Development of a Novel Clinical Trial Design to Evaluate the Effects of Joint Therapeutics on Cartilage Turnover in Healthy Subjects
Received: 01-Jun-2019 / Accepted Date: 10-Jun-2019 / Published Date: 17-Jun-2019 DOI: 10.4172/2165-7025.1000415
Abstract
Background: Articular joint diseases such as Osteoarthritis (OA) and Rheumatoid Arthritis (RA) are quite prevalent throughout the world, particularly in adults 60 years of age and older, and result in significant costs (both financial and quality-of-life) for those that are afflicted. Owing to the lack of drugs that can halt the progression of arthritis there is an obvious current need for additional joint therapeutics. Various biomarkers have been historically evaluated to better guide the development of new therapeutic interventions. Of these biomarkers, c-terminal Cross- Linked Telopeptide of type-II collagen (CTX-II), a marker of cartilage degradation, has shown the most potential. The purpose of this investigation was to develop a clinical trial design to aid in the evaluation of chondroprotective joint therapeutics by taking advantage of the apparent sensitivity of the articular cartilage of healthy, post-menopausal women to increased exercise-induced joint strain.
Methods: Variables such as the timing of urinary CTX-II clearance and the sensitivity of the CTX-II assay were initially investigated. The sensitivity of CTX-II production to differing levels of exercise strain was then investigated in a non-interventional clinical phase in developing the trial design. The joint therapeutic Natural Eggshell Membrane (NEM®) was then evaluated in a subsequent open-label clinical phase as a proof of concept.
Results: There appears to be a reproducible initial increase in uCTX-II clearance within the first 2-4 hours following exercise, followed by somewhat of a plateau, with the maximum (or near maximum) level being observed 24 hours post-exercise in the 2nd void of the morning. The CTX-II assay is sensitive enough to measure changes resulting from exercise in obese and OA subjects with a 20.4% to 43.6% increase from resting. There was an obvious trend in the non-interventional clinical phase for the strenuous nature of the exercise (lifting weight>seated step machine>inclined treadmill) to affect the magnitude of the cartilage turnover of the study subjects. NEM® prevented exercise-induced cartilage turnover in the open-label clinical phase indicating that it is chondroprotective.
Conclusion: This trial design shows great potential to evaluate chondroprotective joint therapeutics including symptomology (i.e. joint pain and stiffness) in healthy individuals, where sparing cartilage may prevent patients from ultimately developing arthritis. By extension, this design may also enable the evaluation of chondroprotective joint therapeutics in an OA population, particularly where cartilage preservation has reached a critical stage.
Keywords: Chondroprotective; High-impact; CTX-II biomarker; Arthritis; Trial design
Introduction
Estimates by the World Health Organization show that a considerable percentage of the global population is afflicted with articular joint disease [1]. There are currently no approved Disease Modifying Osteoarthritis Drugs (DMOADs) [2] and only a handful of Disease Modifying Anti-Rheumatic Drugs (DMARDs) are approved. Both non-biologic and biologic DMARD treatment presently comes with substantial risks, either due to hepatotoxicity or increased occurrences of pathogenic infections and malignancies [3]. The lack of drugs that can safely halt the progression of arthritis (be disease modifying) combined with the fact that it is frequently diagnosed a decade or more into the disease when severity is considerably greater, results in a substantial burden for global healthcare systems. Because so many people develop these costly and debilitating diseases there is an obvious current need for safe and effective joint therapeutics and the future need will be substantially greater. Therefore, the ability to evaluate new joint therapeutics is paramount to the approval of new molecular entities or for new indications for existing drugs to meet this need.
Various biomarkers have been evaluated through the years in an attempt to better understand arthritis progression and/or prognosis and to better guide the development of therapeutic interventions. Researchers have looked at immune cell patterns in the joints [4], serological parameters (cholesterol & triglycerides) and markers of oxidative stress (malondialdehyde & C-reactive protein) [5], synovial fluid cytokine levels (TNF-α, IL-1β, IL-6, etc.) [6], as well as cartilage components in synovial fluid (chondroitin sulfate, glycosaminoglycans, hyaluronic acid, etc.) [7]. Many of these biomarkers suffer from a number of drawbacks, from lack of specificity (e.g. cholesterol) to difficulty in obtaining samples (e.g. synovial fluid). Because of the plethora of biomarkers from which to choose to evaluate arthritis, the Osteoarthritis Biomarkers Network funded by the National Institutes of Health/National Institute of Arthritis, Musculoskeletal, and Skin Disease (NIH/NIAMS) proposed a classification scheme for biomarkers to provide a common format for communication of research in this area. This scheme is termed BIPED which is an acronym for Burden of disease, Investigative, Prognostic, Efficacy of intervention, and diagnostic [8]. These characteristics help to rank biomarkers as to their clinical utility in diagnosing and treating arthritis. Based upon these criteria, indicators of cartilage turnover (i.e. synthesis and degradation) have moved to the top of the list of biomarker candidates likely to be most useful. We chose to investigate c-terminal cross-linked telopeptide of type-II collagen (CTX-II), a marker of cartilage degradation, as an indicator of chondroprotective effects from joint therapeutics. Urinary CTX-II levels are known to be substantially elevated in those afflicted with articular joint diseases, but levels are also known to be elevated in a variety of healthy subsets of the population, as well. Urinary CTX-II levels have been shown to be elevated due to high-impact exercise in healthy college-aged endurance athletes such as cross-country runners by about 85% over age- and weight-matched controls but were not significantly elevated in lowerimpact endurance athletes like swimmers and rowers [9]. Urinary CTX-II has also been shown to be about 2-fold higher in postmenopausal women versus age-matched pre-menopausal women and moderately elevated (~25%) in overweight people (BMI ≥ 25 kg/m2) versus normal-weight controls (BMI<25 kg/m2) [10].
