Research Article Open Access
Determination of Vancomycin in Human Plasma, Bone and Fat by Liquid Chromatography/Tandem Mass Spectrometry
Mei Zhang1,2*, Grant A Moore2 and Simon W Young31Department of Medicine, University of Otago-Christchurch, Christchurch, New Zealand
2Toxicology, Canterbury Health Laboratories, Christchurch, New Zealand
3Department of Orthopaedics, North Shore Hospital, Auckland, New Zealand
- *Corresponding Author:
- Dr. Mei Zhang
Clinical Pharmacology, Department of Medicine
University of Otago - Christchurch/ Toxicology
Canterbury Health Laboratories, Christchurch, New Zealand
Tel: 0064 3 364 0640 ext 89746
Fax: 0064 3 364 1003
E-mail: mei.zhang@cdhb.health.nz
Received date: June 27, 2014; Accepted date: July 16, 2014; Published date: July 18, 2014
Citation: Zhang M, Moore GA, Young SW (2014) Determination of Vancomycin in Human Plasma, Bone and Fat by Liquid Chromatography/Tandem Mass Spectrometry. J Anal Bioanal Tech 5: 196. doi: 10.4172/2155-9872.1000196
Copyright: © 2014 Zhang M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A liquid chromatography/tandem mass spectrometry (LC-MS/MS) assay was developed for the determination of vancomycin in human plasma, bone and fat tissue. For vancomycin in plasma, sample was treated with methanol to precipitate the proteins. After centrifugation, the supernatant was diluted with water, and then injected into the LC-MS/MS system. For vancomycin in bone and fat, the pulverized bone/fat samples were immersed in phosphate buffered saline pH 7.3 at 4°C overnight. After centrifugation, the supernatant of bone/fat tissue suspension was treated in the same way as the plasma samples. Vancomycin and aminopterin, the internal standard, were resolved on a C18(2) column using gradient elution of 0.05% formic acid and methanol. The two compounds were detected using electrospray ionisation in the positive mode. Standard curves were linear over the concentration range 0.05 to 50 mg/L (r2>0.99) in plasma, bone and fat. Bias was ≤ ± 10% from 0.05 to 50 mg/L, intra- and inter-day coefficients of variation (imprecision) were <10%, and the limit of quantification was 0.05 mg/L. The assay has been used successfully in a clinical study to investigate the regional delivery of vancomycin in bone and fat after prophylactic administration of vancomycin through an intraosseous route or systemic route, during total knee arthroplasty.
Keywords
Vancomycin; Plasma; Bone; Fat; LC-MS/MS
Introduction
Total knee arthroplasty (TKA) relieves pain and restores function in patients with often debilitating arthritic disease. However, despite improved surgical techniques, deep infection remains a devastating complication with a reported prevalence of 0.86-2.5% [1-5]. It is believed that the vast majority of early post-operative bone infections are due to contamination at the time of surgery [6]. Prophylactic antibiotics are thought to reduce the risk that contamination will progress to overt clinical infection.
As antibiotic resistance increases, systemic administration of antibiotics may no longer achieve tissue concentrations high enough to provide adequate prophylaxis. Regional administration utilising a tourniquet has been suggested [7], and performing this via the intraosseous route is currently under investigation. The advantages of regional administration include reducing systemic drug concentrations and side effects while achieving higher tissue concentrations at the operative site. Vancomycin (Figure 1) may be well suited to this alternative method of prophylaxis administration, as the use of a lower more targeted dose may reduce systemic side effects such as nephrotoxicity while increasing efficacy due to higher local tissue concentrations. However to date no study has investigated the concentrations of vancomycin in bone and fat tissues during TKA after prophylactic administration through the intraosseous and systemic routes.
Pharmacokinetic studies of vancomycin in plasma, bone and fat tissues require a well validated analytical method to analyse the concentrations of vancomycin, especially for bone tissue due to the heterogeneity of bone. Various analytical methods have been developed for measuring vancomycin in liquid samples only, such as plasma/serum, urine, human drainage tissue fluid and bronchoalveolar lavage fluid, including HPLC methods [8-10] and LC-MS/MS methods [11-15]. A few methods have been reported for the determination of vancomycin in both liquid and solid samples, such as immunoassays for plasma/serum and bone [16,17], HPLC method for plasma, bone and atrial appendage tissue [18], and LC-MS/MS for serum, perivascular fat and atrial wall samples [19]. It appears that no method has been reported for the determination of vancomycin in human plasma, bone and fat tissues using LC-MS/MS.
The aim of this work was to develop a LC-MS/MS method with an efficient extraction recovery for measurement of vancomycin concentrations in plasma, bone and fat samples and to apply it to a clinical study of vancomycin in bone and fat after prophylactic administration of vancomycin via the intraosseous or systemic routes, during TKA.
