Diagnostic Value of CD49d Expression in Patients with Chronic Lymphocytic Leukemia
Received: 29-Oct-2018 / Accepted Date: 11-Mar-2019 / Published Date: 18-Mar-2019 DOI: 10.4172/2168-9652.1000249
Abstract
This study was carried out on 41 newly diagnosed chronic lymphocytic leukemia (CLL) patients for investigation of the diagnostic value of CD49d and its correlation with disease outcome in CLL patients. CD49d expression on CLL cells in peripheral blood samples was estimated by flow cytometry analysis. Patients with ≥ 31.5% of neoplastic cells expressing CD49d were considered high CD49d cases. The cases with high CD49d expression were significantly associated with progression to a more advanced stage while lymphocytic doubling (LD) and mortality were not significantly associated with high CD49d expression. Lymphocytic doubling time (LDT) was significantly shorter in high CD49d versus low CD49d of CLL patients. Additionally, high CD49d group showed significantly shorter progression free survival (PFS) when compared to low CD49d group of CLL patients. However, overall survival (OS) did not differ significantly between both high CD49d and low CD49d of CLL patients. Multivariate analysis revealed that CD49d is an independent prognostic factor for predicting lymphocytic doubling time. Moreover, the advanced Rai stage and high CD49d are independent prognostic factors for shorter progression free survival. Overall, CD49d is selected as the most important flow cytometry-based prognostic biomarker regardless of other prognosticators such as CD38 and ZAP-70. Consistently, bivariate analysis revealed that CD49d identified CLL patients with poorer outcome independent of CD38 and ZAP-70. Overall, CD49d acts as an independent bad prognostic marker for B-CLL patients at different stages and a predictor of overall survival.
Keywords: Chronic lymphocytic leukemia; CD49d; B lymphocytes; Malignancies
Introduction
Chronic Lymphocytic Leukemia (CLL) is a clinically heterogeneous disease, characterized by the progressive accumulation and expansion of a clonal population of neoplastic cells with the morphological appearance of small mature B lymphocytes in blood, bone marrow, and lymphoid organs [1]. It represents the most common form of adult leukemia in the Western world [2]. It is the most frequent B cell leukemia occurring predominantly in elderly patients [3]. In Egypt, according to National Cancer Institute (NCI) hospital-based registry (2002–2010), chronic lymphocytic leukemia accounts for 0.5% of all cancers and 3.08% of Lymphohemopoietic Malignancies [4]. Classification of Chronic Lymphocytic Leukemia patients into different prognostic subgroups based on clinical observations and standard laboratory tests was introduced over thirty years ago by 2 widely accepted clinical staging systems: Rai system [5] and Binet system [6]. These clinical staging systems are the mainstay for assessing prognosis in chronic lymphocytic leukemia patients [7]. However, they are unable to prospectively identify patients at higher risk of disease progression [8]. Age, sex, absolute lymphocytic count, lymphocytic doubling time and serum β2 microglobulin (β2-MG) are simple clinical and laboratory parameters added to clinical stage to improve the prediction of overall survival and time to first treatment in early stage chronic lymphocytic leukemia [9]. The biological markers can predict disease progression and therapeutic outcomes in patients with early stage chronic lymphocytic leukemia [10]. Additionally, the prognostic parameters can predict which early stage patients will experience early disease progression [11]. The level of different adhesion molecules expression in chronic lymphocytic leukemia is apparently reflected on the potential migratory behavior of the leukemic cells to different organs [1].
CD49d is the α4 chain subunit of the CD49d/CD29 (α4β1) integrin heterodimer very late activation-4 receptor, expressed on the surface of CLL lymphocyte cells, and in ~40% of CLL cases [12,13]. It acts as a surface adhesion molecule, associated with the beta integrin CD29 (β1) chain, forming the very late antigen 4 (CD49d/CD29) molecule [14]. CD49d plays a critical role in leukocyte trafficking, homing, activation, and survival and also, mediates cell-to extracellular matrix and cell-tocell interactions via binding with fibronectin or vascular cell adhesion molecule-1(VCAM-1), respectively and facilitates interactions between leucocytes and stromal cells which are crucial processes in the progression of CLL [12]. Furthermore, its expression promotes microenvironmentmediated proliferation of CLL leukemic cells [15]. The CD49d/CD29 molecule contributes to enhanced survival of CLL leukemic cells by mediating migration and adhesion to the protective microenvironment, not by direct prevention of apoptosis [16]. Additionally, this integrin can affect B-cell survival via upregulation of Bcl-2 family members [17]. Therefore, CD49d strongly correlated with more aggressive disease and its expression was stable over the course of the disease [18,19]. It has emerged as one of the most relevant and valuable adverse biological and prognostic predictors of overall survival and progression-free survival in CLL [12,13].
Patients and Methods
A total of 41 newly diagnosed, untreated, and asymptomatic confirmed typical chronic lymphocytic leukemia (CLL) patients were collected from patients admitted to Mansoura Oncology Center, Mansoura University, Egypt. The studied CLL patients comprised of 34 males (82.9%) and 7 females (17.1%) whose advanced ages ranged from 45 to 84 years old with the median age of 59 years. A written informed consent was provided and obtained from every participant patient and control prior to their enrollment and recruited into this study according to the guidelines of Committeé of Medical Ethics of Mansoura University Hospitals. This reported study protocol was reviewed and approved by the local ethics Committeé on Human Research at Mansoura University, Egypt.
The diagnosis of chronic lymphocytic leukemia was established clinically, biologically, morphologically, by using immunophenotyping (IPT) analysis, by standard clinical and laboratory criteria, bone marrow investigations, and by surface marker criteria. It was diagnosed clinically in according to international CLL workshope criteria (IWCLL) [4,20] and based on immunophenotypic analysis for the expression of CD5, CD19 and monoclonal immunoglobulin in accordance with National Cancer Institute Working Group Criteria and Guidelines and then confirmed by a flow cytometry score >3 [21,22]. Chronic lymphocytic leukemia diagnosis is usually an easy issue and relies on the immunophenotyping analysis and not done until an adequate immunophenotype is established [23]. The diagnosis of B-CLL required a persistent lymphocytosis of more than 5.0×109/L and a typical CD19+, CD20+ CD5+, CD23+, Ig light chain (κ or λ light chain) restricted immunophenotype as revealed by flow cytometry of peripheral blood lymphocytes [24]. No single marker is enough to establish the diagnosis of B-CLL and so, a minimum number of monoclonal antibodies (moAb) must be evaluated [25].
