Cytokine Profile of Mesenchymal Stem Cells for their Wound Healing Properties
Received: 26-Nov-2018 / Accepted Date: 17-Dec-2018 / Published Date: 24-Dec-2018 DOI: 10.4172/2576-3881.1000125
Abstract
Wound healing properties of Mesenchymal Stem Cells (MSC) is a topic of great interest and their mechanism of action is mainly attributed to their paracrine effects and their potential to differentiate in the area of contact, thereby resulting in wound healing. Umbilical Cord (UCMSC) derived MSC and Bone marrow derived MSC (BM MSC) are both good candidates for wound healing. However the ease in procuring UCMSC makes it an attractive candidate for wound healing. The capabilities of both MSC towards wound healing is not elucidated clearly and hence we undertake this study to compare UC MSC and BM MSC supernatants and measure certain key cytokines. We also compare its differentiation properties at different passages and study if both these properties are interlinked. We found that UC MSC retains its differentiation properties better than BM MSC, and that their paracrine capabilities seem to be interlinked with their differentiation potencies.
Keywords: Wound healing; Mesenchymal stem cells; Paracrine effect; UCMSC; Bone marrow; Cytokine profile
Introduction
Wound healing is a complex orchestra of events which results in tissue remodelling and a complete closure of wound with minimal skin damage. It involves cell migration, proliferation and deposition of extracellular matrix (ECM), angiogenesis and finally tissue remodelling [1,2,3]. This sequence of events are hampered and deregulated in case of chronic wounds like diabetic foot ulcers. Recent advances in addressing this pressing issue has resulted in unsatisfactory results and failed to prevent morbidity and disability associated with such chronic wounds. Current treatment achieve only about 50% success and their effects are not permanent [2]. Defective cytokine production by local inflammatory cells and fibroblasts, reduced angiogenesis are crucial factors which dominate chronic non healing wounds and pose daunting obstacles to overcome.
Mesenchymal stem cells (MSC) are multipotent cells which have demonstrated wound healing properties in a number of clinical trials. The mechanism of action of MSCs can be attributed to either their ability to transdifferentiate or through their paracrine effects. The ISCT (International Society for Cellular Therapy) definition of MSC is its ability to differentiate into osteocytes, chondrocytes and adipocytes, plus its surface expression of CD73,CD105 and CD90 [4]. Bone marrow MSC have limited ability to differentiate in vitro and lose this ability within a few passages in culture [5], whereas Umbilical Cord Derived MSC (UC-MSC) have shown to retain the ability of differentiation and surface markers upto 108 doublings [4].The paracrine effects of MSC known to promote wound healing are soluble growth factors like Stromal cell Derived Factor 1 (SDF1), Vascular Endothelial Growth Factor (VEGF), Platelet Derived Growth Factor (PDGF), Interleukin 8 (IL8) which supersede its ability to differentiate in the wound area.
In the present study, we attempt to delineate the 2 properties of MSC viz : ability to differentiate and its ability to produce wound healing soluble factors, which could be primarily far more important than its ability to differentiate. Since BM-MSC and UC-MSC have different "stemness" properties in vitro, with UC-MSC retaining the stem like properties in late passages (passage 18, doubling 108) when compared to BM-MSC, it is important to also study whether the 2 kinds of MSCs can still produce soluble factors which are essential for wound healing.
We demonstrate that both BM-MSC and UC-MSC retain their ability to produce Platelet Growth Factor (PDGF), Interleukin 8 (IL-8), Vascular Endothelial Factor (VEGF), Stromal Cell Derived Growth Factor (SDF1), into mid passages in vitro (with Fetal bovine serum), and is in sync with their ability to differentiate into chondrocytes, adipocytes and osteocytes. Their ability to produce these factors decreases significantly by mid passage which correlates with its inability to differentiate.
Materials And Methods
Mesenchymal stem cells isolation and culture
Umbilical cords (UC) were obtained after informed consent was signed by healthy volunteers (Ethical clearance was given by Institutional Committee for Stem cell Research and Therapy (ICSRT): 132/CHE/2010 A – Number 272353). UCs were collected and processed within 48 h of delivery and Bone marrow MSCs (BM-MSCs) were obtained from Lonza. Umbilical derived Mesenchymal Stem cells (UC-MSCs) were isolated and cultured as described previously [6] in 20% Fetal Bovine Serum (FBS) containing Dulbecco’s Modified Eagle’s Medium (DMEM F12) (Gibco). Adherent cells were passaged upon reaching 80% confluency and reseeded at the density of 1000 cells/cm2 in 75 cm2 for further expansion. Medium was changed every 2-3 days and cells were maintained in a humidified atmosphere at 5% CO2 at 37°C. Viable cells were counted using trypan blue stain (Sigma) and Passage 3 (P3) cells (BM-MSCs and UC-MSCs) were used for the experiment.