Materials and Methods
Materials
Vancomycin hydrochloride (Potency: ~1,000 μg per mg ) and aminopterin (~98%) (Figure 1) were purchased from Sigma Co. (Australia). HPLC grade acetonitrile, methanol, and perchloric acid were purchased from BDH (Poole, UK). Phosphate buffered saline pH 7.3 (Dulbecco A) was purchased from Oxoid (Basingstoke, UK). Distilled, deionised water was produced by a Milli-Q Reagent Water System (Millipore, MA, USA). Bone and fat samples were obtained from the patients who received TKA surgery. The vancomycin-free bone and fat samples used as the assay blank and for the preparation of standards were obtained from patients on antibiotics but not vancomycin during TKA surgery. The use of the tissues was approved by the local Ethics Committee. All bone and fat samples were stored at -80°C until analysed. The human plasma used as the assay blank and for the preparation of standards was obtained from New Zealand Blood Services (Christchurch, New Zealand).
Instrumentation and analytical conditions
The LC-MS/MS system consisted of a Shimadzu LC-20AD HPLC system (Shimadzu Corporation, Kyoto, Japan) interfaced with an API 4000™ triple quadrupole mass spectrometer (Applied Biosystems, Foster City, Canada) equipped with a TurboIonSpray® source. Analyst software (Applied Biosystems, Foster City, Canada) was used to control equipment, to coordinate data acquisition, and to analyse data. Vancomycin and the internal standard aminopterin were separated under gradient elution using a Luna C18(2) 5 μm, 50 × 2.0 mm internal diameter analytical column equipped with a C18 4.0 × 2.0 mm internal diameter guard column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of solvent A (0.05% formic acid in water) and solvent B (methanol). The flow rate was set at 0.3 mL/min. The initial condition was 100% solvent A. A linear gradient was performed with mobile phase B increasing from 0% to 90% within 1 min. After 4 min, the mobile phase was returned to the initial condition and reequilibrated for 2 min. The total analysis time was 6 min.
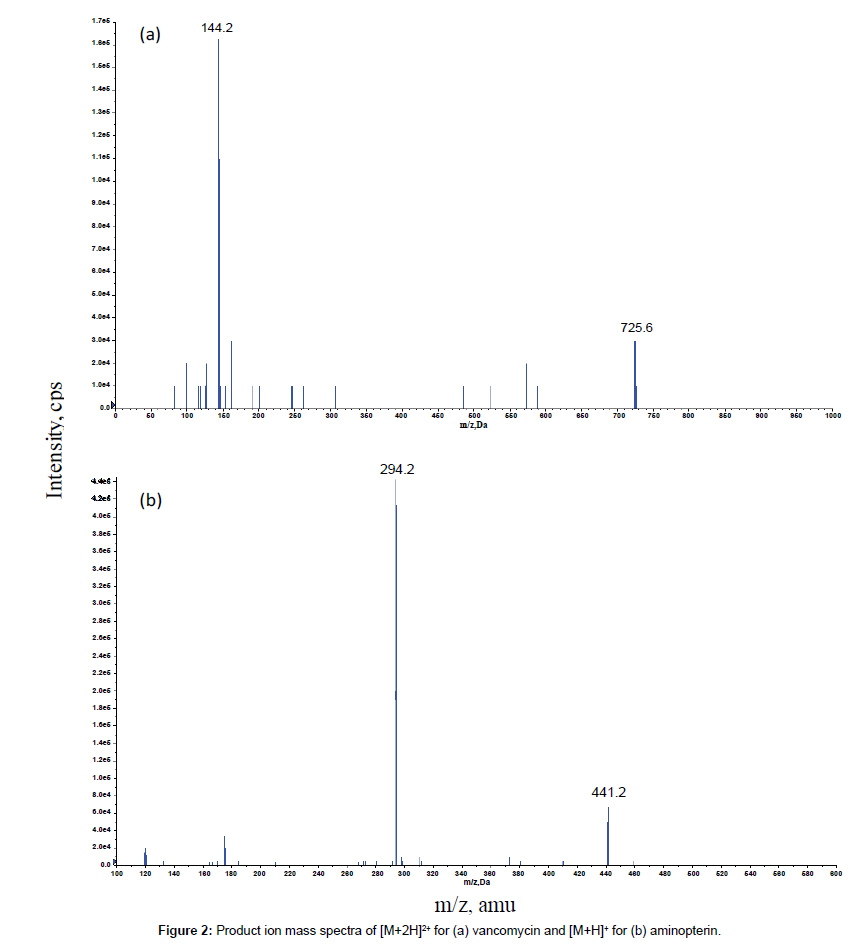
The mass spectrometer was operated in the positive ion mode with curtain gas, Gas 1 and Gas 2 flow rates of 20, 45 and 60 psi, respectively. The ion spray voltage was 5000 V and the source temperature was 500°C. Data acquisition was performed via multiple reaction monitoring (MRM). The optimized precursor-to-product ion transitions monitored for vancomycin [M+2H]2+ and aminopterin [M+H]+ were m/z 725.6 → 144.2 with declustering potential (DP) 51 V and collision energy (CE) 23 V and m/z 441.2 → 294.2 with DP 71 V and CE 29 V, respectively.