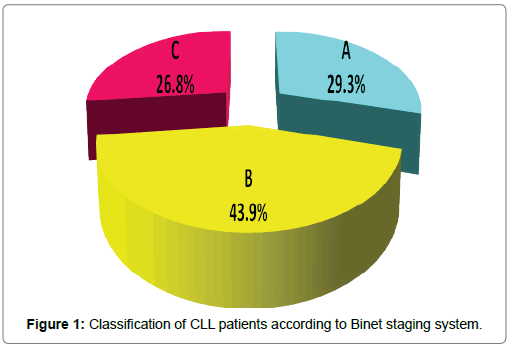
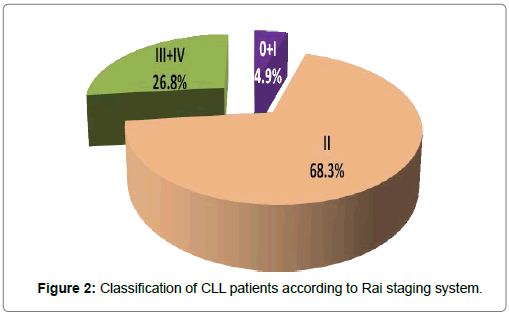
Clinical data of chronic lymphocytic leukemia patients was studied at diagnosis. Lymphadenopathy was present in all cases, was the commonest presentation, followed by splenomegaly was present in 37 (90.2%) cases, and hepatomegaly was present in 26 (63.4%) cases. The CLL cases were classified at diagnosis according to the 2 widely accepted clinical staging systems for assessing prognosis in patients with CLL: Rai et al. [26] and Binet [5] staging systems by using standard clinical and laboratory investigations. Both classifications were used to describe the disease status at the patients’ entry into the study [27]. According to Binet system, a stage included 12 (29.3%) cases, B stage included 18 (43.9%) cases and C stage included 11(26.8%) cases. According to Rai system, 0 and I stages (early stages or low-risk category) included 2 cases (4.9%), II stage (Intermediate-risk category) included 28 (68.3%) cases, III and IV stage (advanced stages or High-risk category) included 11 (26.8%) of cases (Figures 1 and 2). Moreover, clinical outcome and end points of CLL patients such as lymphocyte doubling, progression to more advanced stage and death were studied for prediction of CLL prognosis during the period of follow up. Lymphocytic doubling occurred in 13 cases (31.7%) and progression to a more advanced stage occurred in 17 cases (41.5%). Total mortality occurred in 4 cases (9.8%) and 37 cases are living patients during the entire period of the study. Patients are followed up for periods up to 24 months or until death. Lymphocytic doubling time and survival times of all studied CLL patients was shown in Table 1. Cumulative incidence of CLL cases having lymphocytic doubling time at 6 months was 30.8%. Cumulative incidence of CLL cases surviving without progression or treatment till 12 months was 55.4%. Cumulative incidence of CLL cases surviving at 12 months for overall survival was 95.1%. Mean lymphocytic doubling time was 4.508 months. Mean overall survival 22.98 months. Mean progression free survival was 12.04 months. The estimated mean overall survival from time of diagnosis for the entire cohort was 22.976 months (95% confidence interval (CI): 20.170–25.782).
| CLL (N=41) | |||
|---|---|---|---|
| Cumulative incidence (%) | Mean (months) | CI 95% | |
| Lymphocytic doubling time (LDT) | 30.80% | 4.508 | 2.485-6.530 |
| Progression free survival (PFS) | 55.40% | 12.04 | 10.557-13.523 |
| Overall survival (OS) | 95.10% | 22.976 | 20.170-25.782 |
Cumulative incidence: Cumulative proportion surviving at 12 months for progression free Survival (PFS) and Overall Survival (OS) and at 6 months for Lymphocytic Doubling Time (LDT).
CI 95% Confidence interval at 95%.
Table 1: Lymphocytic doubling time (LDT) and survival times such as progression free survival (PFS) and overall survival (OS) of all studied CLL patients.
Specimen Collection and Handling
All peripheral blood samples were taken at initial diagnosis before any chemotherapeutic approach. One milliliters of whole blood were taken in sterile EDTA tube for complete blood count by automated cell counter (CELL DYN RUBY, Laser instrument, USA) and also, 2 mL of whole blood were taken in sterile EDTA tube for immunophenotyping and determination of the expression of CD49d levels by flow cytometric analysis (Becton Dickinson: BD FACS CantoTM II flow cytometer, company BD Biosciences, San Joe, CA95131, USA). Three ml serum for liver function tests (Alanine Aminotransferse (ALT), Aspartate Aminotransferase (AST), albumin and total serum bilirubin), kidney function tests (Creatinine and uric acid) and lactate dehydrogenase (LDH) carried out on (BT 3500 instrument, Italy). The expression of CD49d was analyzed at time of diagnosis by flow cytometric immunophenotyping analysis, using a panel of antibodies designed to characterize CD5+ B lymphoid neoplasms during the routine clinical evaluation for diagnosis, staging, or follow-up by using a FACS calibur flow cytometer (Becton Dickinson: BD FACS CantoTM II flow cytometer, company BD Biosciences, San Jose, CA95131, USA). Additionally, the CD38 and ZAP-70 expression was analyzed by flow cytometric analysis by using a three-color staining combining method [22].
Statistical Analysis
All of the statistical calculations were done by using excel program (Microsoft Office 2010) and Statistical Package for Social Science program (SPSS ) (SPSS, Inc, Chicago, IL) version 20 [28].
Results And Discussion
Chronic lymphocytic leukemia (CLL) is an indolent disease characterized by a low rate of proliferation, with a unique phenotype, and a high mortality rate [29,23]. Behavior of CLL patients belonging to the same clinical risk category is not uniformly predictable due to the variability in time to progression and survival of early stage CLL [30,31]. Majority of patients with CLL are a symptomatic and have early stage disease at the time of diagnosis but, some experience an aggressive disease course that leads to the premature death within a few months while others live for decades untill 20 years or more and never require therapy [11,32].
