Research Article Open Access
Development of Analytical Methods to Gain Insight into the Role of Terpenes in Biogas Plants
Karine Arrhenius*, Johan Engelbrektsson and Haleh Yaghooby
Department of Chemistry, Materials and Surfaces, SP Technical Research Institute of Sweden, Brinellgatan 4, Sweden
- *Corresponding Author:
- Karine Arrhenius
Department of Chemistry, Materials and Surfaces
SP Technical Research Institute of Sweden, Brinellgatan 4
PO Box 857, SE-501 15 Borås, Sweden
Tel: +460105165728
Fax: +460105165728
E-mail: karine.arrhenius@sp.se
Received date: June 14, 2016; Accepted date: June 21, 2016; Published date: June 27, 2016
Citation: Arrhenius K, Engelbrektsson J, Yaghooby H (2016) Development of Analytical Methods to Gain Insight into the Role of Terpenes in Biogas Plants. J Anal Bioanal Tech 7:324. doi:10.4172/2155-9872.1000324
Copyright: © 2016 Arrhenius K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Terpenes have been shown to be the dominant VOCs present in biogas mostly in plants where food wastes are digested. In particular, p-cymene and D-limonene have been reported to represent up to 90% of all VOCs in the biogas. A number of problems have been linked to terpenes in biogas plants, including odor problems, indoor air quality issues at workplaces and operational problems. In order to study the faith of terpenes, there is a need to develop robust analytical methods to quantify terpenes in all the flows at biogas plants including substrates, gas, and water samples. In this study, reliable analytical methods for the detection and quantification of terpenes in these flows are presented. The methods have a common final step consisting of a TD-GC-MS/FID analysis using Tenax TA for the trapping of terpenes. The methods were then applied to some samples taken at a biogas plant where food waste is digested. The results show that D-limonene was the dominant terpene in the substrate whereas p-cymene was dominant in biogas and process water.
Keywords
Terpenes; Biogas; Substrate; Analysis
Introduction
During the production of biogas from substrates such as food waste, sewage sludge, manure and other organic waste, the organic material is broken down to the main end products, methane and carbon dioxide. However, biogas also contains many other gaseous substances [1,2], but in much lower concentrations. Some of these substances have undesirable odors and may be harmful to the process, the environment, the human health and the equipment. Terpenes, in particular p-cymene, and D-limonene, have been shown to be characteristic for biogas produced in facilities where food waste is digested [3]. It has also been shown that some of these terpenes follow in the vehicles gas, biomethane, when the gas is upgraded to fuel quality [3]. The concentration of terpenes present in biomethane depends on the upgrading technique. These substances are also found in the process water, such as the condensation water recovered after the drying stage [4] and in samples of air in some locales at the plant.
The word terpene is normally used both in its broadest sense to designate all compounds which have a distinct architectural and chemical relation to the simple C5H8 molecule (isoprene), and in a more restricted sense to designate compounds with 10 carbon atoms derived from C10H16 (C10H16O, C10H18O). The latest are often referred to as monoterpenes. Terpenes can be linear (as beta-myrcene) or cyclic (p-menthane), bicylic (3-carene), aromatic monoterpenes containing a benzene ring (p-cymene). In this study, the term terpene is used to design monoterpenes with chemical formula C10H16 or C10H14. Even eucalyptol (C10H18O) is included in this study as it has been found in biogas samples.
p-cymene (C10H14,, boiling point 177°C, solubility in water 23 mg/l) is a naturally occurring aromatic organic compound which is classified as an alkylbenzene related to a monoterpene. It occurs naturally in more than 200 food products [8]. p-cymene is mainly ingested by consumption of food products such as butter, carrots, nutmeg, orange juice, oregano, raspberries and lemon oil, and nearly all spices. It is estimated that approximately 30000 kg of p-cymene is consumed every day as a natural element of these food products.
D-limonene (C10H16, boiling point 176°C, solubility in water 14 mg/l) occurs naturally in lemon rind, dill, fennel, celery and plants, and in many essential oils. It can also be produced synthetically. D-limonene is frequently used as food additive in order to give a lemon flavor, as a fragrance additive in perfumes, body care products and as natural substitute for petroleum-based solvents in paints and detergents.