Standards
A standard stock solution of vancomycin (1.0 mg/mL as free base) was prepared by dissolving 10.25 mg of vancomycin hydrochloride in 10 mL of water and stored at -80°C. Two sets of the same standard stock solutions were prepared for standard curves and for quality control (QC) samples respectively.
For the analysis of vancomycin concentration in plasma, the standard curve of vancomycin was prepared by spiking appropriate aliquots of vancomycin standard stock solutions to drug-free plasma, to give spiked vancomycin standards of 0.05, 0.1, 0.5, 1.0, 5.0, 10, 25 and 50.0 mg/L. Vancomycin plasma quality control (QC) standards were prepared in single 5 mL aliquots in concentrations of 0.05, 1.0, 10 and 50 mg/L and stored at -80°C until analysed.
For the analysis of vancomycin concentration in bone and fat, the supernatants of immersed blank bone and fat tissue suspensions for the preparation of standards were prepared. The blank bone samples were crushed with pliers, finely cut further with a scalpel, then weighed and immersed in the extraction solvent, phosphate buffered saline pH 7.3, (the ratio of bone/ phosphate buffered saline pH 7.3 was around 1:5, w/v) at 4°C overnight. The blank fat samples were finely cut with a scalpel, and then treated in a same way as the bone samples. The immersed bone or fat tissue suspensions were vortexed for 30 seconds and centrifuged at 15,000 g for 10 min to precipitate the tissue particles. The supernatants of the immersed blank bone and fat tissue suspensions were transferred into a clean tube, respectively. The standard curves for the vancomycin analysis in bone and fat were prepared by spiking appropriate aliquots of vancomycin standard stock solutions to the supernatants of immersed blank bone and fat tissue suspensions respectively, to give spiked vancomycin standards of 0.05, 0.1, 0.5, 1.0, 5.0, 10, 25 and 50.0 mg/L. The QC samples of vancomycin in the supernatants of immersed bone and fat tissue suspensions were prepared in single 5 mL aliquots in concentrations of 0.05, 1.0, 10 and 50 mg/L respectively and stored at -80°C until analysed.
The stock internal standard aminopterin solution (1.0 mg/mL) was prepared by dissolving 5.0 mg of aminopterin in 5.0 mL of 0.1 M NaOH. A working solution of the internal standard (0.25 μg/mL) was prepared by diluting the stock solution with water.
Sample preparation
For the analysis of vancomycin concentration in plasma: 50 μL of the internal standard, aminopterin, 0.25 μg/mL, was added to 50 μL of each of the blank, standard, QC and patient samples. The mixtures were vortexed briefly. Two hundred microliters of methanol was added to precipitate the proteins. After centrifugation at 15,000 g for 5 min, a 50 μL aliquot of clear supernatant was mixed with 500 μL of water and transferred to the 96 well microtiter autosampler plate. A volume of 10 μL was injected into the LC-MS/MS system. All the samples were analysed in duplicate.
For the analysis of vancomycin concentration in bone and fat: Based on the method previously described by Graziani et al. [16], phosphate buffered saline pH 7.3 (PBS) was selected as the extraction solvent to extract vancomycin from bone and fat tissue. The bone samples were crushed with pliers, finely cut further with a scalpel, then weighed and immersed in the extraction solvent PBS (the ratio of bone/ PBS was around 1:5, w/v) at 4°C overnight to extract vancomycin from the bone. The fat samples were finely cut with a scalpel, and then treated in the same way as the bone samples. The immersed bone or fat tissue suspension were vortexed for 30 seconds and centrifuged at 15,000 g for 10 min to precipitate the tissue particles. 50 μL of the supernatant was transferred to a clean tube and treated in a same way as the plasma sample.