BM-MSCs and UC-MSCs were cultured at the density of 1000 cells/cm2 in 4 wells of 24 wells plate and sub-cultured till passage 16 (P16). P3, P5 and P16 cells were used for differentiation using adipogenic, chondrogenic and osteogenic differentiation medium and stained after 25 days for Oil Red, Alcian blue and Alizarin Red, respectively.
Protein estimation and cytokines detection
Supernatants were collected at every passage (P3-P16) for MSCs and stored at -80˚C until used. Total protein content in the samples was measured by Biuret method. PDGF, VEGF, SDF-1 and IL-8 levels were determined using ELISA (Ray Bio).
Statistics
All values were expressed as mean ± standard error mean (SEM).
Results
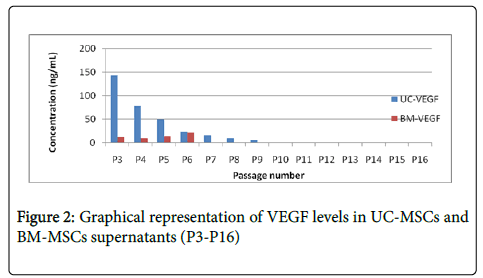
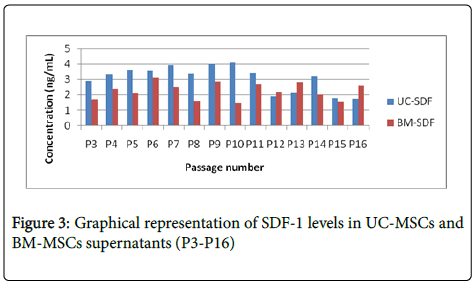
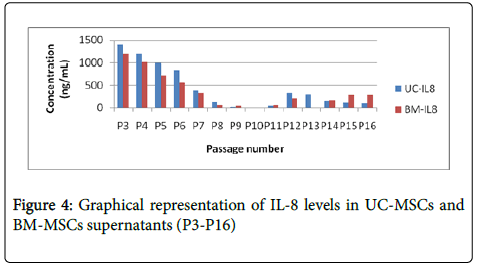
UC MSC and BM MSC cells were cultured in 20% FBS containing DMEM from P3 to P16 and supernatants were collected for all the passages. Levels of PDGF, VEGF, SDF-1 and IL-8 has significantly decreased after passage 8 in both BM MSC and UC MSC supernatants as shown in Figures 1-4, which is correlating with the differentiation potential of the cells as shown in Table 1. Also, BM MSC shows decreased amount of cytokine levels in comparison with UC MSC, wherein VEGF concentrations are seemingly negligible in BM MSC supernatants.
| Umbilical Cord | Bone Marrow | |||||
|---|---|---|---|---|---|---|
| Adipo | Chondro | Osteo | Adipo | Chondro | Osteo | |
| Early Passage | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen |
| Mid Passage | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen | Differentiation Seen |
| Late Passage | – | – | Differentiation Seen | – | – | Differentiation Seen |
Table 1: Ability of UC-MSCs and BM-MSCs for differentiation
Discussion
Mesenchymal stem cells (MSC) are multipotent stem cells which are derived from various sources such as bone marrow, adipose tissue and Umbilical cord. They are spindle shaped adherent population, are immuno-modulatory by nature and are very efficient cells for wound healing. They can differentiate into adipocytes, chondrocytes and osteocytes [4]. They can also differentiate into hepatocytes, cardiomyocyte, astrocyte and neuron [5,7,8].
MSC have shown great promise in regenerative medicine, especially areas affected due to injury /ischemia. MSC migrate to area of ischemic stroke [9] and repair the affected area either by differentiation, cell replacement or paracrine effects or both.
Preclinical studies have demonstrated the efficacy of MSC in wound healing such as excision wound [10], diabetic foot ulcers [11], pressure ulcers [12] and burn injury [13]. MSCs improve wound healing and promote scar- less recovery by accelerating collagen synthesis and angiogenesis. Safety and efficacy of MSC in clinical trials have demonstrated that MSC produce no adverse events and are efficient in the treatment of non-healing ulcers [11], limb ischemia [14] diabetic foot ulcers [10] and radiation burn [15]. Clinically tangible end points like significant increase in angiogenesis and reduction in inflammatory milieu have been effectively demonstrated. The mechanism of action of MSC to produce these clinical effects could be attributed to either their trans differentiation into parenchymal cells [16] or their ability to produce a host of growth factors and cytokines which could result in a paracrine effect [17].