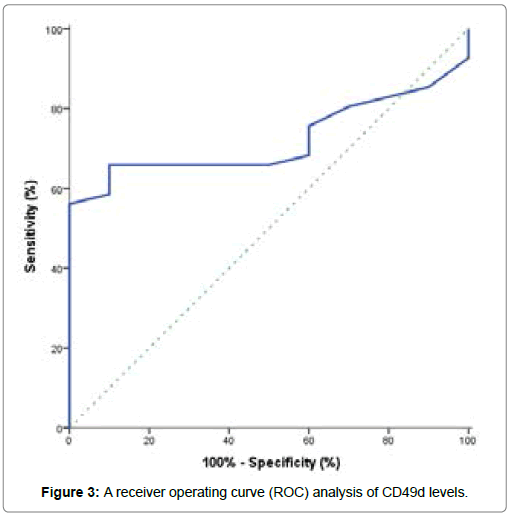
From this study, the CLL patients at diagnosis showed a significant decrease in hemoglobin concentration when compared to control group, but a highly significant decrease in platelets count when compared to control group. Also, a highly significant increase in total leucocytes count, peripheral blood absolute and relative lymphocytic count in CLL patients when compared to control group. Median prolymphocytes in peripheral films was 1% (Table 2). They showed the presence of lymphocytosis at the time of diagnosis [33]. Kamel et al. [1] reported that CLL Rai staging showed a positive correlation with absolute leucocyte count (ALC) and a negative correlation with hemoglobin (Hb) level. On the other hand, CLL patients showed significantly higher lactate dehydrogenase (LDH) concentration when compared to control subjects (P <0.001), as well as no significant differences were observed for alanine aminotransferse (ALT), aspartate aminotransferase (AST), total bilirubin, albumin, creatinine and uric acid in CLL patients when compared to control subjects (P>0.05) (Table 3). We investigated the diagnostic value of CD49d expression in CLL patients, its correlation with disease outcome and also, its role as an independent prognostic marker in CLL patients. The expression of CD49d determined by flow cytometry analysis and identified by several independent groups as a novel predictor of disease progression and overall survival in CLL patients [2,13,15,22,24,31-39]. The performance characteristics of CD49d by using a receiver operating curve (ROC) analysis which conducted to identify the optimal CD49d level for potential prediction of development of CLL was shown in Table 4 and Figure 3. From this curve, the best cut off value for CD49d expression that discriminated CLL patients from controls and yielding the separating of CLL patients into two subgroups with different prognosis was fixed at 31.5%. The values above cut off were considered high CD49d expression while values below cut off were considered low CD49d expression (Figure 4). This is closely similar to previous studies [18,13,19,22,]. They found that 30% cut off value is the best to code CD49d expression status to predict overall survival in CLL patients and patients with ≥ 30% of neoplastic cells expressing CD49d were considered CD49d+ or high CD49d.
| Hematological data | Control (N=25) | CLL (N=41) | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Absolute lymphocytic count (× 109/L) | 1.43 | 4-Jan | 51.4 | 3 -230 | <0.001 |
| Relative lymphocytic count (%) | 32.5 | 20-50 | 81.1 | 43.6-93.7 | <0.001 |
| Prolymphocytes (%) | - | - | 1 | 3-Jan | - |
| Total leucocytic count (TLC) (× 109/L) | 5.19 | 7-Apr | 71 | 8-336 | <0.001 |
| Hemoglobin concentration (Hb) (g/dL) | 14 | 16-Dec | 11 | 15-May | <0.001 |
| Platelet count (× 109/L) | 365.5 | 307-438 | 111 | 8-416 | <0.001 |
Significance probability when P <0.05.
Abbreviation: TLC: Total Leucocytic Count; Hb: Hemoglobin concentration.
Table 2: Comparison between hematological data at diagnosis of studied CLL patients and matched control group.
| Clinical chemistry data | Control (N=25) | CLL (N=41) | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| ALT (U/mL) | 18.5 | 27-Oct | 18 | 6-142 | 0.849 |
| AST (U/mL) | 28.5 | 25-35 | 25.3 | 6-225 | 0.146 |
| Total bilirubin (mg/dL) | 1 | 0.9-1.1 | 2.7 | 0.2-5.2 | 0.615 |
| Albumin (g/dL) | 5 | 5-Apr | 5 | 3.4-6.4 | 0.594 |
| Creatinin (mg/dL) | 1 | 0.7-1.1 | 1 | 0.4-2 | 0.129 |
| Uric acid (mg/dL) | 5 | 6-Mar | 5 | 10-Feb | 0.209 |
| LDH (U/mL) | 140 | 120-170 | 460 | 227-1370 | <0.001 |
Significance probability when P <0.05.
Abbreviation: ALT: Alanine Aminotransferse.
Table 3: Comparison between alanine aminotransferse (ALT), aspartate aminotransferase (AST), total bilirubin, albumin, creatinine, uric acid, and lactate dehydrogenase (LDH) of studied CLL patients and matched control group.
| Cut off (%) | AUC | SE | P | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| 31.5 | 0.721 | 0.068 | 0.032 | 0.587- 0.855 | 56.1 | 100 | 100 | 35.7 | 64.7 |
Abbreviation: AST: Aspartate Aminotransferase; LDH: Lactate Dehydrogenase; AUC: Area Under the Curve; SE: Standard Error; CI: Confidence Interval 95%; PPV: Positive Predictive Value; NPV: Negative Predictive Value.
Table 4: Performance characteristics of CD49d
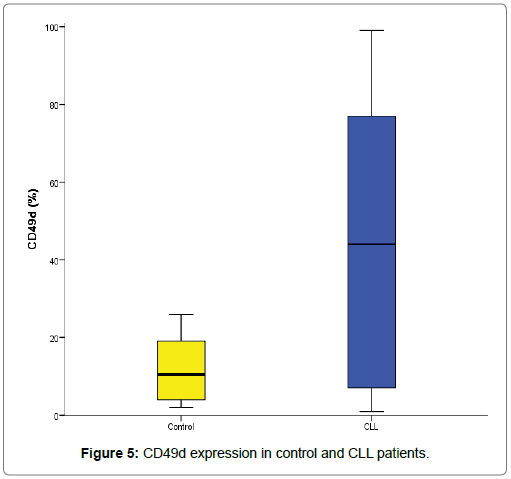
According to this cut off value 31.5%, 23 (56.1%) CLL cases were defined as high CD49d expression while 18 (43.9%) CLL cases as low CD49d expression. CD49d in control ranged from 2-26% with median of 10.5%. Total CLL CD49d ranged from 1-99% with median of 44%. CLL low CD49d ranged from 1-26% with median of 5% while CLL high CD49d ranged from 37-99% with median of 73% (Table 5 and Figure 5). Moreover, our data showed that CD49d expression was increased in CLL patients at diagnosis when compared to control group and there was statistically significant increase of CD49d expression in CLL patients when compared to normal control subjects (P=0.032) (Table 4) [40].
| Control (N= 25) | CLL | |||
|---|---|---|---|---|
| Total | CD49dlow | CD49dhigh | ||
| N= 41 | N=18 | N=23 | ||
| Median (%) | 10.50% | 44% | 5% | 73% |
| Range (%) | 2-26% | 1-99% | 1-26% | 37-99% |
Table 5: CD49d expression in studied groups of CLL patients and matched control group.