Validation
The standard curve was the plot of the peak area ratios (analyte/ internal standard) of vancomycin versus the corresponding concentrations of vancomycin. To evaluate the assay recoveries and matrix effects, three sets of standards were prepared using a modification of the method of Matuszewski et al. [20] for vancomycin and the internal standard aminopterin. The standards of vancomycin were prepared at concentrations of 0.05, 1.0, 10 and 50 mg/L, and aminopterin at 0.25 μg/mL, the concentration used in the assay. The first set was prepared in plasma or the supernatants of immersed blank bone and fat tissue suspensions, the second set in blank extracted plasma or blank extracted bone and fat tissue suspensions (the extracts of blank plasma or bone and fat tissue suspensions after-protein precipitation), and the third set in water. Every set included six samples at each concentration. Absolute recoveries at each concentration were measured by comparing the peak areas of vancomycin and aminopterin in plasma or the supernatants of immersed blank bone and fat tissue suspensions to those in the spiked extracts at the corresponding concentrations (n = 6) [Absolute recovery = (peak area of analyte in spiked plasma sample or spiked blank bone and fat tissue suspension sample)/(peak area of analyte in spiked extract sample of corresponding matrix) × 100%]. The matrix effects were assessed by comparing the peak areas of vancomycin and aminopterin from the spiked extracts with the response of standard solution at the same concentration in water (n = 6) [Matrix effect = (peak area of analyte in spiked extract sample)/(peak area of analyte in spiked water sample) × 100%]. A value of 100% indicates that the responses in water and in spiked extract are the same and no absolute matrix effect is observed. A value of >100% indicates ionization enhancement and a value of <100% indicates ionization suppression. In our work, matrix effect value 100 ± 15% are acceptable. Quality control was assessed by analysis of six samples at each concentration on the same day (intra-day) and of one sample at each concentration on six different days (inter-day). Bias was determined as the measured minus the actual concentration, expressed as a percentage of the actual concentration. Imprecision was measured as intra- and inter-day coefficients of variation. The lowest concentration for vancomycin standard curve was considered to be the lower limit of quantitation (LLOQ) at which the concentration of vancomycin could be determined with acceptable accuracy and precision. According to the US Food and Drug Administration guidance for bioanalytical method validation [21], the mean value determined at LLOQ should not deviate by more than 20% of the actual value, and the precision determined at LLOQ should not exceed 20% of the coefficients of variation (CV). The limit of detection (LOD) was defined as the lowest concentration that can be detected in a sample but not necessarily quantified. The LOD was calculated as 3 times the response compared to blank response.