The fact that large portions of the population develop arthritis combined with the fact that there are currently no approved diseasemodifying or chondroprotective agents results in an obvious urgent need for safe and effective joint therapeutics. To our knowledge, there are presently no clinical models designed to evaluate chondroprotective joint therapeutics. The aim of this research investigation, therefore, was to develop a simple and rapid clinical trial design to aid in the evaluation of chondroprotective joint therapeutics, and we hoped to take advantage of the apparent sensitivity of the articular cartilage of post-menopausal women and/or overweight individuals to evaluate joint therapeutics through increasing joint strain via exercise while monitoring uCTX-II output.
Patients and Methods
Initial investigation of design variables
The initial critical variable investigated was to determine whether or not CTX-II production and urinary clearance would occur in a narrow enough time frame to be useful for our envisaged purpose. Individuals clear proteins at differing rates, so it was also important to determine if the clearance rate was sufficiently consistent between individual subjects to be able to obtain samples at a single point in time. There was also concern as to whether the Enzyme-Linked Immunosorbent Assay (ELISA) would be sensitive enough to measure a change in urinary CTX-II induced by exercise. That is, would the increase in uCTX-II be sufficiently large so that it would not be obscured by the variation inherent in the assays, which have generally been reported to be around 10%-15%.
To help normalize uCTX-II clearance rates, the ratio of urinary CTX-II expressed in micrograms per liter (μg/L) to urinary Creatinine (Cr) expressed in millimoles per liter (mmol/L) was calculated and results were reported as nanograms of CTX-II per millimole of Creatinine (ng/mmol Cr). Urinary CTX-II levels were evaluated using a commercial ELISA kit from Immunodiagnostics Systems, Inc. (Urine CartiLaps® EIA) according to manufacturer instructions. Baseline urine samples were collected from the 2nd void of the morning and were frozen (-20°C) immediately and held until needed for assaying. Thawed samples were subdivided into aliquots to avoid subsequent repeated freeze/thaw cycles that might result in aberrant repeat assay values.
Two females (ages 34 & 37) and one male (age 34) having healthy knee joints (no resting knee pain or stiffness) and one female (age 60) with diagnosed OA of the right knee all with a BMI<25 kg/m2 provided urine samples for basal CTX-II level determination. The subjects subsequently performed exercises (females: 300 stairs per leg over 10-15 minutes; male: jogged 4 miles over ~45 minutes) that would be expected to increase levels of excreted CTX-II. Subjects provided subsequent urine samples approximately every 2-4 hours (for ~12 hours) and again at 24 hours to follow CTX-II clearance temporally to determine when the maximum post-exercise uCTX-II level would be observed.
These initial subjects were all regular exercisers, so we next wanted to evaluate more moderate forms of exercise in non-exercising or infrequently exercising individuals. A post-menopausal female (age 67) with healthy knee joints who did not exercise regularly provided a urine sample for basal CTX-II level determination. The subject subsequently performed exercise for 7-10 minutes on alternating days for two consecutive weeks on an inclined treadmill with an incline of 14 degrees and a pace of 1.7 miles per hour. At the end of each week, the subject provided a urine sample for the comparison of CTX-II levels to baseline. An obese male (age 43) with BMI>25 kg/m2 and having healthy knee joints (no resting knee pain or stiffness) who exercised fewer than 2 times per week provided a urine sample for basal CTX-II level determination. The subject subsequently performed exercise of 50 stairs (standard height) per leg over approximately 10 minutes daily for one week.
Collectively, this data indicated that it was indeed possible to induce meaningful increases in uCTX-II in articularly healthy individuals via a variety of exercises, and that even moderate-intensity exercise resulted in measurable increases in uCTX-II in suspected cartilagesensitive individuals (i.e. post-menopausal and/or obese). With this as a basis, we set out to more formally and systematically evaluate the effects of different moderate-intensity exercises on uCTX-II in healthy, post-menopausal women.
