Research Article Open Access
A Novel Method for Determination of Fenofibric Acid in Human Plasma using HPLC-UV: Application to a Pharmacokinetic Study of New Formulations
Iltaf Shah1, James Barker2, Stephen J Barton2 and Declan P Naughton1*1School of Life Sciences, Kingston University, UK
2School of Pharmacy and Chemistry, Kingston University, UK
- *Corresponding Author:
- Declan P Naughton
Faculty of Science, Engineering and Computing
School of Life Sciences, Kingston University, Penrhyn Road
Kingston-upon-Thames, Surrey, KT1 2EE, UK
Tel: +44 (0)208 417 7097
E-mail: D.Naughton@kingston.ac.uk
Received date: December 16, 2013; Accepted date: February 28, 2014; Published date: March 04, 2014
Citation: Shah I, Barker J, Barton SJ, Naughton DP (2014) A Novel Method for Determination of Fenofibric Acid in Human Plasma using HPLC-UV: Application to a Pharmacokinetic Study of New Formulations. J Anal Bioanal Tech S12:009. doi: 10.4172/2155-9872.S12-009
Copyright: © 2014 Shah I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A high performance liquid chromatography method for the determination of fenofibric acid (FA), the active form of fenofibrate (FBT) in human plasma was developed and validated with 500 μL of human plasma using 4’-chloro- 5-fluro-2-hydroxybenzophenone (CFHB) as internal standard (IS). The assay procedure involved a simple one step liquid/liquid extraction of FA and IS from human plasma into ethyl acetate. The organic layer was separated and evaporated under a gentle stream of nitrogen at 40°C. The residue was reconstituted in the mobile phase and injected on a Symmetry Shieldï�?�?RP18 (150×4.60 mm) 5 μm column. Separation of FA and IS was achieved with a mobile phase consisting of acetonitrile: 0.02 M phosphoric acid (50:50 v/v) at a flow rate of 1 mL/min. Nominal retention times of FA and IS were 6.1 and 9.1 ± 0.5 min, respectively. Absolute recovery of FA using a single step liquid/liquid method was 79.8%. A calibration curve was established for a range of concentrations 0.05 to 10.0 μg/mL with a regression coefficient (r2) of 0.9988. The lower limit of quantification (LLOQ) of FA was 0.05 μg/mL. The intraand inter-day precision for the measurement of FA quality control samples (0.05, 0.12, 1.20 and 8.20 μg/mL), were in the range 4.6-16.9% and 4.4-17.2% relative standard deviations respectively. The accuracy in the measurement of QC samples for FA was in the range of 82.0-104.3% (intra-day) to 95.0-104.9% (inter-day). The method developed was successfully used to investigate the pharmacokinetics and bioequivalence between a micronized Lipidil-Micro™ capsule formulation and a nanonised fenofibrate Lipidil-EZ™ tablet formulation.
Keywords
FA; Fenofibric acid; Fenofibrate; HPLC
Introduction
Fenofibrate (Figure 1a) has been a widely used drug in the treatment of dyslipidaemia [1]. It was originally launched in 1975 as a standard formulation and is now marketed in over 85 countries. Chemically, FBT is 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-propanoic acid,1- methylethyl ester. Fenofibric acid (Figure 1b), the active metabolite of FBT, contributes to a reduction in total cholesterol, LDL cholesterol, apolipoprotien B, total triglycerides and triglyceride rich lipoprotein [2-7]. In addition, treatment with FBT also results in elevation of high-density lipoprotein (HDL) and apoproteins, viz., apoAI and apoAII. The effects of FA on lipid metabolism are mainly mediated through activation of the peroxisome proliferator-activated receptor α [8-10]. Following oral administration, FBT is well absorbed from the gastrointestinal tract, and is rapidly hydrolysed by the CYP3A4 isozyme to FA [11-13]. The maximum plasma concentrations of FA are achieved within 4-5 h following oral dosing of FBT. No unchanged FBT is detected in plasma after oral dosing. The elimination half-life of FA is approximately 20 h [13,14]. FA is primarily conjugated with glucuronic acid and then excreted in urine [4,15]. No unexpected accumulation of FA has been reported during repeat administration [16,17]. After an oral administration of 100 or 200 mg of FBT, the typical peak concentration (Cmax) for FA is approximately 5-15 μg/ mL [18-23]. The use of non-statin drugs such as fibrates has been modest and many health care professionals avoid consideration of combination therapy due to an inordinate fear of toxicity. In order to study the pharmacokinetic parameters of FA in human studies, it is necessary to develop a method for its determination in plasma. There have been several papers on the determination of FBT or its metabolites in biological fluids using techniques such as HPLC, GC-MS and LCMS [7,17,21-26]. Our method uses a simple one step liquid/liquid extraction procedure and HPLC-UV detection with a novel internal standard of 4’-chloro-5-fluro-2-hydroxybenzophenone (CFHB), (Figure 1c). Compared to previously reported methods, the sensitivity and recovery using this method is superior, since the LLOQ is 0.05 μg/ mL and the calibrated range is 0.05 to 10.0 mg/L.