The effects of freezing and thawing on the concentrations of vancomycin were studied using QC samples of plasma, bone and fat at 0.05, 1.0, 10 and 50 mg/L, which were subjected to four freezethaw cycles before analysis. The stability of QC samples at -80°C was evaluated by concentration analysis at weekly intervals for four months. The stability of the stock standard solutions of vancomycin at -80°C for six months was evaluated by comparing the response with that of the freshly prepared standard solutions. The stability of the processed samples at 4°C (the temperature of the autosampler) for 24 hours was evaluated by comparing the results with the original results. In all cases, vancomycin were considered to be stable as long as degradation was <10% of the concentration at day 0. Cefazolin was analysed to investigate possible interference because it is the most likely co-administered drug.
Results and Discussion
Mass spectrometry and Chromatography
The MS/MS parameters were optimised to produce maximum responses for vancomycin and the internal standard aminopterin using electrospray ionisation in the positive ion mode. The protonated molecular ions of [M + 2H]2+ for vancomycin and [M + H]+ for aminopterin were m/z 725.6 and m/z 441.2, respectively. The transitions yielding the most abundant product ions were 725.6 → 144.2 for vancomycin and 441.2 → 294.2 for aminopterin. The product ion spectra of [M + 2H]2+ for vancomycin and [M + H]+ for aminopterin are shown in Figure 2.
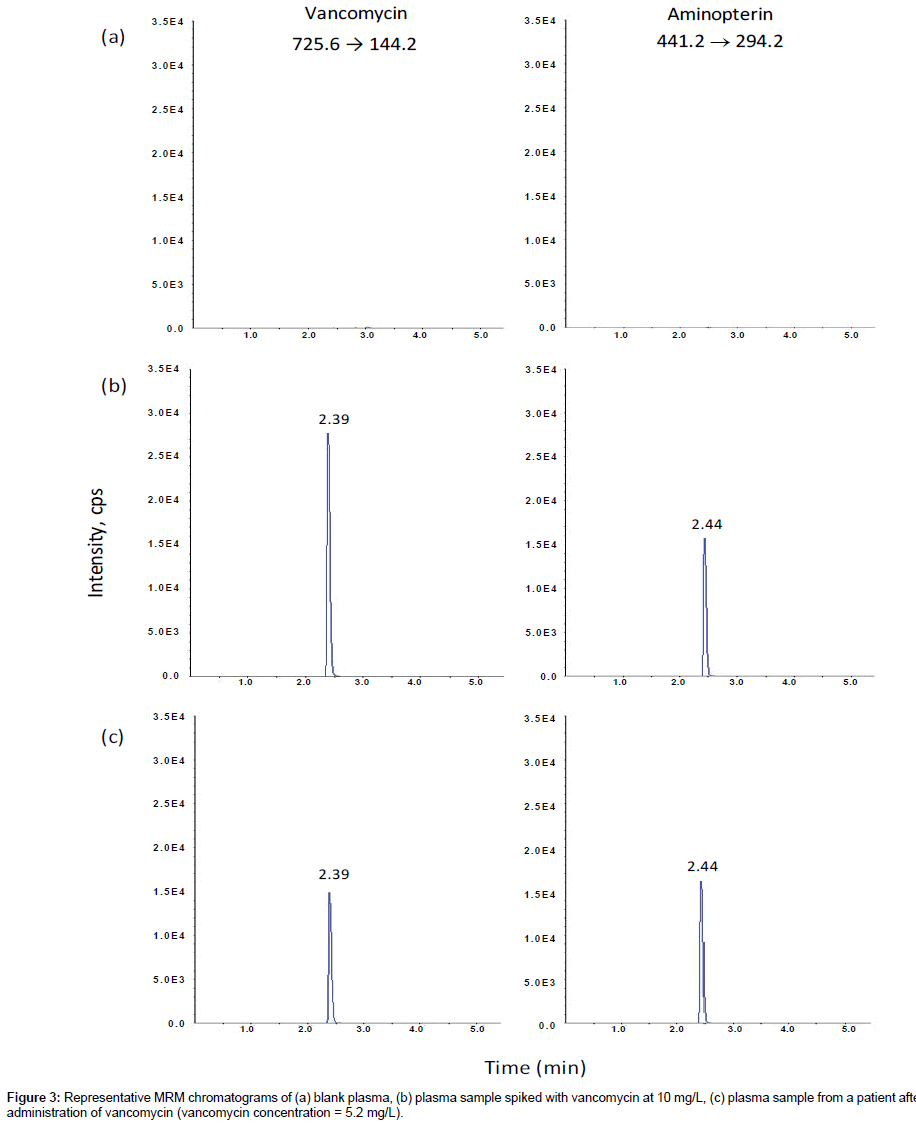
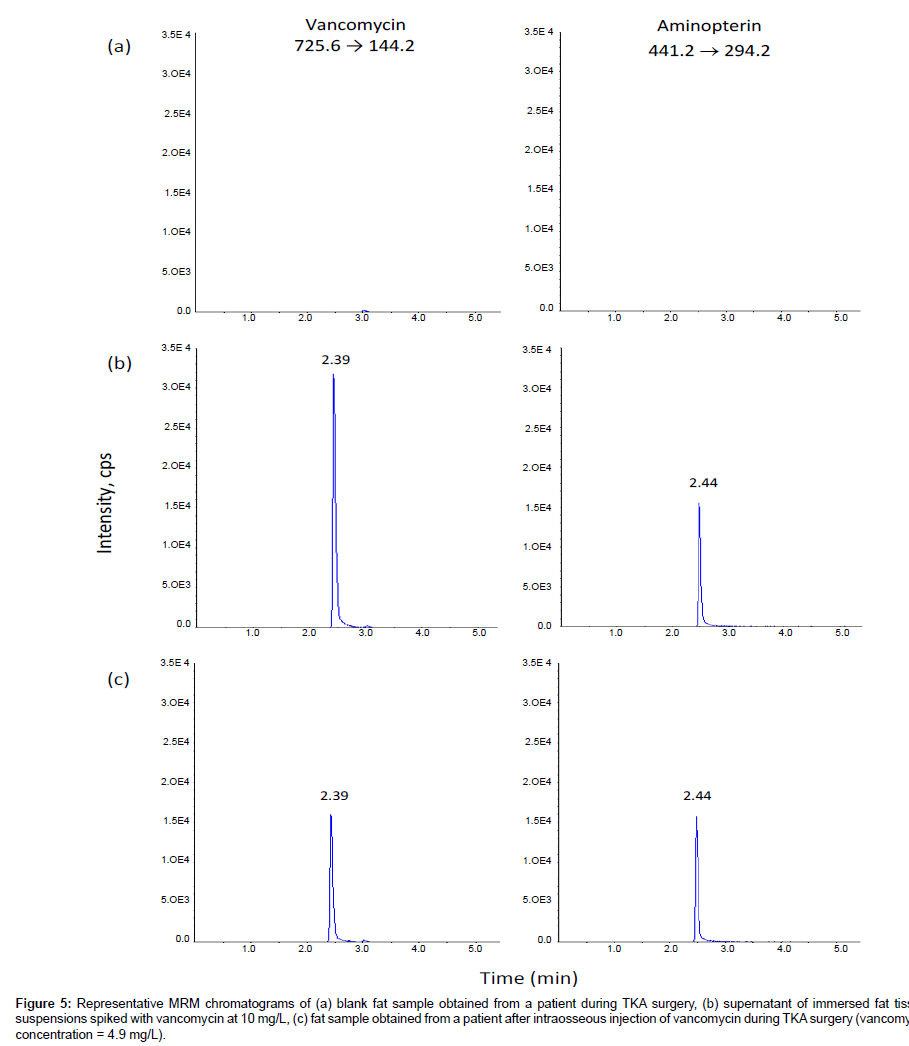
Phenomenex Luna C18(2) and C8 columns gave the best chromatographic resolution and peak sharpness. Choosing the Phenomenex Luna C18(2) column over the Phenomenex Luna C8 column enabled this method to share the same column with another routine method in our laboratory, so that we could run both methods overnight without the need to change columns. The mobile phase consisting of 0.05% formic acid and methanol gave higher signal intensity. Gradient elution with increasing methanol decreased the retention of the components so that they eluted faster with sharper peaks, speeding up the analysis time for each sample. The optimized LC condition chosen was therefore a mobile phase consisting of 0.05% formic acid and methanol with gradient elution on a Phenomenex Luna C18(2) column. Under these conditions, the retention times were approximately 2.39, and 2.44 min for vancomycin and the internal standard aminopterin, respectively (Figures 3-5). Blank samples of plasma, bone and fat from more than six different sources of the same matrix were tested for interference, and vancomycin and the internal standard peaks were free of interference from any other peaks present in the blanks (Figures 3-5). There was no interference of cefazolin.