Non-interventional clinical phase evaluating design variables
A non-interventional clinical trial was conducted utilizing the services of a clinical contract research organization (QPS Bio-Kinetic; Springfield, MO USA) and the exercise facilities of a nearby hospital (The Meyer Orthopedic & Rehabilitation Center-Cox Health Systems; Springfield, MO USA). The study was conducted in accordance with the U.S. Food & Drug Administration’s principles of Good Clinical Practice (Title 21, Code of Federal Regulations, Parts 50 & 56 and ICH E6) and the Declaration of Helsinki (1996 version). The study protocol was approved by a duly authorized Institutional Review Board (IRB) and all subjects provided their written informed consent in order to participate.
A group of 30 post-menopausal females (age range 46-72) with healthy knee joints (no resting knee pain or stiffness), all of whom exercised fewer than 2 times per week, were randomized into one of three different low-impact, moderate-intensity exercise regimen groups. Group A subjects walked for a minimum of 7 minutes on alternating days for two consecutive weeks on a 14 degree inclined treadmill at an approximate pace of 1.7 miles per hour. Group B subjects performed exercise for a minimum of 7 minutes on alternating days for two consecutive weeks on a seated step machine (NuStep® brand) with a workload of 7.0 and a pace of 30-40 steps per minute. Group C subjects performed 3 sets of 8 lifts each of 90 pounds (41 kg) on a seated leg press (Cybex® brand) in a maximum of 7 minutes on alternating days for two consecutive weeks. All subjects provided urine samples for basal CTX-II level determination and urine samples at the end of each week for comparison of CTX-II levels to baseline. Urine samples were obtained from the 2nd void of the morning collected within 12-24 hours after each subject completed the final exercise for the week. Samples were frozen (-20°C) immediately following collection until needed for assaying.
Adverse events
The participants’ self-assessment diaries were reviewed, and the subjects were interviewed at each clinic visit so that any discomfort beyond what would normally be expected for moderate exercise or other adverse events were recorded and reported in accordance with applicable FDA regulations.
With many of the model design questions answered, it was then time to assess whether the model was sensitive enough to enable the evaluation of joint therapeutics. That is, would the change in uCTX-II induced by exercise be sufficiently large to be able to realize a chondroprotective effect resulting from a joint therapeutic.
Pilot Interventional clinical phase testing the trial design
Following a three week resting period, the same groups of 30 postmenopausal females were re-randomized into one of two different treatment groups. In this phase of the study, all subjects performed the same low-impact, moderate-intensity exercise regimen (3 sets of 8 lifts each of 90 pounds (41 kg) on a seated leg press (Cybex® brand) in a maximum of 7 minutes on alternating days for two consecutive weeks). Group 1 subjects consumed one 500 mg capsule per day of a powdered eggshell membrane joint therapeutic composition (commercially available as NEM® brand eggshell membrane; ESM Technologies, LLC, Carthage, MO USA) for 7 days prior to beginning the exercise regimen and continued to take the treatment during the two-week exercise period (3 weeks total). Group 2 subjects consumed one 500 mg capsule per day of the same powdered eggshell membrane joint therapeutic composition but began taking it on day 1 of the two-week exercise period and continued to do so throughout the remaining time (2 weeks total). As in period 1 of the study, all subjects provided urine samples for basal CTX-II level determination and urine samples at the end of each week for the comparison of CTX-II levels to baseline.
Adverse events
The participants’ self-assessment diaries were reviewed, and the subjects were interviewed at each clinic visit so that any discomfort beyond what would normally be expected for moderate exercise or other adverse events were recorded and reported in accordance with applicable FDA regulations.
Results
Initial investigation of design variables
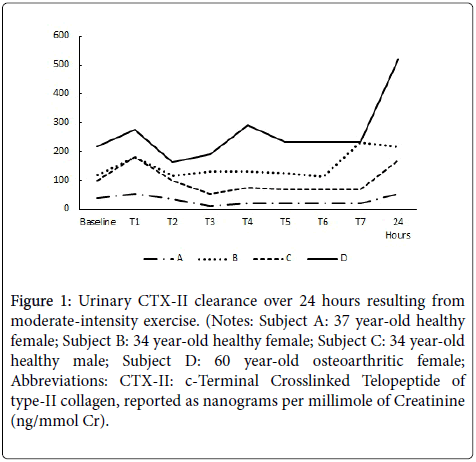
The results of the temporal clearance evaluation for the two females (ages 34 & 37) and one male (age 34) having healthy knee joints (no resting knee pain or stiffness) and one female (age 60) with diagnosed OA of the right knee all with a BMI<25 kg/m2 are reported in Figure 1. There appears to be a reproducible initial increase in uCTX-II within the first 2-4 hours following exercise, followed by somewhat of a plateau. However, for all subjects, the maximum (or near maximum) level of uCTX-II was observed 24 hours post-exercise (2nd void of the morning). Not surprisingly, the female with OA had the highest basal uCTX-II and appeared to be the most sensitive to joint strain resulting from exercise.
