. Incomplete data could skew the results of this long-term study [17]. While Schneider et al. [23] also assessed body composition using DEXA, Kavanagh et al. [19] used the air displacement plethysmography method, and Otto et al. [22] used BIA. LM loss contributed to 45% of the TWL for the LRYGB group and 37% loss in the LSG group in Schneider et al. [23]; however, Golzarand et al. [18] found that FFM loss contributed to 24.9±7.1% of TWL in the LRYGB group compared to 24.5±6.6% in the LSG group. Schneider et al. [23] also found that study participants without DM had significantly lower LM (48.7±10.5 kg vs. 57.9±14.4 kg, P = 0.037), but a higher decrease in LM postoperative than their counterparts with DM (-16.3±15.7 kg vs. -12.6±5.8 kg, P = 0.55).
Five studies determined that FM and % FM were comparable in patients that underwent LRYGB or LSG at six months and one, two, and three years postoperative [18-20,23,24]. The TWL postoperative was primarily from FM loss versus LM or FFM loss [18,19,21]. The majority of the study participants were female, which could be why most of the TWL was from FM [17,18,20-24]. Research indicates that females have higher % FM and less FFM or LM than males [39,40]; therefore, the results might be different if there were more males included in these studies. Similarly, Buhler et al. [17], Otto et al. [22], and Kim et al. [20], all found no significant differences in % BF in either the LRYGB or the LSG group. Percent BF loss ranged from 30- 44%. Despite the variation in body composition tools (DEXA, BIA, and displacement plethysmography) used, the results were consistent across studies. Overall, the results of these studies indicate that FM, % FM, and % BF loss are comparable for patients with clinically severe obesity who undergo LRYGB and LSG [17-20,22-24]. Additionally, all study participants across all studies lost both FM and % BF as well as FFM or LM [17-24].
Discussion
This review of comparative studies reaffirms that LRYGB and LSG are both acceptable tools to facilitate weight loss in patients with clinically severe obesity that have been unsuccessful in traditional behavioral and/or medical management [17-24]. Although no statistically significant difference was found between LRYGB and LSG in Kim et al. [20], Otto et al. [22], Golzarand et al. [18], Kavanagh et al. [19], Buhler et al. [17], or Venancio et al. [24], LRYGB performed marginally better in maximizing % EWL in the medium-term study conducted by Martinaitis et al. [21] and the long-term study by Schneider et al. [23]. Additional medium-term and long-term studies that compare the clinical outcomes (i.e. anthropometric and body composition) associated with LRYGB and LSG are necessary to understand which bariatric procedure might be better at sustaining weight and FM loss while minimizing loss of LM in the long term.
Weight loss post-bariatric surgery consists of a combination of FM, FFM, and/or LM loss. While the loss of FM is desirable, loss of LM could be detrimental to achieving sustained weight loss and quality of life by reducing resting metabolic rate and decreasing functional capacity as well as muscle strength [41]. Research by Nuijten et al. [42] indicated that people who are older, male, have higher preoperative BMI, and have undergone LRYGB or LSG are more likely to experience excessive FFM loss postoperative. Adequate protein consumption and physical activity are standard recommendations for patients in the postoperative phase to reduce and/or prevent loss of LM. Although research studies and expert consensus indicate that patients that have undergone bariatric surgery need to consume adequate protein while incorporating weight-bearing physical activity into their daily lives, systematic reviews exploring whether adequate and/or high protein intake can slow down or decrease LM loss have been inconclusive [41,43]. More research is necessary to establish the amount of protein required for people undergoing bariatric surgery to prevent LM loss.
Implications for Research and Future Practice
Limitations of the studies utilized in this review include heterogeneity in the sample sizes, baseline characteristics, higher female to male study participant ratio, lack of randomization, and different methodologies used to assess body composition. Also, the results of these eight studies may not be generalizable to males since they were underrepresented in all of the studies or to other bariatric surgery institutions as all of the research was conducted at a single center primarily by one bariatric surgeon [17-24]. Martinaitis et al. [21] was the only study that included people with a BMI > 50 kg/m2 and had a small subset of people that were 60 years of age or older (n = 11); therefore, more research is needed in this area to determine which bariatric procedure is more effective in people with clinically severe obesity and geriatric populations. In addition, most studies included in this review did not provide their follow-up rates except Kim et al. [20] which may be a limitation as individuals lost to follow-up may have dropped out of studies due to weight regain [44]. Bariatric surgery attrition may skew study results toward more favorable study outcomes [44]. Lastly, there was a paucity of medium-term and long-term studies investigating the differences in anthropometric and body composition parameters. All of these variables could lead to study bias and limit generalizability and interpretation of these results. Large-scale, multi-center, long-term RCTs are necessary to offset some of these limitations. Additionally, researchers should be strongly encouraged to use the ASMBS recommendations for standardized reporting outcomes, making it easier to compare results across multiple studies.