The aim of the study was to delineate the "stem-like" properties exhibited by MSCs and correlate it to its ability to produce soluble factors like VEGF, PDGF, SDF1, IL-8, which are historically known to promote wound healing. We studied two sources of MSC, BM MSC derived from bone marrow, the other was Umbilical Cord derived MSC and the parameters of analysis was its ability to display stem like properties of differentiation and its ability to produce soluble factors which promote wound healing. We observe that BM MSC and Cord derived MSC lose their property of differentiation within 5 passages (16.6 doubling) which coincides with its inability to produce paracrine factors.
We chose four cytokines: viz. Platelet Growth Factor (PDGF), Interleukin 8 (IL-8), Vascular Endothelial Factor (VEGF), Stromal Cell Derived Growth Factor (SDF1) for this study. VEGF promotes endothelial cell motility, proliferation and survival, Endothelial precursors (EPC) mobilization and homing, promotes integrin expression and up regulates vasculogenic cytokines. SDF1 is implicated in EPC recruitment. PDGF is implicated in EPC migration and expansion [18]. IL-8 attracts polymorph nuclear leukocytes and helps in epidermal wound healing.
MSCs have an innate ability to heal wounds and are effective in all the phases of wound healing. The first phase which is inflammatory phase is when the wound occurs. MSCs exert an immunosuppressive milieu which regulates proliferation of T cells, B cells NK cells [19-25]. The next phase of wound healing i.e., proliferation occurs 2-3 days which results in re-epithilization and angiogenesis. Wounds treated with MSC (BM MSC or Adipose Derived MC), have resulted in increased re-epithelialisation, angiogenesis, and granulation tissue formation [26-33].
Mechanism of action of MSCs is shown to be both by differentiation and paracrine signalling in the proliferation phase. That MSC differentiate into either fibroblasts, endothelial cells, pericytes [34,35] and epidermal keratinocytes [26,29,33] have been demonstrated in studies involving fluorescent labelled BM MSC injected either topically or intravenously, in mouse models. Co-culture experiments involving BM MSC and heat shocked sebaceous glands resulted in differentiation of MSC to sebaceous glands in skin near the wound area [31], although the engraftment is low and decreases with time. However the observations are not conclusive as there is difference of opinion wherein many studies have refuted this differentiation of MSCs near the wound area [28]. Additionally, most studies have indicated co localization of GFP with specific cell markers, implicating fusion of MSC with these resident cells as a possible mechanism, rather than differentiation [36].
There is growing evidence that the predominant mechanism for wound healing mediated by BM MSC, Adipose MSC, Umbilical cord MSC [37-40] is by secreted factors like PDGF, VEGF, SDF1, IL-8 and chemokines, which promote and accelerate re-epithelialisation and wound repair [33,37,41]. MSC secrete exosomes which helps in activation, migration and proliferation of different cells involved in wound healing which would promote angiogenesis, epithelialisation and proliferation of fibroblast.
Conclusion
In our study we have demonstrated that the differentiation ability and paracrine modalities of BM MSC and Umbilical cord derived MSC are correlated with each other. BM MSC and Cord Derived MSC exhibit significantly low amounts of PDGF, IL 8, SDF1 and VEGF by mid passage, as shown in Figures 1-4 which is when they also are unable to differentiate, in our culture conditions (Table 1). We have demonstrated BM-MSCs and UC-MSCs gradually lose their stemness properties in vitro and it seems to be correlated to their ability to produce paracrine soluble factors crucial for wound healing. Further studies to improve culture conditions will help elucidate more effective methods to improve the regenerative potentials of MSCs.
References
- Ding D, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transpl 20: 5-14.
- Lee SH, Jin SY, Song JS, Seo KK, Cho KH (2012) Paracrine effects of adipose derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 24: 136-43.
- Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, et al. (2012) Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res 83: 267-275.
- Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, et al. (2013) Isolation and Characterisation of Mesenchymal Stem Cells from Different Regions of the Human Umbilical Cord. BioMed Research International 8: 916136.
- Boroujeni ME , Gowda P , Johnson J , Rao J, Saremy S (2012) The Proliferation and Differentiation Capacity of Bone Marrow Derived- Human Mesenchymal Stem Cells in Early and Late Doubling. Asian J Biochem 7: 27-36.
- Purushothama H, Gururaj Rao, SGA Rao, Jyothsna Rao (2017) Complete in vitro characterization of umbilical cord wharton’s jelly derived mesenchymal stem cells. Int J Biochem Biotechnol 13: 325-350.
- Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, et al (2010) Hocking AMMesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 316: 48-54.
- Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, et al (2010) Mesenchymal stem cells’ interaction with skin: wound-healing effect on fibroblast cells and skintissue. Wound Rep Regen 18: 655-661.
- Maharlooei MK, Bagheri M, Solhjou Z, Jahromi BM, Akrami M, et al (2011) Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res ClinPrac 93: 228-234.
- Liu L, Yu Y, Hou Y, Chai J, Duan H, et al (2014) Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous woundhealing of severe burned rats. PLoS One 9: 1-8.
- Kato J, Kamiya H, Himeno T, Shibata T, Kondo M, et al (2014) Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabet Its Complic 28: 588-595.
- Marei HE, Hasan A, Rizzi R, Althani A, Afifi N, et al (2018) Potential of Stem Cell-Based Therapy for Ischemic Stroke Stem cell and stroke 9: 34.
- Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, et al (2014) Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther 5: 28.
- de la Garza-Rodea AS, Knaän-Shanzer S, Van Bekkum DW (2011) Pressure Ulcers: Description of a New Model and Use of Mesenchymal Stem Cells for Repair. Dermatology 223: 266-284.
- Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, et al (2007) Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13: 1299-1312.
- Bura A, Planat-Benard V, Bourin P, Silvestre J, Gross F5, et al (2014) Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 16: 245-257.
- Vojtassák J, Danisovic L, Kubes M, Bakos D, Jarábek L, et al (2006) Autologousbiograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett 27: 134-137.
- Eric Bey MD,Marie Prat PhD,Patrick Duhamel MD,Marc Benderitter PhD,Michel Brachet MD,François Trompier PhD,Pierre Battaglini PhD,Isabelle Ernou BA,Laetitia Boutin BA,Muriel (2010) Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations
- Hollander A, Macchiarini P, Gordijn B, Birchall M (2009) The first stem-cell based tissue-engineered organ replacement: implications for regenerative medicine and society. Regen Med 4: 147–148.
- Hidefumi I, Jun IJ, Yasuhiko T (2012) Liver anti-fibrosis therapy with mesenchymal stem cells secreting hepatocyte growth factor. J Biomater Sci Polym Ed 23: 2259-2272.
- R. Kahn, R. M. Robertson, R. Smith, and D. Eddy, “The impact of prevention on reducing the burden of cardiovascular disease,†Circulation, vol. 118, no. 5, pp. 576–585, 2008.
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, et al (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology 30: 42-48.
- Â Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenicstimuli. Blood 99: 3838-3843.
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141-150.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107: 367-372.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24: 74-85.
- Â Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815-1822.
- Ali EA, Elaine H, Justin M, Ryosuke I, Jeremy B, et al (2008) Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol 127: 348-358.
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, et al (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180: 2581-2587.
- McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, et al (2006) Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen 14: 471-478.
- Javazon EH, Keswani SG, Badillo AT, Crombleholme TM, Zoltick PW, et al (2007) Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair and Regen 15: 350-359.
- Â Wu Y, Chen L, Scott PG, Tredget EE (2007) Tredget. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25: 2648-2659.
- Maria P Alfaro, Matthew Pagni, Alicia Vincent, James Atkinson, Michael F Hill, et al (2008) The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proceedings of the National Academy of Sciences 105: 18366-18371.
- Li H, Fu X, Ouyang Y, Cai C, Wang J, et al (2006) Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res 326: 725-736.
- Cagri AU, Morikuni T, Hiko H, Hiroshi M (2014) The effect of bone-marrow-derived stem cells and adipose-derived stem cells on wound contraction and epithelization. Adv Wound Care 3: 405-413.
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, et al (2007) Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48: 15-24.
- Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, et al (2012) Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33: 80-90.
- Deng W, Han Q, Liao L, Li C, Ge W, et al (2005) Engrafted bone marrow-derived Flk-1+ mesenchymal stem cells regenerate skin tissue. Tissue Eng 11: 110-119.
- Hocking AM, Gibran NS (2010) Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 316: 2213-2219.
- Liwen C, Edward ET, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3: 4.
- Park BS, Jang KA, Sung JH, Park JS, Kwon YH, et al. (2008) Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 34: 1323-1326.
Citation: Rao S, Umrao A, Purushothama H, Rao G, Rao J (2018) Cytokine Profile of Mesenchymal Stem Cells for their Wound Healing Properties. J Cytokine Biol 3:125. DOI: 10.4172/2576-3881.1000125
Copyright: © 2018 Rao S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4000
- [From(publication date): 0-2018 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 3095
- PDF downloads: 905