Figure 1: Urinary CTX-II clearance over 24 hours resulting from moderate-intensity exercise. (Notes: Subject A: 37 year-old healthy female; Subject B: 34 year-old healthy female; Subject C: 34 year-old healthy male; Subject D: 60 year-old osteoarthritic female; Abbreviations: CTX-II: c-Terminal Crosslinked Telopeptide of type-II collagen, reported as nanograms per millimole of Creatinine (ng/mmol Cr).
For the post-menopausal female (age 67) with healthy knee joints who did not exercise regularly, CTX-II had increased from baseline (156 ng/mmol Cr) by 20.4% at week 1 (188 ng/mmol Cr) and at week 2 CTX-II (199 ng/mmol Cr) was slightly further elevated at 27.1% above baseline. For the obese male (age 43) with BMI>25 kg/m2 and having healthy knee joints (no resting knee pain or stiffness) who exercised fewer than 2 times per week basal CTX-II was 70 ng/mmol Cr. On Day 4, CTX-II (100 ng/mmol Cr) had increased by 43.6% from baseline and on day 8, CTX-II (87 ng/mmol Cr) was increased by a lesser 24.3% from baseline.
Non-interventional clinical phase evaluating design variables
Table 1 contains baseline demographic data for the three groups (A, B & C) of post-menopausal females from the 30-subject noninterventional clinical phase. The three groups were not statistically different in any of the baseline demographic data using the nonparametric Kruskal-Wallis Test for multiple groups. It is important to note that their urinary CTX-II levels are consistent with being healthy post-menopausal females, having average uCTX-II levels at the very lowest end of the range of what would be expected for arthritic subjects [11,12] and well within the expected normal range for women of their age, BMI and hormonal status [10].
| Group A | Group B | Group C | |
|---|---|---|---|
| Age (yrs) | 58.2 ± 4.7 | 52.4 ± 6.2 | 57.8 ± 7.1 |
| Weight (kg) | 73.4 ± 11.1 | 83.5 ± 18.2 | 77.2 ± 12.2 |
| BMI | 26.8 ± 3.4 | 30.1 ± 4.4 | 28.6 ± 4.2 |
| uCTX-II (ng/mmole Cr) | 259 ± 102 | 219 ± 98 | 288 ± 84 |
Notes: Values are reported as mean ± standard deviation (n=10 per group). There were no statistical differences between treatment groups in any of the listed parameters.
Abbreviations: BMI: Body Mass Index; calculated as weight in kilograms divided by the square of height in meters. uCTX-II: urinary c-Terminal Crosslinked Telopeptide of type-II collagen, reported as nanograms per millimole of Creatinine (ng/mmol Cr).
Table 1: Period 1 participant baseline demographic data.
Using the non-parametric Friedman Test for repeated measures coupled with a post-hoc Conover analysis to determine which time points (Baseline, Week 1, or Week 2) differed, it was determined that Group C differed statistically (p<0.05) at both week 1 (331 ± 117) and week 2 (313 ± 108) from baseline (288 ± 84). Group B showed a clear statistical trend (p<0.10) for week 1 & week 2 to differ from Baseline. Group A was not statistically different at any time point.
Only one or two subjects in Group A (not shown) experienced any pain or stiffness from walking on an inclined treadmill (as described previously). Pain and stiffness results for Group B and Group C are also reported in Table 2. There were substantial increases in pain in both groups both immediately after exercise (500%-900%) and 12 hours post-exercise (600%-900%). Similarly, there were substantial increases in stiffness in both groups both immediately after exercise (200%-350%) and 12 hours post-exercise (233%-350%). For Group B, these results were statistically significant (p<0.05) for 12-hour postexercise pain (week 1 & week 2) and showed a statistical trend (p~0.10) for immediate pain (both weeks), immediate stiffness (week 1), and 12-hour post-exercise stiffness (both weeks). For Group C, these results were statistically significant (p<0.05) for all categories (immediate pain & stiffness and 12-hour post-exercise pain & stiffness) at Week 1 and for week 2 immediate pain & stiffness and showed a statistical trend (p~0.10) for 12-hour post-exercise pain & stiffness.