From these results, we observed a wide interpatient variation of CLL B-cell CD49d protein expression ranged between 0 and 100% CD19+ cells and the great majority of CLL patients in our series either expressed CD49d at low levels or expressed CD49d at very high levels. This is in agreement with the results [22,41]. They showed that most cases either expressed CD49d at a high level or showed negligible expression, confirming that CD49d is easier to interpret as positive or negative. Additionally, they reported that CD49d expression was variably expressed on B-CLL cells and showed bimodal distribution with most patients, either very high or very low levels of expression and this makes CD49d most interesting for a good new reproducible marker as reported in previous studies [13,24,32]. Concerning to age and gender distributions, there were no significant differences of age and gender distributions according to CD49d positivity in studied groups of CLL patients (P>0.05) (Table 6). They reported that there were not significant differences in age or gender between CLL patients with CD49d expression ≥ 30% and <30% [41-46]. Hendy et al. [42] reported that there was no correlation was detected between CD49d and age or sex. As well as, there were no significant differences found between low CD49d and high CD49d CLL groups regarding to clinical characteristics data (P >0.05) (Table 7).
| CD49dlow | CD49dhigh | P | ||
|---|---|---|---|---|
| N=18 | N=23 | |||
| Age (Years) | Median | 57.5 | 59 | 0.285 |
| Range | 45-69 | 48-84 | ||
| Gender | Males, N (%) | 17 (94.4%) | 17 (73.9%) | 0.112 |
| Females, N (%) | 1 (5.6%) | 6 (26.1%) | ||
Age is presented by median and range. Gender is presented by number and percentage. Significance probability when P <0.05.
Table 6: Age and gender distributions according to CD49d positivity in studied groups of CLL patients
| Clinical characteristics | CD49dlow | CD49dhigh | P | |||
|---|---|---|---|---|---|---|
| N= 18 | N= 23 | |||||
| N | % | N | % | |||
| Lymphadenopathy | 18 | 100 | 22 | 95.7 | 0.6 | |
| Splengomegaly | 15 | 83.3 | 18 | 78.3 | 0.7 | |
| Hepatomegaly | 13 | 72.2 | 13 | 56.5 | 0.3 | |
| Binet stage | A | 3 | 16.7 | 8 | 34.8 | 0.5 |
| B | 11 | 61.1 | 8 | 34.8 | ||
| C | 4 | 22.2 | 7 | 30.4 | ||
| Rai stage | 0+I+II | 14 | 77.8 | 16 | 69.6 | 0.8 |
| III+IV | 4 | 22.2 | 7 | 30.4 | ||
Significance probability when P <0.05.
Table 7: Comparison between CD49d positivity according to clinical characteristics of studied groups of CLL patients.
According to stages, CD49d expression lacks of a significant association at stage Binet A in our cohort, but those patients with high CD49d expression had a distinct poor disease outcome in contrast to those with low CD49d expression and the lack of association could be explained with the lower patient number in Binet A stage (11 patients with Binet A: 3 patients with low CD49d and 8 patients with high CD49d). They reported that CD49d protein expression did not provide prognostic information in both patients with early stage (Binet A) and with more advanced stage disease (Binet B/C) [24]. Baumann et al. [18] reported that a high expression of CD49d (>30%) was associated with progressive disease such as advanced clinical stage and so, patients with higher expression of CD49d had more advanced Rai and Binet clinical stages as well as more constitutional symptoms. Zucchetto et al. [38] observed significant associations between CD49d expression and Rai staging. Hendy et al. [42] reported that there was a positive correlation between the CD49d expression and prognostic parameters in CLL patients such as CLL staging.
Our result is in disagreement with [43,22,33]. They showed that patients with high CD49d expression (≥ 30%) had a significantly higher median Rai stage and significantly associated with a more advanced Binet and Rai stage disease stages at the time of diagnosis. Seiler et al. [40] reported that the differences of CD49d expression were related to clinical stage and showed that CLL patients in early disease stage had significantly lower CD49d expression compared with patients in the advanced disease stage. The mean CD49d expression of the advanced stage group was different from that of the normal control group and also, CD49d expression was significantly different in early and advanced stages independently from treatment status.
Concerning to lymphadenopathy, Strati et al. [19] reported that CD49d positivity expression was strongly associated with the presence of nodal presentation and subsequent development of lymphadenopathy in CLL patients at the time of diagnosis as well as during the course of disease, confirming that patients with high CD49d positive expression would experience a clinical course dominated by lymphadenopathy. There were a significantly higher percentage of leukemic cells expressing CD49d observed among patients presenting with lymphadenopathy at the time of diagnosis. Additionally, they reported that among CLL Rai 0 patients, CD49d positivity was associated with shorter time to subsequent development of lymphadenopathy and this association was maintained on multivariate analysis after adjusting for either FISH or IGHV status. Baseline lymphadenopathy was more frequent among CD49d-positive patients than CD49d-negative patients. Till et al. [44] reported that there was an association between high CD49d expression and the presence of lymphadenopathy and disease stage.
Moreover, the authors reported that the level of CD49d expression was linked to the presence of bulky lymphadenopathy and/or adverse outcome in CLL [22,33,43]. Pasikowska et al. [45] demonstrated that CLL cells collected from lymph nodes have higher CD49d expression than those in the peripheral blood, Baumann et al. [18] reported that increased CD49d expression was associated a higher number of involved lymph node regions and a tendency towards more frequently bulky lymphadenopathy. Additionally, Kamel et al. [1] showed a significant relation between CD49d with lymphadenopathy and this is consistent with other studies that linked CD49d to the presence of bulky lymphadenopathy and/or adverse outcome in CLL, reported that there was a positive correlation between the CD49d expression and prognostic parameters in CLL patients such as lymphadenopathy [35,32,42].
Regarding to organomegally, they reported that high CD49d expression was significantly associated with splenomegaly and with hepatomegaly [22,33,43]. Strati et al. [19] confirmed the association between CD49d positivity expression and initial presentation with splenomegaly and they reported that the baseline splenomegaly was more frequent among CD49d-positive patients than among CD49d negative patients. Baumann et al. [18] reported that increased CD49d expression was associated a tendency towards the presence of liver and spleen enlargement.