Lipidil is registered in Australia for the treatment of dyslipidaemia and hypercholesterolemia. The method developed was used to investigate the bioavailability of two different oral formulations of fenofibrate: the first formulation consisted of a 200 mg capsule of Lipidil-Micro™ containing micrononized fenofibrate material, while the second was a 145 mg of nanonized Lipidil-EZ™ tablet. This study was performed by comparing plasma concentration level profiles of FA from 41 healthy volunteers.
Materials and Methods
Chemicals and reagents
Fenofibric acid was obtained from Eskay Industries (Pune, India). 4’-chloro-5-fluro-2-hydroxybenzophenone, phosphoric acid (85%), hydrochloric acid (37%) and ethyl acetate were supplied by Sigma Chemicals Ltd., (Poole, Dorset, UK). Acetonitrile, methanol and water were HPLC grade and obtained from Hichrom Ltd., (Reading, Berkshire, UK). The control human drug-free plasma was purchased from Charter House Clinical Research Unit, (London, UK).
Instrumentation
The LC system consisted of a Model 1100 series liquid chromatography equipped with a quadratic pump, vacuum degasser, thermostatic column compartment, auto-sampler and a UV detector; all purchased from Hewlett-Packard (Stockport, Cheshire, UK). The separation of fenofibric acid and CFHB (IS) was carried out using a mobile phase consisting of a mixture of acetonitrile and 0.02 M phosphoric acid (50:50, v/v) on a Symmetry Shield™ RP18 (150×4.60 mm) 5 μm column obtained from (Waters UK Ltd., Hertfordshire, UK) kept at ambient temperature. Before use, the mobile phase was degassed for 15 min in an ultrasonic bath. The flow rate was 1 mL/min and UV detection was carried out at 287 nm. All data obtained were processed and stored on a Vectra XA computer from Hewlett-Packard using the HP Chemstation (Rev.A.04.02 HP-1990-1996) software.
Preparation of stock and standard solutions
Stock solutions of FA and IS were prepared by dissolving appropriate amounts of the compounds in methanol to give final concentrations of 10 mg/mL and 1 mg/mL respectively. These were stored at 2-8°C, prior to use. Further dilutions of these stocks were made with methanol to give working solutions of 10 μg/mL for IS and 1 mg/mL and 10 μg/mL for FA. These working solutions were used to spike plasma samples either for a calibration curve (0.05 - 10 μg/mL) or for quality control during the validation. Calibration samples were prepared by spiking 475 μL of control human plasma with the appropriate amount of analytes (25 μL FA and 125 μL IS) on the day of analysis. Samples for determination of recovery, precision and accuracy were prepared by spiking control plasma in bulk at appropriate concentration [0.05 μg/ mL (QC-lowest), 0.12 μg/mL (QC low), 1.20 μg/mL (QC medium) and 8.20 μg/mL (QC high)]. These solutions were prepared and stored at -20°C, until analysis.
Sample preparation procedure
To 500 μL plasma sample, 125 μL of IS solution was added and mixed for 30 seconds with a vortex mixer. After the addition of 3 mL ethyl acetate and 1 mL of 1 M HCl, the mixture was vortexed for 5 min, followed by centrifugation for 5 min at 5,500×g. The top organic layer was separated and evaporated to dryness at 40°C using a stream of nitrogen. The residue was reconstituted in 100 μL of mobile phase and 25 μL was injected into the HPLC system.
Specificity and selectivity
The interference of endogenous fluid in plasma was investigated by analysing between three and six independent blank plasma samples and plasma spiked at the lower limit of quantitation (LLOQ).
Calibration curve
A calibration curve was acquired by plotting the peak area ratio of analytes FA:IS against the nominal concentration of calibration standards. The concentrations used were 0.05, 0.15, 0.30, 0.65, 1.25, 2.50, 5.00 and 10.00 μg/mL FA. The calibration needed to have a correlation coefficient (r2) of 0.98 or better in order to be acceptable, according to US Food and Drugs Administration Guidelines (FDA). The acceptance criterion for the standards was within ± 15% deviation from a nominal value, except at LLOQ, which was set at ± 20%.