| Time Point | uCTX-II (% change) |
Immediate Pain (% change) |
12-hour Pain (% change) |
Immediate Stiffness (% change) |
12-hour Stiffness (% change) |
|
|---|---|---|---|---|---|---|
| Group B | Baseline | 219 ± 98 (N/A) | 0.1 ± 0.3 (N/A) | 0.1 ± 0.3 (N/A) | 0.2 ± 0.4 (N/A) | 0.2 ± 0.4 (N/A) |
| Week 1 | 233 ± 103 (+6.4)# | 0.6 ± 1.0 (+500)# | 0.7 ± 1.1 (+600)* | 0.9 ± 1.0 (+350)# | 0.9 ± 1.0 (+350)# | |
| Week 2 | 218 ± 102 (-0.5)# | 0.8 ± 1.5 (+700)# | 0.8 ± 1.1 (+700)* | 0.6 ± 1.1 (+200) | 0.9 ± 1.1 (+350)# | |
| Group C | Baseline | 288 ± 84 (N/A) | 0.1 ± 0.3 (N/A) | 0.1 ± 0.3 (N/A) | 0.3 ± 0.5 (N/A) | 0.3 ± 0.5 (N/A) |
| Week 1 | 331 ± 117 (+14.9)* | 1.0 ± 0.8 (+900)* | 1.0 ± 1.1 (+900)* | 1.0 ± 0.8 (+233)* | 1.2 ± 1.0 (+300)* | |
| Week 2 | 313 ± 108 (+8.7)* | 0.8 ± 0.8 (+700)* | 0.7 ± 1.1 (+600)# | 1.2 ± 1.0 (+300)* | 0.9 ± 1.1 (+233)# |
Notes: Values are reported as mean ± standard deviation (% Change from baseline) (n=10 per group). Group B performed seated step machine exercise. Group C performed seated leg press exercise.
Abbreviations: uCTX-II: urinary c-Terminal Crosslinked Telopeptide of type-II collagen, reported as nanograms per millimole of Creatinine (ng/mmol Cr). *p<0.05; # p~0.10 versus baseline.
Table 2: Pain, Stiffness, and uCTX-II levels at baseline and after 1 week & 2 weeks of performing one of two low-impact, moderate-intensity exercise regimens.
Adverse events
No serious adverse events were reported in this study period. There were twelve Adverse Events (AEs) reported in period 1 including: (7) headaches and one instance each of congestion, cold-like symptoms, sore throat, nausea, and leg cramp. No AEs required discontinuation of the exercise regimen nor did they lead to withdrawal from the study.
Pilot interventional clinical phase testing the trial design
Table 3 contains the baseline demographic data for the two groups of re-randomized study subjects from the interventional clinical phase (Period 2). The groups were not statistically different in any of the baseline demographic data using the non-parametric Mann-Whitney U Test for independent groups. It is also significant that their uCTX-II levels had returned to levels similar to what was found in Period 1 of the study following the 3-week resting period. Again, CTX-II levels remained consistent with being healthy post-menopausal females. Table 4 presents the uCTX-II results for the two groups of subjects at baseline and after preforming the designated exercise regimen for two consecutive weeks.
| Group 1 | Group 2 | |
|---|---|---|
| Age, yrs | 56.2 ± 8.0 | 56.1 ± 4.8 |
| Weight, kg | 78.4 ± 12.1 | 77.7 ± 16.7 |
| BMI | 28.4 ± 3.7 | 28.6 ± 4.7 |
| uCTX-II (ng/mmole Cr) | 220 ± 97 | 174 ± 76 |
Notes: Values are reported as mean ± standard deviation (n=15 per group). There were no statistical differences between treatment groups in any of the listed parameters.
Abbreviations: BMI: Body Mass Index, calculated as weight in kilograms divided by the square of height in meters. uCTX-II: urinary c-Terminal Crosslinked Telopeptide of type-II collagen, reported as nanograms per millimole of Creatinine (ng/mmol Cr).
Table 3: Period 2 participant baseline demographic data.
| Time Point | uCTX-II (% change) |
Immediate Pain (% change) |
12-hour Pain (% change) |
Immediate Stiffness (% change) |
12-hour Stiffness (% change) |
|
|---|---|---|---|---|---|---|
| Group 1 | Baseline | 220 ± 97 (N/A) | 0.4 ± 1.1 (N/A) | 0.4 ± 1.1 (N/A) | 0.5 ± 1.0 (N/A) | 0.5 ± 1.0 (N/A) |
| Week 1 | 222 ± 92 (+1.3) | 1.2 ± 1.9 (+200) | 1.1 ± 1.7 (+168) | 0.8 ± 1.3 (+50) | 1.0 ± 1.7 (+88) | |
| Week 2 | 209 ± 93 (-4.8) | 0.7 ± 1.4 (+83) | 0.6 ± 1.0 (+50) | 0.7 ± 1.2 (+25) | 0.7 ± 1.0 (+25) | |
| Group 2 | Baseline | 174 ± 76 (N/A) | 0.4 ± 0.7 (N/A) | 0.4 ± 0.7 (N/A) | 0.6 ± 1.0 (N/A) | 0.6 ± 1.0 (N/A) |
| Week 1 | 178 ± 87 (+2.3) | 0.9 ± 0.8 (+117) | 0.7 ± 0.9 (+83) | 0.9 ± 0.9 (+44) | 0.8 ± 1.1 (+33) | |
| Week 2 | 161 ± 58 (-5.7) | 0.9 ± 0.7 (+117) | 0.3 ± 0.6 (-17) | 0.6 ± 0.6 (0) | 0.5 ± 0.7 (-11) |
Notes: Values are reported as mean ± standard deviation (% change from baseline) (n= 15 per group).