A number of problems have been linked to terpenes in biogas plants, including odor problems, indoor air quality issues at workplaces and operational problems. The concentration of terpenes in the biomethane can be high enough to impact/mask the odorisation of the fuel by compounds as tetrahydrothiophene (THT) despite terpenes odor threshold being 1,000 times lower than those of THT [5]. At high concentrations, terpenes may also condensate in the natural gas pipelines and influence the integrity of the plastic pipelines and the components in the gas grid [5].Regarding terpenes impact on the equipment, some Swedish plants have suspected these substances to partly have dissolved electrical cables and other rubber/plastic parts in valves and pumps, resulting in difficulties to operate valvesand consequentlyneed to replace damaged components with shorter time intervals. Furthermore, compensators with rubber which are sitting after blowers have been reported to have been damaged by terpenes. Regarding issues related to workplace environment, monoterpenes are irritating to skin and mucuous membranes and can cause both allergic and nonallergic contact dermatitis. The occupational exposure limit in Sweden is 150 mg/m3 for both total and individual monoterpenes[6,7].
Therefore, there is a need to develop robust analytical methods to quantify terpenes in all the flows at biogas plants including substrates, gas, and water samples.
A common analysis technique to analyseterpenes in gas is based on thermal desorption-gas chromatography/mass spectrometry and/or flame ionisation detector (TD-GC-MS/FID) [3]where compounds are trapped on sorbents. Sorbents retain the compound(s) of interest but not the matrix. This leads to an enrichment of the targeted compounds, which can be desorbed by heating, or extracted with solvents, for analysis. The advantage of using sorbent tubes is that they are relatively simple to use, store and transport to the laboratory. The volumes required for sampling biogas reported in the literature are between 0.5 liters (50 mL/min over 10 min) and 10 litres (1 l/min over 10 min [9]).The tubes once thermally desorbed can be easily reused after precautionary conditioning at elevated temperature and under a flow of ultra-pure inert gas following well-established procedures (Method TO-17 [10]).The factors to consider when selecting suitable sorbents include the strength of the sorbent-sorbate interaction, the temperature, the artefacts, the hydrophobicity, the inertness and the mechanical strength (friability) [11].
Tenax TA (a porous polymer based on the monomer 2.6-diphenylene oxide),is commonly used for terpenes as it can adsorb nonpolar or slightly polar substances with boiling point in the range 70-350°C. However, it has been reported that sampling of monoterpenes from air in Tenax may introduce analytical errors owing to oxidation and acid rearrangements on the adsorbent.Particularly terpenes which react rapidly with ozone, e.g. myrcene and limonene have been shown to be partially lost when sampling air with high photooxidant levels [12].
In a previous study, purge and trap method combined with TD-GC-MS/FID analysis has been proven to be satisfactory [4] to quantify non-polar VOC in process water at biogas plants. As already reported, the rate of removal of compounds from the water samples is dependent upon four factors; the surface area of the bubbles that sparge the sample, the temperature of the sample, the time in which the gas contacts the sample and the Henry´s law constant of the compounds. The relatively high Henry´s law constant for D-limonene (0.026 atm-cu m/mole) and p-cymene (0.011 atm-cu m/mole) should result in high purge efficiency.
In this study, reliable analytical methods for the detection and quantification of terpenes in gas samples, process water samples and substrates have been developed and then applied to some samples taken at a biogas plant where food wastes are digested.The methods have a common final step consisting of a TD-GC-MS/FID analysis. Tenax TA is used for trapping of terpenes from biogas, biomethane, air as well as terpenes which have been purged from liquid or solid sample matrices.
Material and Methods
Chemicals
Α-pinene, camphene, β-myrcene, β-pinene, α-terpinene, D-limonen, γ-terpinene (each at 2 % w/v in methanol) were purchased from LGC Standards (Wesel, Germany).
3-carene (>98,5%), p-cymene (99%), eucalyptol (>99%) were purchased from Sigma-Aldrich.
Diethyl ether (CHROMASOLV®, for HPLC≥99.9%) were purchased from Sigma-Aldrich.