Despite anatomical differences between the LRYGB and LSG, variations in baseline anthropometrics, body composition, and demographics, six of eight studies concluded that there were no significant differences found for weight loss and body composition [17-20,22,24]. The findings re-emphasize that individuals who undergo bariatric surgery lose FM, but more importantly, they experience a loss of LM, which has implications for clinical practice [17-24]. Clinicians should not only counsel patients to consume 60 to 100 grams of protein daily to prevent or lessen the loss of LM [45], but they should also explain the "why" to increase patient adherence to recommendations. Also, clinicians should encourage their patients to begin a physical activity or exercise regimen preoperative and postoperative when medically feasible. For example, the ASMBS recommends 20 minutes of mild activity (i.e., aerobic and resistance training) approximately four times per week preoperative and 30 minutes of moderate-intensity physical activity daily postoperative [46]. Consuming adequate protein and being physically active may be beneficial tools for maximizing weight loss and improving body composition metrics.
Conclusion
Anthropometric and body composition outcomes are comparable between LRYGB and LSG [17-20,22,24]. More long-term RCTs investigating this topic are needed to determine which bariatric procedure is the most effective and whether there are differences in anthropometric and body composition outcomes based on gender, age, ethnicity, degree of obesity, co-morbidities, protein consumption, and physical activity level.
Conflict of Interest: The authors declare that they have no conflict of interest.
Funding: None
References
- https://www.cdc.gov/nchs/fastats/obesity-overweight.htm
- https://www.cdc.gov/obesity/data/adult.html
- Cawley J, Biener A, Meyerhoefer C, Ding Y, Zvenyach T, et al. (2021) Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm 27(3):354-366.
- NIH conference (1991) Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 115(12):956-61.
- https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- Arterburn DE, Telem DA, Kushner RF, Courcoulas AP (2020) Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 324(9):879-887.
- Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, et al. (2021) Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet 397(10287):1830-1841.
- McGrice M, Don Paul K (2015) Interventions to improve long-term weight loss in patients following bariatric surgery: challenges and solutions. Diabetes Metab Syndr Obes 8:263-274.
- Vitiello A, Angrisani L, Santonicola A, Iovino P, Pilone V, et al. (2019) Bariatric Surgery Versus Lifestyle Intervention in Class I Obesity: 7-10-Year Results of a Retrospective Study. World J Surg 43(3):758-762.
- Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, et al. (2018) Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 319(3):255-265.
- Shoar S, Saber AA (2017) Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis 13(2):170-180.
- Han Y, Jia Y, Wang H, Cao L, Zhao Y (2020) Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int J Surg 76:101-110.
- Peterli R, Wölnerhanssen BK, Vetter D, Nett P, Gass M, et al. (2017) Laparoscopic Sleeve Gastrectomy Versus Roux-Y-Gastric Bypass for Morbid Obesity-3-Year Outcomes of the Prospective Randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg 265(3):466-473.
- Buchwald H, Avidor Y, Braunwald E, Jensen, MD, Pories W, et al. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724-37.
- Christou NV, Sampalis JS, Liberman M, Look D, Auger S, et al. (2004) Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 240: 416-23.
- Academy of Nutrition and Dietetics (2016) Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process.
- Bühler J, Rast S, Beglinger C, Peterli R, Peters T, et al. (2021) Long-Term Effects of Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass on Body Composition and Bone Mass Density. Obes Facts 14: 131-140.
- Golzarand M, Toolabi K, Djafarian K (2019) Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: a Comparative Prospective Study. Obes Surg 29: 406-413.
- Kavanagh R, Smith J, Avgenackis E, Jones D, Nau P (2020) A Comparison of the Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Body Mass Composition as Measured by Air Displacement Plethysmography. Obes Surg 30: 451-455.
- Kim G, Tan CS, Tan KW, Lim SPY, So JBY, et al. (2019) Sleeve Gastrectomy and Roux-En-Y Gastric Bypass Lead to Comparable Changes in Body Composition in a Multiethnic Asian Population. J Gastrointest Surg 23: 445-450.
- Martinaitis L, Tuero C, Fortún Landecho M, Cienfuegos JA, Moncada R, et al (2019) The long-term benefits of bariatric surgery in elderly and super-obese populations. Rev Esp Enferm Dig 111: 371-377.
- Otto M, Elrefai M, Krammer J, Weiß C, Kienle P, et al. (2019) Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Lead to Comparable Changes in Body Composition after Adjustment for Initial Body Mass Index. Obes Surg 26: 479-85.
- Schneider J, Peterli R, Gass M, Slawik M, Peters T, et al. (2016) Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis 12: 563-570.