Precision and accuracy
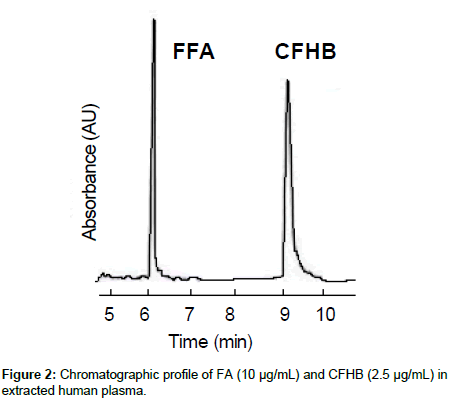
The inter-and intra- assay precision and accuracy were estimated by analysing six replicates containing FA at four levels QC, i.e., 0.05, 0.12, 1.20 and 8.20 μg/mL. The criterion for acceptability of the data was accuracy within ± 15% deviation from nominal value, except at LLOQ, which was set at ± 20%. A typical chromatogram of FA and CFHB in extracted human plasma is shown in Figure 2.
Recovery
The absolute recovery of FA and IS, through the liquid/liquid extraction procedure was determined by comparing the responses of analytes extracted from the six replicate QC samples at each level with the un-extracted samples. The recovery of the IS was determined at a single concentration.
Stability experiment
The stability of FA and IS in the biomatrix over 48 h was determined at ambient temperatures for four concentrations. The results obtained from the initial measurement were used as a reference to determine the relative stability of the analytes at subsequent points. Freeze stability of FA and IS in human plasma was assessed by analyzing the QC samples stored at -20°C. The stability of FA in human plasma following repeated freeze/thaw cycles was accessed using QC sample spiked with FA. The samples were stored at -20°C between freeze/thaw cycles. Samples were considered to be stable if %SD and precision (% RSD) is ± 15% of nominal value of QC’s, except for LLOQ, where it should be no more than 20% of the nominal value.
Pharmacokinetics study
The method developed was used to investigate the plasma profile after two oral doses of FBT: a Lipidil-Micro™ 200 mg of micronised fenofibrate capsule as a reference and a Lipidil-EZ™ 145 mg nanonised fenofibrate tablet (Solvey Pharmaceuticals, Brussels, Belgium). A bioequivalence study on 41 healthy young male volunteers was conducted. The subjects received one capsule containing 145 mg of Lipidil-EZ™ and blood samples were collected during 72 h. The plasma concentrations of FA were determined for both formulations.
Results and Discussion
Method development, specificity and selectivity
Various combinations of mobile phases were tried in order to get the best peak resolution and sensitivity for the analyte. The best resolution was achieved using 0.02 M phosphoric acid: acetonitrile (50:50, v/v) at 1 mL/min flow rate. The selectivity of the analytical method was investigated in order to prove that the method could be used for quantitative analysis of FA. Potential interfering substances in a biological matrix include endogenous fluid and metabolites of FBT. The selectivity was studied by analysing four individual blank plasma samples from different volunteers. No endogenous interference was observed at the analyte retention time.
Calibration curve
The calibration curve was constructed using eight calibrators with range of 0.05-10 μg/mL. The standard curve had a reliable reproducibility over the standard concentrations for FA across the calibration range. A calibration curve was prepared by determining the best fit peak area ratio (peak area analyte/peak area IS) versus concentration using a quadratic curve fit y=ax2+b. The average regression coefficient (r2) was 0.9988.
Accuracy and precision
The intra and inter day precision and accuracy are given in Table 1 along with lower limit of detection and linear range.
| Analyte | QC | Linear range | LOD | r2 | Intra-day | Inter-day | ||
|---|---|---|---|---|---|---|---|---|
| Precision% CV | Accuracy% | Precision% CV | Accuracy% | |||||
| (μg/mL) | ||||||||
| Fenofibric Acid | 0.05 | 0.05-10 | 0.025 | 0.9988 | 16.9 | 82.0 | 17.2 | 99.1 |
| 0.12 | 6.0 | 96.2 | 13.4 | 104.9 | ||||
| 1.20 | 6.7 | 96.3 | 7.6 | 95.0 | ||||
| 8.20 | 4.6 | 104.3 | 4.4 | 101.6 | ||||
Table 1: Summary of assay validation results.
Recovery
Un-extracted and extracted quantity controls were measured at 0.12, 1.2 and 8.2 μg/mL by injecting six replicates at each level. The absolute mean recovery was 79.8% for FA.
Stability
Over a 48 h period, mean accuracy of FA at QC 0.12, 1.2 and 8.2 μg/mL was 83.9%, 101.8% and 103.8% and mean precision was 12.7%, 5.5% and 6.3%, respectively.