Methods
The analyses were performed on a gas chromatograph (Agilent technologies 6890N) equipped with a flame ionization detector and a mass spectrometer 5975C inert MSD in the so called electron impact mode. The GC column was a non-polar capillary column (5% phenyl polysilphenylene-siloxane, BPX5, 50 m long, 0.32 mm internal diameter, 1 µm film thickness). The desorption of the Tenax tubes were carried out on a Markes TD100 desorbed, where the adsorbed substances were released by heating the sorbent tubes during 7 min at 275°C and then transferred to a cold trap for focusing. The trap was then rapidly heated up again and terpenes were released and reached a gas chromatography (GC) column for separation. The column effluent was split into two streams for the detection of individual compounds, one stream passing through the flame ionization detector and the other stream through the mass spectrometer. Terpenes were quantified by means of an external calibration curve (response ratio versus amount ratio) obtained from the FID signal.
Results and discussion
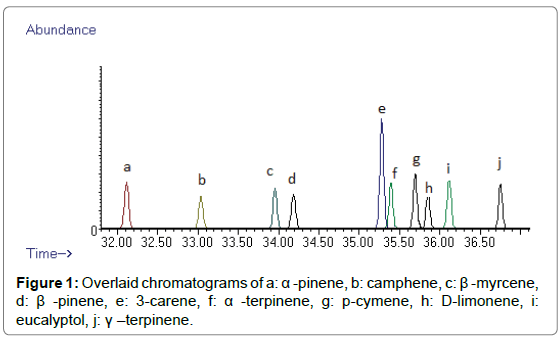
To ensure the correct identification of the terpenes studied and a good chromatographic separation, the retention time for each terpene was determined by analyzing all targeted compounds individually in solution at 200 ng/µl. Overlaid chromatograms are shown in Figure 1. A reasonable separation was achieved even if p-cymene (g) and D-limonene (h) are elutedquite close to each other.
Retention time
Gas samples: The trapping efficiency on Tenax TA of terpenes present in gas samples was tested by preparing a gas containing terpenes at known concentrations.An ALTEF gas-tight bag (Restek, Bellefonte, Pennsylvania, USA) filled with methane (N45, 99.995%, Air Liquide) was spiked with terpenes so the concentration in the gas was 75 mg/m3 for p-cymene, 45 mg/m3 for D-limonene, 18 mg/m3 for α-pinene, 9 mg/m3for camphene, β-myrcen, β-pinene, α-terpinene , γ-terpinene, 1 mg/m3 for eucalyptol and 3-carene.These concentrations were chosen as they are representative of concentrations found in real biogas samples[3].100 ml of the gas was then sampled onto a Tenax tube, directly (t=0 hours) using a pump. The experiment is repeated after 5 hours and after 120 hours storage in the bag. The recovery yields expressed as the ratio between the measured concentration and the targeted concentration are presented in Table 1.
| Recovery yields (%)
|
t = 0 hour
|
t = 5 hours
|
t = 120 hours
|
|---|---|---|---|
| α -pinene
|
96 |
91 |
106 |
| camphene
|
105 |
98 |
113 |
| β-myrcene
|
112 |
106 |
95 |
| β-pinene
|
101 |
98 |
105 |
| 3-carene
|
95 |
93 |
89 |
| α-terpinene
|
94 |
87 |
74 |
| p-cymene
|
86 |
78 |
53 |
| D-limonene
|
86 |
83 |
71 |
| eucalyptol
|
100 |
99 |
87 |
| γ-terpinene
|
107 |
101 |
79 |
Table 1: Recovery yields for terpenes stored in gas bag after 0, 5 and 12 hours.
The results show that the terpenes are quantitatively recovered on Tenax tubes (recovery > 85%). The slightly lower recovery yield observed for p-cymene and D-limonene at t=0 is probably due to adsorption effects on the wall of the bag as these two compounds are at higher concentrations than the other terpenes. The terpenes concentrations decrease at t=120 hours for most of the tested compounds probably due to time dependent adsorption effects.