Our data showed that there were no significant differences found between low CD49d and high CD49d CLL groups regarding to hematological data (P >0.05) (Table 8). They reported that no correlation found between the CD49d expression and peripheral cell count but, early stage patients had lower levels of peripheral cell counts compared with the advanced stage patients [40]. They reported that high CD49d was associated with significant decrease in platelets count and hemoglobin concentration [22]. Additionally Baumann et al. [18] reported that patients with a higher CD49d expression was associated with a lower blood lymphocyte count, absolute leukocyte count, and a higher number of lymphoid areas involved by the disease and also, patients with higher expression of had lower hemoglobin levels. Hendy et al. [42] reported that there was a positive correlation between the CD49d expression and prognostic parameters in CLL patients such as bone marrow lymphocytic count.
| Hematological data | CD49dlow | CD49dhigh | p | ||
|---|---|---|---|---|---|
| N=18 | N=23 | ||||
| Median | Range | Median | Range | ||
| Absolute lymphocytic count (× 109/L) | 51.1 | 6-230 | 51.4 | 3-183 | 0.979 |
| Relative lymphocytic count (%) | 77.1 | 48.1-93.7 | 84.2 | 43.6-91.6 | 0.763 |
| Prolymphocytes (%) | 1 | 0-3 | 1 | 0-3 | 0.329 |
| Total leucocytic count (TLC) (× 109/L) | 86.35 | 9-335 | 56.4 | 8-300 | 0.694 |
| Hemoglobin concentration (Hb) (g/dL) | 11 | 15-Jun | 11 | 14-May | 0.307 |
| Platelet count (× 109/L) | 115.5 | 26-229 | 98.2 | 8-416 | 0.854 |
Significance probability when P <0.05.
Abbreviation: TLC: Total Leucocytic Count; Hb: Hemoglobin Concentration.
Table 8: Comparison between CD49d positivity regarding to hematological data of studied groups of CLL patients.
On the other hand, there were no significant differences found between low CD49d and high CD49d CLL groups regarding to clinical chemistry data (P >0.05) (Table 9). They reported that high CD49d was associated with significant increase in lactate dehydrogenase versus low CD49d expression [22]. Additionally, Baumann et al. reported that a high expression of CD49d (>30%) was associated with progressive disease and a higher tumour burden as indicated by high serum lactate dehydrogenase or β2-microglobulin levels and so, patients with higher expression of CD49d had higher lactate dehydrogenase [18].
| Clinical Chemistry data | CD49dlow | CD49dhigh | P | ||
|---|---|---|---|---|---|
| (N= 18) | (N= 23) | ||||
| Median | Range | Median | Range | ||
| ALT (U/mL) | 17 | Jun-40 | 24 | 8-142 | 0.09 |
| AST (U/mL) | 20 | Oct-35 | 25 | 6-225 | 0.18 |
| Total bilirubin (mg/dL) | 1 | 0.5-2.0 | 1 | 0.4-41 | 0.725 |
| Albumin (g/dL) | 5 | 3.9-5.6 | 4.5 | 3.4-6.4 | 0.137 |
| Creatinin (mg/dL) | 1 | 2-Jan | 1 | 0.4-2 | 0.324 |
| Uric acid (mg/dL) | 5.6 | 4-9.7 | 5 | 10-Feb | 0.147 |
| LDH (U/mL) | 440 | 277-871 | 498 | 290-1370 | 0.486 |
Significance probability when P <0.05.
Abbreviation: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; LDH: Lactate Dehydrogenase
Table 9: Comparison between CD49d groups regarding to clinical chemistry data such as alanine aminotransferse (ALT), aspartate aminotransferase (AST), total bilirubin, albumin, creatinine, uric acid, and lactate dehydrogenase (LDH) of studied groups of CLL patients.
Many studies suggested that the use of an additional prognostic marker may be more useful than their individual use, such as combined analysis of CD38 and ZAP-70 or CD38 and CD49d [46]. Therefore, several disease characteristics in association with CD49d expression status of the patients’ B-CLL cells was evaluated in order to determine its effect on the disease course and the prognostic value of CD49d alone and in combination with established prognostic indicators. Our data showed that there were no significant associations found between CD49d versus Zeta chain associated protein 70 (ZAP-70) and cluster of differentiation CD38 positivity in relation to each other in studied groups of CLL patients (P >0.05) (Table 10). They reported that CD49d surface expression was significantly and positively correlated with both CD38 and ZAP-70 expression [13,15,22,32,34,35,38,39,42,43]. This disagreement may be due the low number of events or cases in our CLL series. Additionally, Gooden [41] et al. reported that flow cytometric expression for CD49d showed the greatest discrimination between positive and negative cases compared with CD38 and ZAP- 70. Strati et al. [19] showed that the expression of CD49d correlates with some other prognostic factors. Specifically, higher expression of CD49d is associated with CD38 [47-53] and ZAP-70. Baumann et al. [18] reported that a high expression of CD49d (>30%) was significantly associated with aggressive disease biology and biological markers such as increased ZAP-70 or CD38 expression. Kamel et al. [1] revealed a positive correlation was encountered between CD38 and CD49d adhesion molecules and this is in agreement with other studies. The authors observed statistically significant correlation between CD49d and CD38 [42,38].
| CD49dlow | CD49dhigh | P | |||
|---|---|---|---|---|---|
| N=18 | N=23 | ||||
| N | % | N | % | ||
| CD38low | 7 | 38.9 | 12 | 52.2 | 0.397 |
| CD38high | 11 | 61.1 | 11 | 47.8 | |
| ZAP70low | 8 | 44.4 | 9 | 39.1 | 0.732 |
| ZAP70high | 10 | 55.6 | 14 | 60.9 | |
Significance probability when P <0.05.
Abbreviation: ZAP70: Zeta Chain associated Protein Kinase 70; CD: Cluster of Differentiation
Table 10: Comparison between cluster of differentiation CD38 and Zeta chain associated protein kinase 70 (ZAP70) positivity in studied groups of CLL patients according to CD49d positivity in studied groups of CLL patients.
We studied clinical outcomes and end points such as lymphocyte doubling, progression to a more advanced stage and mortality according to CD49d positivity in studied groups of CLL patients as shown in Table 11 for prediction whether CD49d expression has prognostic relevance in CLL patients and so, prediction of CLL prognosis during the period of follow up. Progression to a more advanced stage was significantly associated with high CD49d expression (P=0.004) while lymphocytic doubling and mortality were not significantly associated with high CD49d expression (P >0.05) and so, the higher CD49d expression was found to be associated with increased risk of CLL and disease progression. They reported that higher CD49d expression groups showed significantly higher number of cases with increased risk of CLL and disease progression and so, CD49d high expression identified as an adverse and an independent prognostic factor for lymphocyte doubling and progression to a more advanced stage or disease progression by using univariate log-rank analysis [22]. Additionally, the progressive CLL correlates with the expression of prognostic biomarker CD49d which involved in migration and tissue invasion [43,48,35]. However, lymphocytic doubling and mortality were not significantly associated with high CD49d expression [22]. They identified CD49d as adverse prognostic factor for lymphocytic doubling.
| Clinical outcome | CD49dlow | CD49dhigh | P | |||
|---|---|---|---|---|---|---|
| N=18 | N=23 | |||||
| No | % | No | % | |||
| Lymphocytic doubling (LD) | 4 | 22.2 | 9 | 39.1 | 0.248 | |
| Progression to a more advanced stage | 3 | 16.7 | 14 | 60.9 | 0.004 | |
| Died | 1 | 5.6 | 3 | 13 | 0.618 | |
Significance probability when P <0.05. LD: Lymphocytic doubling.