Applications
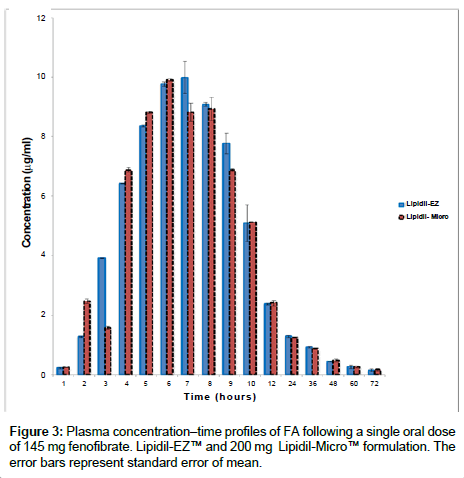
The method was applied to the plasma samples provided by 41 healthy, young, male volunteers who took part in a bioequivalence study. An ethics committee approved the protocol and the volunteers provided informed written consent to participate. The bioequivalence study was conducted according to the principles of the Declaration of Helsinki [27]. The study demonstrated bioequivalence between a micronised fenofibrate (original 200 mg capsule formulation of Lipidil- Micro™) and a new formulation of 145 mg Lipidil-EZ™ tablets. Bioequivalence was assessed by measuring plasma concentrations of FA. Plots of the plasma FA levels (μg/mL) versus post-dose sampling time (h) for the micronized and the nanonized formulations are presented in Figure 3. Pharmacokinetic parameters (AUC0→72, AUC0→∞, Cmax, tmax) calculated from these data are presented in Table 2 and clearly demonstrate the bioequivalence of the Lipidil-EZ™ and the micronized formulation Lipidil-Micro™. Results were obtained from each sample in duplicate and the mean calculated. The concentration profiles of the tested drug and reference drug were similar.
| Parameters | Lipidil-EZ™ | Lipidil-Micro™ |
|---|---|---|
| AUC0 →72(µgmL-1h) | 151.1 ± 53 | 152.1 ± 51.1 |
| AUC0 →∞ (µgmL-1 h) | 161.2 ± 49.2 | 160.1 ± 50.2 |
| Cmax(µgmL-1) | 9.1 ± 1.5 | 10.1 ± 2.0 |
| tmax(h) | 7.1 ± 1.3 | 6.0 ± 1.2 |
Table 2: Pharmacokinetic parameters, where AUC shows area under curve and Cmax shows the peak plasma concentration of the drugs after administration and tmax means time to reach Cmax.
These pharmacokinetic studies in healthy volunteers have demonstrated that a dose of 145 mg of the new nanonized Lipidil-EZ™ tablet is sufficient to give plasma levels equivalent to those previously achieved by a dose of 200 mg of the micronised fenofibrate Lipidil- Micro™ capsule.
Conclusion
A simple, precise, accurate, economical and robust method was developed and validated to determine fenofibric acid in human plasma using a novel internal standard (4’-chloro-5-fluro-2- hydroxybenzophenone) to meet the requirements for a pharmacokinetic investigation of this compound. Moreover, the sensitivity of this method is higher, since the determined LLOQ is 0.05 μg/mL compared to previously reported methods [21-25]. This method gives narrow peaks, satisfactory selectivity and a short run-time as compared to the published methods. FA and CFHB were extracted using a single step liquid/liquid extraction, thus reducing cost, time and effort. The developed method has been successfully applied to the determination of FA in plasma levels for investigating the bioequivalence of a new oral formulation of FBT (Lipidil-EZ™ by Solvey Pharmaceuticals).
References
- Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG (2002) Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care 25: 1198-1202.
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, et al. (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98: 2088-2093.
- Wong RP, Davis TM (2012) In vitro antimalarial activity and drug interactions of fenofibric acid. Antimicrob Agents Chemother 56: 2814-2818.
- Després JP (2001) Increasing high-density lipoprotein cholesterol: an update on fenofibrate. Am J Cardiol 88: 30N-36N.
- Ramachandran S, Abbas A, Saraf S, Raju J, Jewkes C, et al. (2012) Significant increase in high-density lipoprotein cholesterol with fibrates is associated with low pretreatment high-density lipoprotein cholesterol: findings from an outpatient clinic setting. Metab Syndr Relat Disord 10: 189-194.
- Weisweiler P (1989) Low-dose colestipol plus fenofibrate: effects on plasma lipoproteins, lecithin:cholesterolacyltransferase, and postheparin lipases in familial hypercholesterolemia. Metabolism 38: 271-274.