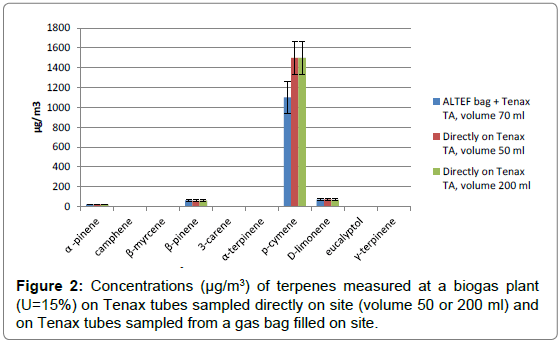
The method was then applied at different biogas plantswhere food waste is used as main substrate. Different volumes (50, 70, 200 ml) of gas have been sampled directly on Tenax tube and indirectly from an ALTEF bag that was filled on site. Results from biogas sampled from a digester are presented in Figure 2.
The results show a good agreement between the two methods (sampling directly on site or sampled from a gas tight bag) with the exception of p-cymene for which lower concentrations is obtained for the sample taken from the gas bag. Due to the high concentration of p-cymene in the sample, it can be assumed that the difference is due to partial adsorption of the compound on the walls of the bag.
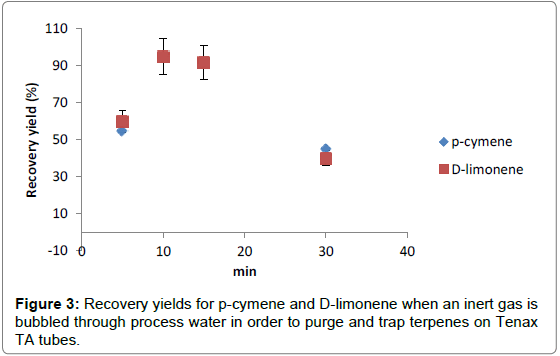
Water samples: When upgrading biogas to biomethane, process water is generated at some point depending on the upgrading technique. With water scrubber, water is used to capture carbon dioxide. With amine scrubber or pressure swing adsorption upgrading, process water is generated during the drying step. The terpenes present in process water was extracted by bubbling an inert gas as nitrogen (Alphagaz 1, 99.999%, Air Liquide) through a known volume (typically 30 ml) of water introduced into a 100-ml glass bottle with a gas phase on the top of the water. The terpenes were then captured on Tenax tubes. Two extractions were performed in succession; the purpose of the second extraction was to check whether terpenes were quantitatively removed from the water. To test the method, three water solutions containing 3 mg/l, 8 mg/l and respectively 15 mg/l of D-limonene and p-cymene were prepared. Four extraction times were tested: 5, 10, 15 and 30 minutes. The recovery yields are presented on Figure 3.
Good recovery yields (>90%) were obtained when bubbling 10 and 15 minutes. However, when bubbling shorter time as5 min or long time as 30 min, the recovery yieldwas found to be poor; these observations being the same at the three levels of concentration.
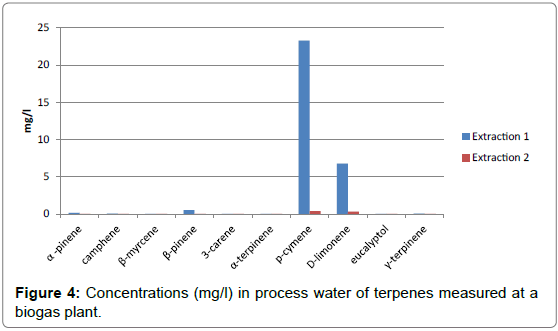
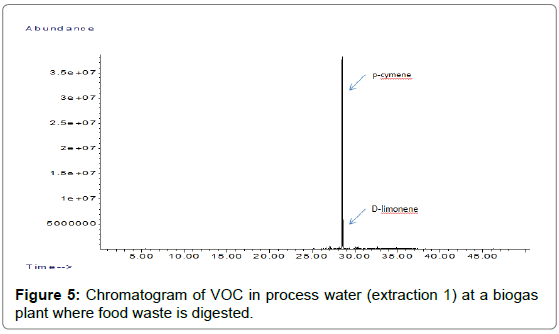
The method was then applied at one biogas plantwhere food waste is used as main substrate. The samples were collected and filled to the top of two 100-mL glass bottles and then immediately sent to the laboratory. The samples arrived to the laboratory the day after sampling and were then stored in refrigerator until analysis were performed. The procedure described above was then applied to the samples. The bottles filled on site were shaken vigorously before transferring into another bottle for the sampling on tubes. In some cases, the sample presents two phases, a water phase on the bottom of the bottle and an organic phase on the top of the bottle. In that case, the organic phase needs to be removed and analysed separately. The fraction between the organic phase and the water phase needs to be determined. Results from the plant are presented in Figures4 and 5.