Abbreviations: uCTX-II: urinary c-Terminal Crosslinked Telopeptide of type-II collagen, reported as nanograms per millimole of Creatinine (ng/mmol Cr). *p<0.05; # p~0.10 versus baseline.
Table 4: Pain, stiffness and uCTX-II levels at baseline and after 1 week & 2 weeks of performing a low-impact, moderate-intensity exercise regimen while consuming a joint therapeutic composition.
There were no statistical differences for either group versus baseline for urinary CTX-II, which is in contrast to results found in Group C in Study Period 1 (performing the same exercise regimen) (Table 2), indicating that the eggshell membrane joint therapeutic composition is chondroprotective (cartilage-sparing) in this clinical trial designed to induce cartilage turnover via exercise.
Because there were also no statistically significant differences between Group 1 and Group 2 for any of the pain or stiffness categories (Table 4), the same 10 subjects from Group C were compared directly to corresponding results obtained in Study Period 1 performing the same exercise regimen when not consuming a joint therapeutic composition (Table 5). There were large reductions in pain and stiffness absolute treatment effects (-258% to -867%) from consuming the NEM joint therapeutic while performing the exercise regimen in study period 2 versus performing the same exercise regimen while untreated in study period 1. These differences were statistically significant (p<0.05) for Week 1 12-hour pain and for week 2 both Immediate & 12-hour stiffness. All other pain and stiffness criteria failed to reach statistical significance, however they all showed trends for improvement (p<0.10). There were moderate reductions (-11.7% to -19.1%) in the uCTX-II absolute treatment effect as well, however this failed to reach statistical significance. Week 2 uCTX-II did, however, show a trend for improvement (p<0.10).
| Weeks | Treatment | Absolute | ||
|---|---|---|---|---|
| Post-treatment | Untreated | NEM | Treatment Effect | |
| uCTX-II | Baseline (n=10, 10) | 288 ± 84 | 237 ± 82 | - |
| 1 (n=10, 10) | 331 ± 117 | 227 ± 85 | -19.10% | |
| 2 (n=10, 10) | 313 ± 108 | 230 ± 70 | -11.7%# | |
| Immediate | Baseline (n=10, 10) | 0.1 ± 0.3 | 0.6 ± 1.0 | - |
| Pain | 1 (n=10, 10) | 1.0 ± 0.8 | 0.8 ± 0.9 | -867%# |
| 2 (n=10, 10) | 0.8 ± 0.8 | 0.7 ± 0.8 | -683%# | |
| 12-hour | Baseline (n=10, 10) | 0.1 ± 0.3 | 0.6 ± 1.0 | - |
| Pain | 1 (n=10, 10) | 1.0 ± 1.1 | 0.7 ± 0.9 | -883%* |
| 2 (n=10, 10) | 0.7 ± 1.1 | 0.4 ± 0.7 | -633%# | |
| Immediate | Baseline (n=10, 10) | 0.3 ± 0.5 | 0.8 ± 1.1 | - |
| Stiffness | 1 (n=10, 10) | 1.0 ± 0.8 | 0.9 ± 1.1 | -220%# |
| 2 (n=10, 10) | 1.2 ± 1.0 | 0.6 ± 0.8 | -325%* | |
| 12-hour | Baseline (n=10, 10) | 0.3 ± 0.5 | 0.8 ± 1.1 | - |
| Stiffness | 1 (n=10, 10) | 1.2 ± 1.0 | 0.9 ± 1.3 | -287%# |
| 2 (n=10, 10) | 0.9 ± 1.1 | 0.6 ± 0.8 | -258%* | |
Notes: Values are reported as mean ± standard deviation. Absolute Treatment Effect is the net difference of NEM treatment versus untreated for the change in mean treatment effect from baseline expressed as a percent. Negative values for pain or function indicate superior improvement in the treatment group.
Abbreviations: *p<0.05; # p<0.10 versus untreated
Table 5: Urinary CTX-II, pain, and stiffness levels at baseline and after 1 week & 2 weeks of performing a low-impact, moderate-intensity exercise regimen while untreated and while consuming a joint therapeutic composition.
Adverse events
No serious adverse events were reported in this study period. There were twenty-three AEs reported in period 2 including: (13) headaches, (4) cold-like symptoms, and one instance each of constipation, sore throat, nausea, shoulder pain, swelling, and leg cramp. No AEs required discontinuation of the treatment product nor did they lead to withdrawal from the study.