Table 11: Clinical outcome such as lymphocytic doubling (LD), progression to a more advanced stage, and mortality according to CD49d positivity in studied groups of CLL patients.
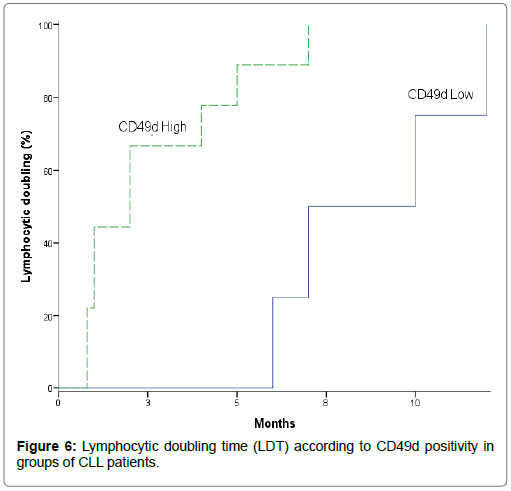
Moreover, lymphocyte doubling time and survival times were used as useful indications of disease progression and disease kinetics. Table 12 and Figure 6 showed lymphocyte doubling time in studied CLL patients according to CD49d positivity, leads to investigate the prognostic relevance and the effect of CD49d expression level on lymphocyte doubling time. Lymphocytic doubling time was significantly shorter in high CD49d versus low CD49d of CLL patients (Mean: 2.622 vs. 8.750 months). In addition, cumulative proportion of CLL patients having lymphocytic doubling at 6 months was higher in high CD49d when compared to low CD49d (Cumulative proportion: 88.9 vs. 25%) (P=0.010) [13,22,31,32,34]. They reported that patients with high CD49d expression had significantly shorter time to develop lymphocyte doubling and significantly lower cumulative proportion at 24 months still without lymphocyte doubling. Baumann et al. [18] indicated that a trend towards higher disease kinetics as assessed by the lymphocyte doubling time was observed in patients with increased CD49d compared to those with lower expression.
| CD49dlow | CD49dhigh | P | |||||
|---|---|---|---|---|---|---|---|
| N= 18 | N= 23 | Log Rank (Mantel-Cox) | |||||
| Cumulative proportion (%) | Mean (months) | CI 95% | Cumulative proportion (%) | Mean (months) | CI 95% | ||
| LDT | 25% | 8.75 | 6.051-11.449 | 88.90% | 2.622 | 1.172-4.072 | 0.01 |
Significance probability when P <0.05.
Cumulative proportion: proportion of patients having lymphocytic doubling at 6 months; CI 95%: Confidence interval at 95%.
Abbreviation: LDT: Lymphocytic Doubling Time;
Table 12: Lymphocytic doubling time (LDT) according to CD49d positivity in studied groups of CLL patients.
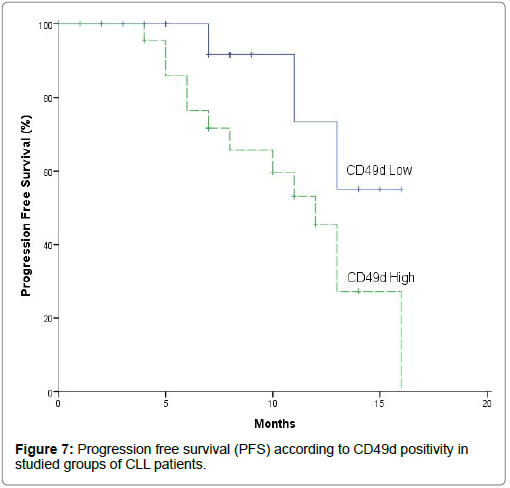
Survival times such as progression free survival and overall survival were studied according to CD49d positivity (Table 13, Figures 7 and 8) by using Kaplan-Meier survival curves to investigate the effect of CD49d expression level on disease course in patients with high and low CD49d expression in terms of progression free survival and overall survival [33]. Concerning to progression free survival, high CD49d group showed significantly shorter progression free survival when compared to low CD49d group of CLL patients (Mean: 11.005 vs. 13.783 months) (p=0.046). In addition cumulative proportion of CLL patients having progression-free survival (survived without progression) at 12 months was lower in high CD49d when compared to low CD49d (Cumulative proportion: 45.5% vs. 73.3%) (P=0.046) [13,22,31,32,34]. They reported that patients with high CD49d expression had significantly shorter progression free survival and significantly lower cumulative proportion at 24 months survived without progression versus those with low CD49d expression. Furthermore, CLL cases with high CD49d protein expression are characterized by unfavorable clinical course [24].
| CD49dlow | CD49dhigh | p | |||||
|---|---|---|---|---|---|---|---|
| N= 18 | N= 23 | Log Rank (Mantel-Cox) | |||||
| Cumulative survival (%) | Mean (month) | CI 95% | Cumulative survival | Mean (months) | CI 95% | | |
| (%) | |||||||
| PFS | 73.30% | 13.783 | 11.764 - 15.802 | 45.50% | 11.005 | 9.067-12.94 | 0.046 |
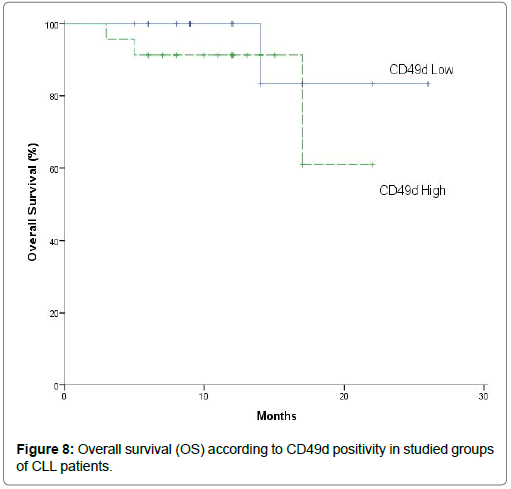
| OS | 100% | 24 | 20.422 - 27.578 | 91.30% | 18.913 | 15.834-21.99 | 0.37 |
Significance probability when P <0.05.