The concentrations of terpenes found in the process water clearly mirror the concentration of terpenes found in the biogas, with p-cymene and to a lesser extent, D-limonene clearly dominant. The terpenes are quantitatively recovered during the first extraction and only a small part of the terpenes is recovered during the second extraction. The concentration of p-cymene found in this particular sample was close to its solubility in water (23 mg/l).
Substrate samples
Biogas substrateis a much more complex matrix composed of a great variety of organic compounds. A crucial step of sample preparation is the isolation and enrichment of the compounds of interest. Samples of homogenized and hygienized substrate from biogas plants where food waste are digested were collected in plastic bottles and send directly to the laboratory where they were placed in the freeze until analysis.
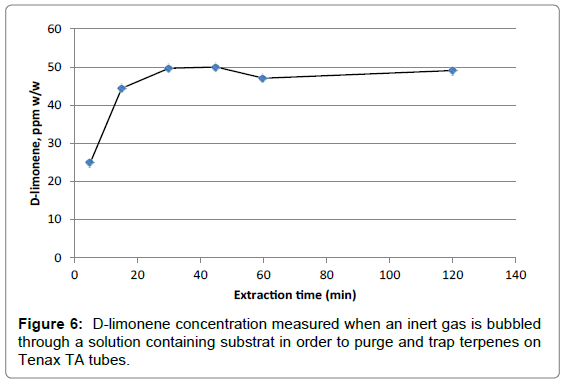
Approximately 5 g of homogenized substrate was dissolved in 500 ml water in a 1000 ml glass bottle. The solution was then diluted twenty times (0.5 mgsubstrat/ml water). A magnetic stirrer was used to cause a stir bar immersed in the bottle to spin quickly. To test the method, six extraction times were tested: 5, 15, 30, 45, 60 and120 minutes. The concentration of D-limonene, expressed in ppm w/w,obtained at the different extraction times is presented on Figure 6.
The concentration of D-limonene was found to be around 50 ppm w/w when bubbling 30 minutes or longer time. However, when bubbling shorter time as 5 min or 15 min, the concentration of D-limonene was found to be lower than 50 ppm w/w; indicating that the extraction was not yet efficient at these extraction times.
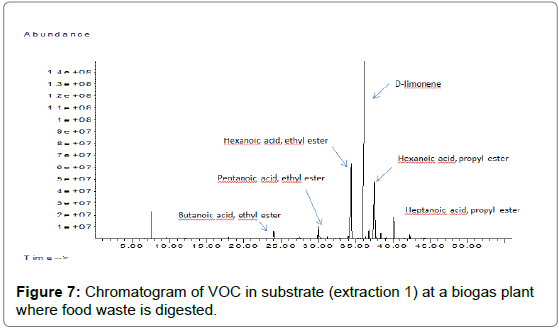
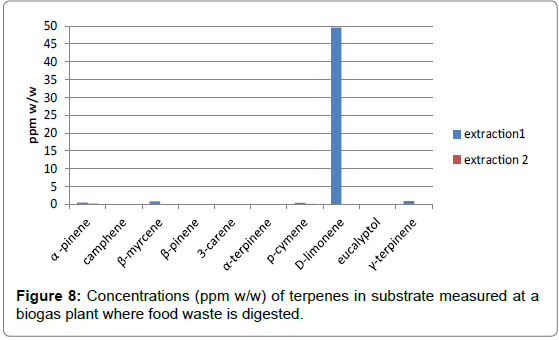
The results obtained when bubbling 60 minutes are also presented on Figures 7 (chromatogram) and 8 (ppm w/w terpenes measured in substrate).
Figure 8: Concentrations (ppm w/w) of terpenes in substrate measured at a biogas plant where food waste is digested.
The results show that D-limonene is clearly the dominant terpene found in the substrate tested. Other terpenes (β-myrcene, γ-terpinene) were found but at much lower concentrations.