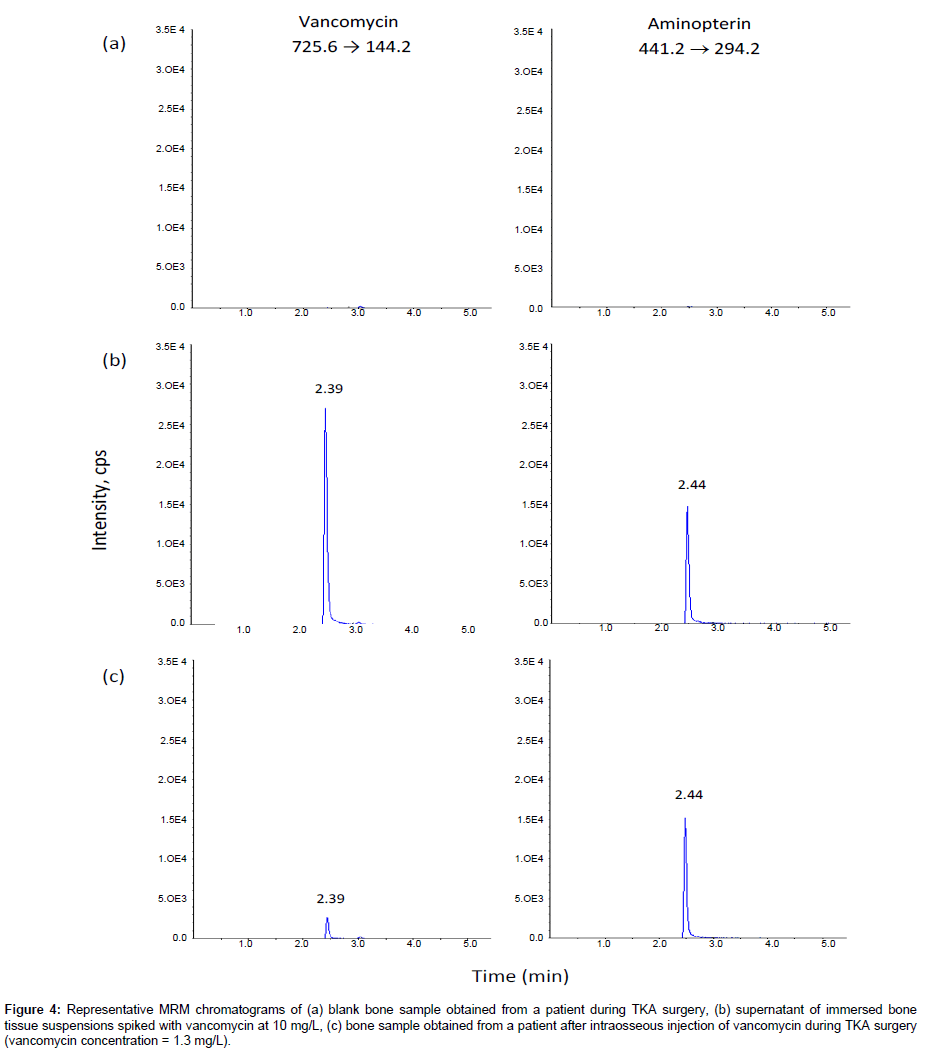
Figure 4: Representative MRM chromatograms of (a) blank bone sample obtained from a patient during TKA surgery, (b) supernatant of immersed bone tissue suspensions spiked with vancomycin at 10 mg/L, (c) bone sample obtained from a patient after intraosseous injection of vancomycin during TKA surgery (vancomycin concentration = 1.3 mg/L).
Figure 5: Representative MRM chromatograms of (a) blank fat sample obtained from a patient during TKA surgery, (b) supernatant of immersed fat tissue suspensions spiked with vancomycin at 10 mg/L, (c) fat sample obtained from a patient after intraosseous injection of vancomycin during TKA surgery (vancomycin concentration = 4.9 mg/L).
Sample preparation
Protein precipitation is the simplest and most rapid sample cleanup method of liquid sample preparation for the determination of drugs. Methanol is one of the most widely used precipitating agents. Precipitation with methanol (4:1 ratio of methanol to sample) gave the best sample cleanup and highest recoveries for vancomycinin and the internal standard aminopterin.