Abbreviation: PFS: Progression-free survival; OS: Overall survival; Cumulative proportion: proportion of patients surviving at 12 months; CI 95%: Confidence interval at 95%.
Table 13: Survival times such as progression-free survival (PFS) and overall survival (OS) according to CD49d positivity in studied groups of CLL patients.
However, overall survival did not significantly differ between both high CD49d and low CD49d of CLL patients (P= 0.370) and so, there were not significantly differences in overall survival between high-risk patients with high and low CD49d expression [22]. They reported that overall survival showed no significant differences between high and low CD49d expression and so, high CD49d expression not identified as adverse prognostic factor for overall survival. This marker was not significantly for predicting overall survival by using univariate log-rank analysis. The lack of association between CD49d and overall survival in our study could be related to the relatively small sample size of cases in the individual categories and short follow-up of early stage patients [12,24,32-35,40,46,51]. They reported that CD49d protein expression as a continuous variable was strongly and significantly correlated with overall survival. Also, they showed a significantly shorter survival in CD49d high CLL patients compared with CD49d low CLL patients. High CD49d protein expression levels were associated with an unfavorable clinical outcome as indicated by having a significantly shorter overall survival and time from diagnosis to treatment than patients with low CD49d protein expression levels who had a significantly longer overall survival. And so, patients with low CD49d protein expression had a distinct trend for better overall survival in stage Binet A and Binet B/C [39,50,51].
On the other hand, the prognostic value of CD49d expression was compared with other clinical parameters and already established flow cytometric prognostic factors namely age, absolute lymphocytic count, Rai staging, lactate dehydrogenase, cluster of differentiation CD38, zeta chain associated protein kinase and CD49d positivity as covariates in studied CLL patients by using multivariate analysis for prediction of lymphocytic doubling time as dependent parameter and to predict the value of CD49d expression as an independent prognostic factors for lymphocyte doubling time. Multivariate analysis revealed that high CD49d expression is an independent significantly prognostic factors for developing and predicting shorter lymphocyte doubling time and so, lymphocytic doubling time as dependent parameter on CD49d expression (P=0.013, HR=1.568, 95% CI=2.199-9.386) (Table 14). They reported that the prognostic value of CD49d is independent of other prognostic parameters [32,34].
| Covariates Age (years) | P (0.247) | HR (0.913) | 95% CI | |
|---|---|---|---|---|
| 0.783 | 1.065 | |||
| Rai (advanced versus early stages) | 0.907 | 0.849 | 0.055 | 13.101 |
| Absolute lymphocytic count (× 109/L) | 0.956 | 1 | 0.984 | 1.018 |
| LDH (U/L) | 0.572 | 0.999 | 0.997 | 1.002 |
| CD38 (high versus low) | 0.115 | 2.321 | 0.427 | 4.916 |
| ZAP70 (high versus low) | 0.602 | 0.533 | 0.05 | 5.647 |
| CD49d (high versus low) | 0.013 | 1.568 | 2.199 | 9.386 |
Significance probability when P <0.05.
Abbreviation: HR: Hazzard Ratio; CI 95%: Confidence Interval at 95%; LDH: Lactate Dehydrogenase; CD: Cluster of Differentiation; ZAP70: Zeta Chain Associated Protein Kinase 70.
Table 14: Multivariate analysis for prediction of lymphocytic doubling time (LDT) as dependent parameter studied with other covariates such as age, absolute lymphocytic count, Rai staging, lactate dehydrogenase (LDH), cluster of differentiation CD38, zeta associated protein kinase (ZAP70) and CD49d positivity in studied CLL patients.
The relevance of CD49d expression as prognosticator for progressive disease was evaluated as progression free survival compared with other prognostic factors namely age, absolute lymphocytic count, Rai staging, lactate dehydrogenase, cluster of differentiation CD38, zeta chain associated protein kinase and CD49d positivity as covariates in studied CLL patients by using multivariate analysis for prediction of progression free survival as dependent parameter and the value of CD49d expression as an independent prognostic factors for progression free survival. Multivariate analysis showed that Rai stage and high CD49d expression are significantly independent prognostic factors for predicting shorter progression free survival among the other biologic risk factors (P=0.042, HR=3.892, CI95%=1.050-14.422; P=0.028, HR=1.304, CI95%=1.034-4.225 respectively) (Table 15).
| Covariates | P | HR | 95% CI | |
|---|---|---|---|---|
| Age (years) | 0.401 | 0.963 | 0.883 | 1.051 |
| Rai (advanced versus early stages) | 0.042 | 3.892 | 1.05 | 14.422 |
| Absolute lymphocytic count (109/L) | 0.328 | 1.005 | 0.995 | 1.015 |
| LDH (U/L) | 0.376 | 1.001 | 0.999 | 1.003 |
| CD38 (high versus low) | 0.665 | 1.421 | 0.29 | 6.97 |
| ZAP70 (high versus low) | 0.103 | 3.894 | 0.761 | 19.925 |
| CD49d (high versus low) | 0.028 | 1.304 | 1.034 | 4.225 |
Significance probability when P <0.05. HR: Hazzard ratio. CI 95%: Confidence interval at 95%.
Abbreviations: LDH: Lactate Dehydrogenase. CD: Cluster of Differentiation. ZAP70: Zeta Chain Associated Protein Kinase 70.
Table 15: Multivariate analysis for prediction of progression free survival (PFS) as dependent parameter studied with other covariates by applying age, absolute lymphocytic count, Rai staging, lactate dehydrogenase (LDH), cluster of differentiation CD38, zeta associated protein kinase (ZAP70) and CD49d positivity as covariates in studied CLL patients.
They showed that high CD49d expression is an independent prognostic marker for lymphocyte doubling and for progression to a more advanced stage [22,24,31,34,35,]. Binet et al. [30] found that high CD49d expression was a significantly independent prognostic factor and ZAP-70 ≥ 20%, CD49d ≥ 45%, Binet B/C have an independent power for predicting unfavorable prognosis by using multivariate Cox regression analysis. Baumann et al. [18] showed that CD49d was predictive for overall survival, along with other factors such as IGHV mutational status and ZAP-70 expression in the multivariate analysis.