Discussion
Articular joint diseases are very prevalent throughout the world, particularly in adults 60 years of age and older, and result in significant costs (both financial and quality-of-life) for those that suffer from the debilitating diseases. Owing to the lack of drugs that can halt the progression of arthritis (be disease modifying) there is an obvious current need for additional joint therapeutics. We report here the development of a clinical trial design to aid in the evaluation of chondroprotective joint therapeutics.
Initial design aspects were investigated, and it was found that exercise-induced urinary CTX-II is excreted similarly over time in various healthy and arthritic individuals (i.e. young adult females, post-menopausal non-OA and OA females, young adult normal weight and obese males). The levels of uCTX-II varied somewhat over time following exercise, however collecting the 2nd void of the morning approximately 24 hours post-exercise possibly smooths some of the individual variation in protein clearance and appears to reproducibly generate maximal uCTX-II values. Interestingly, uCTX-II levels return to baseline within 48-72 hours following exercise. Therefore, it is important that the subjects exercise repeatedly (e.g. every day, alternating days, etc.) to increase the likelihood of capturing the chondroprotective effect from a joint therapeutic under investigation.
The cartilage of various healthy subsets of the population (no joint disease or symptoms) was found to be sensitive to low-impact, lowintensity exercise via the timely production and urinary excretion of CTX-II. This differs markedly from previous reports [9] of elevated uCTX-II levels resulting from high-impact, high-intensity exercise (i.e. cross-country runners) but not in low-impact, high-intensity exercises (i.e. crew rowers or competitive swimmers). We believe that this difference may be attributable to an inherent sensitivity to strain in the cartilage of either post-menopausal women or infrequent exercisers, or a combination of these factors. This difference may also be attributable to the joints of the subjects adjusting and adapting to the exercise strain after sufficient repetition, as would occur in endurance athletes. In the present design, we hoped to exploit both factors and maximize the likelihood of achieving measurable exercise-induced cartilage effects by utilizing infrequently-exercising, post-menopausal women as study subjects.
Cartilage is primarily composed of Extracellular Matrix (ECM), a composite network of proteins such as type-II collagen interacting with negatively charged polysaccharides such as hyaluronic acid and chondroitin sulfate (usually present as proteoglycans), all of which are synthesized and secreted by chondrocytes. During normal cartilage turnover in healthy articular joints ECM production balances ECM breakdown thereby ensuring the continuous renewal of this critical joint-cushioning tissue. However, pathological conditions such as arthritis are characterized by an imbalance in cartilage turnover, in which catabolic processes predominate over anabolic processes. ECM synthesis cannot keep pace with degradation and a loss of the structural integrity of the articular cartilage results. Products of this degradation imbalance can be found in both blood and urine of arthritic patients. Initially cartilage breakdown occurs via slow but extensive proteoglycan loss in the ECM matrix resulting in loss of cartilage thickness. The process culminates in the breakdown of the fibrillar collagen support matrix and ultimately leads to chondrocyte apoptosis. Proteoglycan loss has been shown to be reversible in a number of canine studies involving induced OA, however collagen matrix degradation appears to be irreversible [13]. Therefore, it is critical for a chondroprotective joint therapeutic to spare the collagen matrix within cartilage, and this effect can be demonstrated via monitoring of uCTX-II a collagen fragment generated directly from the degradation of the cartilage collagen matrix.
We first set out to evaluate different types of exercise and possible effects these might have on cartilage turnover in these subjects. The exercises were chosen to provide some strain on the knee. That is, walking on an inclined treadmill was chosen as opposed to walking on a flat surface to provide at least some level of additional knee strain. However, the exercises were carefully selected specifically to avoid significant knee joint impact. This was done not only to isolate the effect of joint strain versus joint impact on cartilage, but also to make the exercises more amenable to performance by older study subjects. When reviewing data from the non-interventional period of the study across all groups, there is a clear trend for the strenuous nature of the exercise (lifting weight>seated step machine>inclined treadmill) to affect the magnitude of the cartilage turnover of the study subjects. In fact, walking on an inclined treadmill appeared to be beneficial in reducing cartilage turnover. This unexpected finding resulting from joint strain compared to the known effect from the nature of exercise impact (low-versus high) on the joint not only enables the evaluation of exercise induced cartilage turnover from low-intensity exercise in an older target population, but may also inform future designs of exercise for joint rehabilitation. If indeed the loss of the cartilage collagen matrix is irreversible in human joints as has been shown in a canine model [13], care should be taken to limit both joint impact and strain while rehabilitating a joint following injury and/or surgery. Further research will be needed to determine if this concern is borne out.