Method validation
For the analysis of vancomycin concentration in plasma, bone and fat, standard curves were adequately fitted by 1/x² weighted quadratic regressions (r>0.999) over the concentration range of 0.05 to 50 mg/L. The LOD was around 0.02 mg/L and the LLOQ was around 0.05 mg/L. The accuracy and precision were assessed at LLOQ and the low, medium and high level QCs. There was no constant direction to the bias (i.e. + or -) for LLOQ and QCs and the mean values were within ± 10% of the spiked values (Tables 1-3). Imprecision was small, as indicated by both intra- and inter-day coefficients of variation of <10% at concentrations of LLOQ and QCs (Tables 1-3). The absolute recoveries of vancomycin from plasma and the supernatants of immersed bone and fat tissue suspensions at concentrations of 0.05, 1.0, 10 and 50 mg/L were similar and consistent, with mean values of around 100%. The absolute recoveries of the internal standard aminopterin from plasma and the supernatants of immersed bone and fat tissue suspensions at the concentration employed were similar with mean values of around 100%.
| Sample | Concentrationspiked (mg/L) | Concentration found (mg/L)(Mean ± SD) | Bias(%) | ImprecisionCV (%) | |
|---|---|---|---|---|---|
| Intra-day | |||||
| LLOQ | 0.050 | 0.048 ± 0.0036 | -4.00 | 7.5 | |
| QC1 | 1.00 | 1.01 ± 0.04 | 0.83 | 3.9 | |
| QC2 | 10.0 | 9.43 ± 0.34 | -5.70 | 3.6 | |
| QC3 | 50.0 | 49.5 ± 0.91 | -0.92 | 1.9 | |
| Inter-day | |||||
| LLOQ | 0.050 | 0.050 ± 0.0034 | -0.67 | 6.9 | |
| QC1 | 1.00 | 1.00 ± 0.060 | 0.33 | 6.0 | |
| QC2 | 10.0 | 10.0 ± 0.38 | 0.38 | 3.8 | |
| QC3 | 50.0 | 49.5 ± 0.75 | -1.1 | 1.5 | |
Table 1: Intra- and inter-day assay variance of the determination of vancomycin in plasma (n = 6).
| Sample | Concentrationspiked (mg/L) | Concentration found (mg/L)(Mean ± SD) | Bias(%) | Imprecision CV (%) | |
|---|---|---|---|---|---|
| Intra-day | |||||
| LLOQ | 0.050 | 0.049 ± 0.0040 | -0.60 | 8.1 | |
| QC1 | 1.00 | 0.92 ± 0.036 | -8.19 | 3.9 | |
| QC2 | 10.0 | 10.0 ± 0.32 | 0.17 | 3.2 | |
| QC3 | 50.0 | 51.6 ± 1.24 | 3.27 | 2.4 | |
| Inter-day | |||||
| LLOQ | 0.050 | 0.052 ± 0.0046 | 4.00 | 8.8 | |
| QC1 | 1.00 | 1.03 ± 0.054 | 2.85 | 5.2 | |
| QC2 | 10.0 | 10.2 ± 0.26 | 1.51 | 2.6 | |
| QC3 | 50.0 | 50.1 ± 1.71 | 0.20 | 3.4 | |
Table 2: Intra- and inter-day assay variance of the determination of vancomycin in supernatant of immersed bone tissue suspension (n = 6).
| Sample | Concentration spiked (mg/L) |
Concentration found (mg/L) (Mean ± SD) |
Bias (%) |
Imprecision CV (%) |
|
|---|---|---|---|---|---|
| Intra-day | |||||
| LLOQ | 0.050 | 0.046 ± 0.0044 | -8.00 | 9.6 | |
| QC1 | 1.00 | 1.02 ± 0.027 | 2.67 | 2.2 | |
| QC2 | 10.0 | 9.55 ± 0.23 | -4.51 | 2.4 | |
| QC3 | 50.0 | 51.7 ± 1.31 | 3.45 | 2.53 | |
| Inter-day | |||||
| LLOQ | 0.050 | 0.051 ± 0.0053 | 6.25 | 10.0 | |
| QC1 | 1.00 | 1.05 ± 0.062 | 5.87 | 5.9 | |
| QC2 | 10.0 | 10.0 ± 0.32 | 0.45 | 3.2 | |
| QC3 | 50.0 | 52.5 ± 1.10 | 5.0 | 2.1 | |
Table 3: Intra- and inter-day assay variance of the determination of vancomycin in supernatant of immersed fat tissue suspension (n = 6).
The matrix effects (mean ± SD%) for plasma and the supernatants of immersed bone and fat tissue suspensions at concentrations of 0.05, 1.0, 10 and 50 mg/L were similar and consistent, with mean values of around 100%. The matrix effects for the internal standard aminopterin for plasma and the supernatants of immersed bone and fat tissue suspensions at the concentration employed were similar with mean values of around 100%. No significant matrix effects were evident.
Vancomycin was found to be stable in plasma and the supernatants of immersed bone and fat tissue suspensions for at least four freezethaw cycles when stored at -80°C. The QC samples were stable for at least four months at -80°C. Stock standard solutions of vancomycin remained stable for at least six months at -80°C. The processed samples were stable for at least 24 hours at 4°C. In all cases, the concentrations of vancomycin of stored samples deviated <10% from the freshly prepared samples.