- Desager JP (1978) Gas--liquid chromatographic determination of procetofenic acid in human plasma and urine. J Chromatogr 145: 160-164.
- Hu L, Wu H, Niu F, Yan C, Yang X, et al. (2011) Design of fenofibratemicroemulsion for improved bioavailability. Int J Pharm 420: 251-255.
- Ling H, Luoma JT, Hilleman D (2013) A Review of Currently Available Fenofibrate and Fenofibric Acid Formulations. Cardiol Res 4: 47-55.
- Breyer S, Semmler A, Miller T, Hill A, Geissler S, et al. (2012) Radioiodinated dechloro-4-iodofenofibrate: a hydrophobic model drug for molecular imaging studies. Int J Pharm 431: 78-83.
- Van Speybroeck M, Mellaerts R, Mols R, Thi TD, Martens JA, et al. (2010) Enhanced absorption of the poorly soluble drug fenofibrate by tuning its release rate from ordered mesoporous silica. Eur J Pharm Sci 41: 623-630.
- Vecera R, Zacharová A, Orolin J, Strojil J, Skottová N, et al. (2011) Fenofibrate-induced decrease of expression of CYP2C11 and CYP2C6 in rat. Biopharm Drug Dispos 32: 482-487.
- Vogt M, Kunath K, Dressman JB (2008) Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm 68: 283-288.
- Miller DB, Spence JD (1998) Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet 34: 155-162.
- Guivarc'h PH, Vachon MG, Fordyce D (2004) A new fenofibrate formulation: results of six single-dose, clinical studies of bioavailability under fed and fasting conditions. Clin Ther 26: 1456-1469.
- Patel T, Patel L, Patel T, Makwana S, Patel T (2010) Enhancement of dissolution of Fenofibrate by Solid dispersion Technique. Int J Res Pharm Sci 1: 127-132.
- Streel B, Hubert P, Ceccato A (2000) Determination of fenofibric acid in human plasma using automated solid-phase extraction coupled to liquid chromatography. J Chromatogr B Biomed Sci Appl 742: 391-400.
- Ardila Quintero JA, RomãoSartori E, Rocha-Filho RC, Fatibello-Filho O (2012) Square-wave voltammetric determination of bezafibrate in pharmaceutical formulations using a cathodically pretreated boron-doped diamond electrode. Talanta 103: 201-206.
- de Melo J, Hurtado FK, Poitevin FS, Flores FC, Zimmermann ES, et al. (2010) HPLC determination of bezafibrate in human plasma and its application to pharmacokinetics studies. J Chromatogr Sci 48: 362-366.
- Srivastava RA (2009) Fenofibrate ameliorates diabetic and dyslipidemic profiles in KKAy mice partly via down-regulation of 11beta-HSD1, PEPCK and DGAT2. Comparison of PPARalpha, PPARgamma, and liver x receptor agonists. Eur J Pharmacol 607: 258-263.
- Bhavesh D, Shah S, Shivprakash (2009) Determination of fenofibric acid in human plasma by ultra performance liquid chromatography-electrospray ionization mass spectrometry: application to a bioequivalence study. Biomed Chromatogr 23: 922-928.
- Kumar A, Monif T, Khuroo AH, Iyer SS, Singh AK, et al. (2010) Development and validation of a LC-ESI-MS/MS method in human plasma for quantification of fenofibric acid, involving chromatographic resolution of fenofibric acid acyl-β-D-glucuronide. Anal Methods 2: 1584-1591.
- Salama FM, Nassar MW, Sharaf El-Din MM, Attia KA, Kaddah MY (2011) Determination of fenofibrate and the degradation product using simultaneous UV-derivative spectrometric method and HPLC. Am J Anal Chem 2: 332-343.
- Trivedi RK, Kallem RR, Mullangi R, Srinivas NR (2005) Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC-MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal 39: 661-669.
- Martin PD, Dane AL, Schneck DW, Warwick MJ (2003) An open-label, randomized, three-way crossover trial of the effects of coadministration of rosuvastatin and fenofibrate on the pharmacokinetic properties of rosuvastatin and fenofibric acid in healthy male volunteers. Clin Ther 25: 459-471.
- Straka RJ, Burkhardt RT, Fisher JE (2007) Determination of fenofibric acid concentrations by HPLC after anion exchange solid-phase extraction from human serum. Ther Drug Monit 29: 197-202.
- World Medical Association General Assembly (2004) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J IntBioethique 15: 124-129.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16229
- [From(publication date):
specialissue-2014 - Aug 11, 2025] - Breakdown by view type
- HTML page views : 11454
- PDF downloads : 4775