We first set out to evaluate different types of exercise and possible effects these might have on cartilage turnover in these subjects. The exercises were chosen to provide some strain on the knee. That is, walking on an inclined treadmill was chosen as opposed to walking on a flat surface to provide at least some level of additional knee strain. However, the exercises were carefully selected specifically to avoid significant knee joint impact. This was done not only to isolate the effect of joint strain versus joint impact on cartilage, but also to make the exercises more amenable to performance by older study subjects. When reviewing data from the non-interventional period of the study across all groups, there is a clear trend for the strenuous nature of the exercise (lifting weight>seated step machine>inclined treadmill) to affect the magnitude of the cartilage turnover of the study subjects. In fact, walking on an inclined treadmill appeared to be beneficial in reducing cartilage turnover. This unexpected finding resulting from joint strain compared to the known effect from the nature of exercise impact (low-versus high) on the joint not only enables the evaluation of exercise induced cartilage turnover from low-intensity exercise in an older target population, but may also inform future designs of exercise for joint rehabilitation. If indeed the loss of the cartilage collagen matrix is irreversible in human joints as has been shown in a canine model [13], care should be taken to limit both joint impact and strain while rehabilitating a joint following injury and/or surgery. Further research will be needed to determine if this concern is borne out.
As the study was intended to investigate design variables, it suffered from a number of limitations. The limited enrollment (30 subjects) hindered the determination of statistical significance and may have limited realization of further clinically meaningful results. The inclusion of a comparative treatment agent would likely have provided additional information but would have required a significantly larger initial study population. A follow-up randomized, double-blind, placebo-controlled study with some minor modifications will allow us to better validate the current study design and may provide additional valuable insight into the use of uCTX-II in evaluating chondroprotective joint therapeutics in healthy individuals. Importantly, based on results from our preliminary investigation this design would be expected to function equally well, if not better, in an OA population wherein their cartilage would be even more sensitive to the effects of exercise [14].
Conclusion
With one third of those 65 and older in the U.S. having been diagnosed with osteoarthritis, and that number expected to grow immensely as the overall population ages, it is important for patients to have treatment options that are both effective and safe. With the current lack of effective disease modifying drugs (i.e. DMOADs) and with existing drugs having significant safety issues (i.e. DMARDs), there is a substantial need to improve our ability to develop joint therapeutics to meet current and future demands. The clinical trial design presented here shows great potential to evaluate chondroprotective joint therapeutics in healthy individuals, where sparing cartilage may very well prevent patients from ultimately developing arthritis. The design also demonstrated the potential to evaluate joint therapeutic efficacy in improving symptomology (i.e. joint pain and stiffness). By extension, this design may also enable the evaluation of chondroprotective joint therapeutics in an OA population, particularly where cartilage preservation has reached a critical stage.
Acknowledgements
The sponsor would like to thank all of the study participants.
Disclosure
The study sponsor was ESM Technologies, LLC. KJR & MB are employed by the sponsor.
DM & SAD are employed by the CRO and have no other competing interests.
References
- Woolf AD, Pfleger B (2003) Burden of Major Musculoskeletal Conditions. Bull World Health Organ 81: 646-656.
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA (2005) Osteoarthritis - An Untreatable Disease? Nat Rev Drug Discov 4: 331-334.
- Ruderman EM (2012) Overview of safety of non-biologic and biologic DMARDs. Rheumatol 51: vi37-vi43.
- Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, et al. (2002) Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage 10: 277-281.
- Mishra R, Singh A, Chandra V, Negi MPS, Tripathy BC, et al. (2012) A comparative analysis of serological parameters and oxidative stress in osteoarthritis and rheumatoid arthritis. Rheumatol Int 32: 2377-2382.
- Punzi L, Calò L, Plebani M (2002) Clinical Significance of Cytokine Determination in Synovial Fluid. Crit Rev Clin Lab Sci 39: 63-88.
- Belcher C, Yaqub R, Fawthrop F, Bayliss M, Doherty M (1997) Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis 56: 299-307.
- Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, et al. (2006) Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage 14: 723-727.
- O’Kane JW, Hutchinson E, Atley LM, Eyre DR (2006) Sport-related differences in biomarkers of bone resorption and cartilage degradation in endurance athletes. Osteoarthritis Cartilage 14: 71-76.
- Mouritzen U, Christgau S, Lehmann HJ, Tankó LB, Christiansen C (2003) Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis 62: 332-336.
- Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, et al. (2001) Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis 60: 619-626.
- Karsdal MA, Byrjalsen , Bay-Jensen AC, Henriksen K, Riis BJ, et al. (2010) Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis - the effect of sex, Kellgren-Lawrence (KL) score, Body Mass Index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation. BMC Musculoskel Disord 11: 125.
- Brandt KD (2003) Response of joint structures to inactivity and to reloading after immobilization. Arthritis Rheum 49: 267-271.
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, et al. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58: 26-35.
Citation: Ruff KJ, Back M, Morrison D, Duncan SA (2019) Development of a Novel Clinical Trial Design to Evaluate the Effects of Joint Therapeutics on Cartilage Turnover in Healthy Subjects. J Nov Physiother 9: 415. DOI: 10.4172/2165-7025.1000415
Copyright: © 2019 Ruff KJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3878
- [From(publication date): 0-2019 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 2961
- PDF downloads: 917