Application of the assays
The method has been used in a clinical study to investigate the regional delivery of vancomycin in bone and fat after prophylactic administration of vancomycin through intraosseous route or systemic route, during TKA. We also used this method to monitor the vancomycin concentrations in plasma for patients on vancomycin therapy. Clinical guidelines for vancomycin use in humans recommend trough serum concentrations of 15-20 mg/L [22]. We have analysed nearly 500 plasma, bone and fat samples. vancomycin concentrations were 0.05–30.5 mg/L with the majority between 0.30–25.0 mg/L, which was well covered by the concentration range of the vancomycin standard curve. All patient samples were analysed in duplicate and the variations between duplicate analysis results were <10%. To ensure the accuracy and reproducibility of the method for patient samples, some patient samples were reanalysed. All the repeat values were ≤ 15% difference from the initial value.
Conclusion
A validated LC-MS/MS method for the determination of vancomycin in plasma, bone and fat has been described. The method has been used in a clinical study, and proven to be rapid, sensitive, specific and accurate.
References
- Bengtson S, Knutson K (1991) The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand 62: 301-311.
- Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, et al. (2004) Infection after total knee arthroplasty. J Bone Joint Surg Br 86: 688-691.
- Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR (2010) The microbiology of the infected knee arthroplasty. Int Orthop 34: 505-510.
- Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ (2006) The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br 88: 943-948.
- Wilson MG, Kelley K, Thornhill TS (1990) Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am 72: 878-883.
- Fletcher N, Sofianos D, Berkes MB, Obremskey WT (2007) Prevention of perioperative infection. J Bone Joint Surg Am 89: 1605-1618.
- de Lalla F, Viola R, Pellizzer G, Lazzarini L, Tramarin A, et al. (2000) Regional prophylaxis with teicoplanin in monolateral or bilateral total knee replacement: an open study. Antimicrob Agents Chemother 44: 316-319.
- Jesús Valle MJ, López FG, Navarro AS (2008) Development and validation of an HPLC method for vancomycin and its application to a pharmacokinetic study. J Pharm Biomed Anal 48: 835-839.
- Abu-Shandi KH (2009) Determination of vancomycin in human plasma using high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 395: 527-532.
- Hagihara M, Sutherland C, Nicolau DP (2013) Development of HPLC methods for the determination of vancomycin in human plasma, mouse serum and bronchoalveolar lavage fluid. J Chromatogr Sci 51: 201-207.
- Cass RT, Villa JS, Karr DE, Schmidt Jr DE (2001) Rapid bioanalysis of vancomycin in serum and urine by high-performance liquid chromatography tandem mass spectrometry using on-line sample extraction and parallel analytical columns. Rapid Commun Mass Spectrom 15: 406-412.
- Shibata N, Ishida M, Prasad YVR, Gao W, Yoshikawa Y, et al. (2003) Highly sensitive quantification of vancomycin in plasma samples using liquid chromatography–tandem mass spectrometry and oral bioavailability in rats. J Chromatogr B Analyt Technol Biomed Life Sci 789: 211-218.
- Cheng C, Liu S, Xiao D, Hollembaek J, Yao L, et al. (2010) LC-MS/MS method development and validation for the determination of polymyxins and vancomycin in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878: 2831-2838.
- König K, Kobold U, Fink G, Leinenbach A, Dülffer T, et al. (2013) Quantification of vancomycin in human serum by LC-MS/MS. Clin Chem Lab Med 51: 1761-1769.
- Shou D, Dong Y2, Shen L3, Wu R2, Zhang Y2, et al. (2014) Rapid Quantification of Tobramycin and Vancomycin by UPLC-TQD and Application to Osteomyelitis Patient Samples. J Chromatogr Sci 52: 501-507.
- Graziani AL, Lawson LA, Gibson GA, Steinberg MA, MacGregor RR (1988) Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother 32: 1320-1322.
- Massias L, Dubois C, de Lentdecker P, Brodaty O, Fischler M, et al. (1992) Penetration of vancomycin in uninfected sternal bone. Antimicrob Agents Chemother 36: 2539-2541.
- Greene SV, Abdalla T, Morgan SL, Bryan CS (1987) High-performance liquid chromatographic analysis of vancomycin in plasma, bone, atrial appendage tissue and pericardial fluid. J Chromatogr 417: 121-128.
- Payne CJ, Thomson AH, Stearns AT, Watson DG, Zhang T, et al. (2011) Pharmacokinetics and tissue penetration of vancomycin continuous infusion as prophylaxis for vascular surgery. J Antimicrob Chemother 66: 2624-2627.
- Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75: 3019-3030.
- US Department of Health and Human Services Food and Drug Administration (2000) Guidance for Industry: Bioanalytical Method Validation. Center for Drug Evaluation and Research (CDER).
- Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, et al. (2009) Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49: 325-327.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16620
- [From(publication date):
July-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 11786
- PDF downloads : 4834