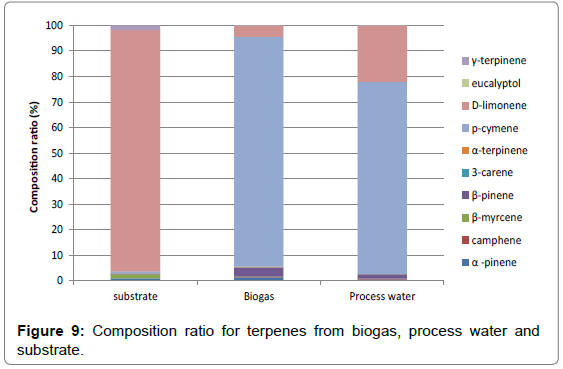
The composition ratio for terpenes from biogas, process water and substrate from the same plant is presented in Figure 9. The results show that p-cymene is probably formed from D-limonene during the digestion process as it is the dominant compound in biogas but not in the substrate.
Conclusions
In this study, reliable analytical methods for the detection and quantification of terpenes in gas samples, process water samples and substrates have been developed. The methods have a common final step consisting of a TD-GC-MS/FID analysis using Tenax TA for the trapping of terpenes. Spiked samples of water were used to evaluate and optimize the extraction variables (extraction time and ratio of water to raw material). The quantitative recovery of terpenes in water samples or substrate samples has been demonstrated by the absence of high amount of terpenes in the second extraction. The quantitative trapping of terpenes from biogas samples have been demonstrated by sampling different volumes of gas and obtaining equivalent results.
The methods were then applied to some samples taken at a biogas plant where food wastes are digested, showing that D-limonene was the dominant terpene in the substrate while p-cymene was found to be predominant in both biogas and process water. The methods can be used to gain insight into the role of terpenes in biogas plants where food wastes are digested which in turn can be used to propose measures in order to minimize problems (odor problems, indoor air quality issues at workplaces and operational problems) that are related to terpenes.
References
- Allen MR, Braithwaite A, Hills C (1997) Trace Organic Compounds in Landfill gas at Seven U.K. Waste Disposal Sites. Environ Sci Technol 31: 1054-1061.
- Rasi S (2009) Biogas composition and upgrading to biomethane. Jyväskylä Studies in Biological and Environmental Science, p: 202.
- Arrhenius K, Johansson U (2012) Characterisation of contaminants in biogas before and after upgrading to vehicle gas, Report SGC246.
- Nilsson Påledal S1, Arrhenius K2, Moestedt J3, Engelbrektsson J2, Stensen K3 (2016) Characterisation and treatment of VOCs in process water from upgrading facilities for compressed biogas (CBG). See comment in PubMed Commons below Chemosphere 145: 424-430.
- Polman EA, Top H, Gerritsen B, Rekers A (2015) Development of existing and new measurement technologies for determination of the gas composition, EDGaR, GT15-0029
- Eriksson KA, Levin JO, Sandström T, Lindström-Espeling K, Lindén G, et al. (1997) Terpene exposure and respiratory effects among workers in Swedish joinery shops. Scand J Work Environ Health 23: 114-120.
- National Swedish Board of Occupational Safety and Health (NSBOSH) (1993) Occupational exposure limit, Stockholm, NSBOSH. Ordinance AFS 1993: 9
- CIVO-TNO, Volatile compounds in food. Database (2000) Boelens Aroma Chemical Information Service, Zeist, Netherlands.
- Raich-Montiu J, Ribas-Font C, de Arespacochaga N, Roig-Torres E, Broto-Puig F, et al. (2014) Analytical methodology for sampling and analyzing eight siloxanes and trimethylsilanol in biogas from different wastewater treatment plants in Europe. Anal Chim Acta 812: 83-91.
- Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition, Compendium Method TO-17, Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes, 1999.
- Ramirez N, Marcé RM, Borrull F (2011) Determination of volatile organic compounds in industrial wastewater plant air emissions by multi-sorbent adsorption and thermal desorption-gas chromatography-mass spectrometry. Intern J Environ Anal Chem 91: 911-928.
- Strömvall AM, Petersson G (1992 ) Protection of terpenes against oxidative and acid decomposition on adsorbent cartridges. J Chrom 589: 385-389.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12180
- [From(publication date):
August-2016 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 11183
- PDF downloads : 997