- Venâncio FA, Almeida LA, Zovico PV, Barauna VG, Miguel GP, et al. (2016) Roux-en-Y Gastric Bypass and Sleeve Gastrectomy Differently Affect Oxidative Damage Markers and their Correlations with Body Parameters. Obes Surg 31: 1680-1687.
- Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, et al. (2015) Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis 11: 489-506.
- Wolfe BM, Kvach E, Eckel RH (2016) Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res 118: 1844-55.
- Khalaj A, Tasdighi E, Hosseinpanah F, Mahdavi M, Valizadeh M, et al. (2020) Two-year outcomes of sleeve gastrectomy versus gastric bypass: first report based on Tehran obesity treatment study (TOTS). BMC Surg 20(1): 160.
- Jain D, Sill A, Averbach A (2018) Do patients with higher baseline BMI have improved weight loss with Roux-en-Y gastric bypass versus sleeve gastrectomy? Surg Obes Relat Dis 14(9): 1304-1309.
- ASMBS American Society for Metabolic and Bariatric Surgery (2022) Metabolic and Bariatric Surgery.
- Jabbour G, Salman A (2021) Bariatric Surgery in Adults with Obesity: the Impact on Performance, Metabolism, and Health Indices. Obes Surg 31: 1767-1789.
- van Rijswijk AS, van Olst N, Schats W, van der Peet DL, van de Laar AW (2021) What Is Weight Loss After Bariatric Surgery Expressed in Percentage Total Weight Loss (%TWL)? A Systematic Review. Obes Surg 31: 3833-3847.
- Corcelles R, Boules M, Froylich D, Hag A, Daigle CR, et al. (2016) Total Weight Loss as the Outcome Measure of Choice After Roux-en-Y Gastric Bypass. Obes Surg 26: 1794-8.
- Willoughby D, Hewlings S, Kalman D (2018) Body Composition Changes in Weight Loss: Strategies and Supplementation for Maintaining Lean Body Mass, a Brief Review. Nutrients 10.
- de Aquino LA, Pereira SE, de Souza Silva J, Sobrinho CJ, Ramalho A (2012) Bariatric surgery: impact on body composition after Roux-en-Y gastric bypass. Obes Surg 22: 195-200.
- Haghighat N, Ashtari-Larky D, Aghakhani L, Asbaghi O, Hoseinpour H, et al. (2021) How Does Fat Mass Change in the First Year After Bariatric Surgery? A Systemic Review and Meta-Analysis. Obes Surg 31: 3799-3821.
- Zalesin KC, Franklin BA, Lillystone MA, Shamoun T, Krause KR, et al. (2010) Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord 8: 15-20.
- Tamboli RA, Hossain HA, Marks PA, Eckhauser AW, Rathmacher JA, et al. (2010) Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 18: 1718-1724.
- Sheean P, Gonzalez MC, Prado CM, McKeever L, Hall AM, et al. (2020) American Society for Parenteral and Enteral Nutrition Clinical Guidelines: The Validity of Body Composition Assessment in Clinical Populations. JPEN J Parenter Enteral Nutr 44: 12-43.
- Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, et al. (2018) Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ 9: 28.
- Wu BN, O'Sullivan AJ (2011) Sex Differences in Energy Metabolism Need to Be Considered with Lifestyle Modifications in Humans. J Nutr Metab 391809.
- Romeijn MM, Holthuijsen DDB, Kolen AM, Janssen L, Schep G, et al. (2021) The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: a systematic review. Nutri J 20: 27.
- Nuijten MAH, Monpellier VM, Eijsvogels TMH, Janssen IMC, Hazebroek EJ, et al. (2020) Rate and Determinants of Excessive Fat-Free Mass Loss After Bariatric Surgery. Obes Surg 30: 3119-3126.
- Ito MK, Gonçalves VSS, Faria SLCM, Moize V, Porporatti AL, et al. (2017) Effect of Protein Intake on the Protein Status and Lean Mass of Post-Bariatric Surgery Patients: a Systematic Review. Obes Surg 27: 502-512.
- Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, et al. (2014) Long-term follow-up after bariatric surgery: a systematic review. JAMA 312: 934-42.
- ASMBS American Society for Metabolic and Bariatric Surgery (2022) Life After Bariatric Surgery.
- King WC, Bond DS (2013) The importance of preoperative and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev 41: 26-35.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Brown T, Gottesman K, Newkirk M, Ziegler J (2022) Comparison of Anthropometric and Body Composition Outcomes between Laparoscopic Rouxen- Y Gastric Bypass and Sleeve Gastrectomy: A Narrative Review. J Obes Weight Loss Ther 12: 501. DOI: 10.4172/2165-7904.1000501
Copyright: © 2022 Brown T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3648
- [From(publication date): 0-2022 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 3102
- PDF downloads: 546