Conclusion
From this study, we concluded that CD49d surface expression acts as the strongest flow cytometry-based predictor and prognostic marker of disease progression, lymphocytic doubling time, progression free survival and overall survival in a large multicenter study. Its expression could be useful as a good promising novel and a powerful diagnostic marker for chronic lymphocytic leukemia (CLL) patients. Additionally, CD49d proved to be an independent bad prognostic marker for CLL patients at different stages and a predictor of poorer overall survival, prognosis, response to therapy, and more advanced disease. Therefore, the introduction of CD49d detection in routine prognostic assessment of CLL patients suggested both pathogenesis and therapeutic implications for CD49d expression in CLL. Humanized anti-CD49d monoclonal antibodies (humanized anti-α4-integrin antibody) could offer the potential to impair CLL cell migration to the proliferation centers in the lymphoid tissues and adhesion of leukemic cells and so, overcome apoptotic resistance and thereby shutdown the production of new CLL clones.
Recommendation
The flow cytometric determination of CD49d expression levels as a reliable prognostic marker should be recommended in the future routine clinical practice for the baseline prognostic stratification and assessment of newly diagnosed CLL patients.
References
- Kamel AM, El-Sharkawy NM, Osman RA, Abd El-Fattah EK, El-Noshokaty E, et al. (2016) Adhesion molecules expression in CLL: Potential impact on clinical and hematological parameters. J Egypt Natl Canc Inst 28: 31-37.
- Gooden CE, Jones P, Bates R, Shallenberger WM, Surti U, et al. (2018) CD49d shows superior performance characteristics for flow cytometric prognostic testing in chronic lymphocytic leukemia/Small Lymphocytic Lymphoma. Cytometry Part B Clin Cytom 94: 129-135.
- Zenz T, Mertens D, Kuppers R, Dohner H, Stilgenbauer S (2010) From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer 10: 37-50.
- Hallek M, Cheson BD, Catovsky D, Carligaris-Cappio F, Dighiero G, et al. (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National cancer institute – working group 1996 guidelines. Blood 111: 5446-5456.
- Rai KR (1987) A critical analysis of staging in CLL. In: Gale RP, Rai KR, editors. Chronic lymphocytic leukemia: recent progress and future directions; vol. 59. New York: Alan. R. Liss p. 253-264.
- Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, et al (1981) A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 48: 198-206.
- Seiler T, Dohner H, Stingelbauer S (2006) Risk stratification in chronic lymphocytic leukemia. Semin Oncol 33: 186-194.
- Campo S, Campo GM, Avenoso A, D’Ascola A, Musolino A, et al (2006) Lymphocytes from patients with early stage of B-cell chronic lymphocytic leukaemia and long survival synthesize decorin. Biochimie 88: 1933-1939.
- Bulian P, Tarnani M, Rossi D, Forconi F, Del Poeta G, et al. (2011) Multicentre validation of a prognostic index for overall survival in chronic lymphocytic leukemia. Hematol Oncol 29: 91-99.
- Kay NE, O’Brien SM, Pettitt AR, Stilgenbauer S (2007) The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia 21: 1885-1891
- Shanafelt TD, Geyer SM, Kay NE (2004) Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patient with CLL. Blood 103, 4: 1202-1210.
- Brachtl G, Hofbauer PJ, Greil R, Hartmann TN (2014) The pathogenic relevance of the prognostic markers CD38 and CD49d in chronic lymphocytic leukemia. Ann. Hematol 93: 361-374.
- Bulian P, Shanafelt TD, Fegan C, Zucchetto A, Cro L, et al. (2014) CD49d is the strongest flow cytometry–based predictor of overall survival in chronic lymphocytic leukemia. J Clin Oncol 32: 897-904.
- Dal Bo M, Bulian P, Bomben R, Zucchetto A, Rossi FM, et al. (2016) CD49d prevails over the novel recurrent mutations as independent prognosticator of overall survival in chronic lymphocytic leukemia. Leukemia 30, 10: 2011-2018.
- Zucchetto A, Vaisitti T, Benedetti D, Tissino E, Bertagnolo V, et al. (2012) The CD49d/CD29 complex is physically and functionally associated with CD38 in B-cell chronic lymphocytic leukemia cells. Leukemia 26: 1301-1312.
-  Kriston C, Plander M, Márk Ã, Sebestyén A, Bugyik E, et al. (2018) In contrast to high CD49d, low CXCR4 expression indicates the dependency of chronic lymphocytic leukemia (CLL) cells on the microenvironment. Ann Hematol 97: 2145-2152.
- Hayashida K, Shimaoka Y, Ochi T, Lipsky PE (2000) Rheumatoid arthritis synovial stromal cells inhibit apoptosis and up-regulate Bcl-xL expression by B cells in a CD49/CD29-CD106-dependent mechanism. J Immunol 164: 1110-1116.
- Baumann T, Â Delgado J, Santacruz R, MezTrillos AM, Rozman M, et al (2016) CD49d (ITGA4) expression is a predictor of time to first treatment in patients with chronic lymphocytic leukaemia and mutated IGHV status. Br J Haematol 172: 48-55.
- Strati P, Parikh SA, Chaffee KG, Achenbach SJ, Slager SL, et al.(2017) CD49d associates with nodal presentation and subsequent development of lymphadenopathy in patients with chronic lymphocytic leukaemia. Br J Haematol 178: 99-105.
- Ortin X, Giraltb M, Romeu M, Lejeune M, Nogues MR, et al. (2012) Oxidative Stress in patients with early stage chronic lymphocytic leukemia. Assessment and correlation with prognostic factors J. Hematol 1: 77-88.
- Bajle V, Milicevie Z, Spremo-Potparevic B (2010) A negative adaptive response is expressed in peripheral blood lymphocytes that are exposed to mitomycin C and cycloheximide. J BUNO 16: 111-117.
- Ibrahim L, Elderiny WE, Elhelw L, Ismail M (2015) CD49d and CD26 are Independent Prognostic Markers for Disease Progression in Patients with Chronic Lymphocytic Leukemia. Blood Cells Mol Dis 1: 154-60.
- Rawstron AC, Shingles J, de Tute R, Bennett F, Jack AS, Hillmen P (2010) Chronic lymphocytic leukaemia (CLL) and cLL type monoclonal B-Cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytometry B Clin Cytom 78 Suppl 1: S42-S46.
- Nuckel H, Switala M, Collins CH, Sellmann L, Grosse-Wilde H, et al. (2009) High CD49d protein and mRNA expression predicts poor outcome in chronic lymphocytic leukemia. Clin Immunol 131: 472-480.
- Costa ES, Pedreira CE, Barrena S, Lecrevisse Q, Flores J, et al. (2010) Automated pattern-guided principal component analysis vs expert-based immunophenotypic classification of B-cell chronic lymphoproliferative disorders: a step forward in the standardization of clinical immunophenotyping. Leukemia 24: 1927-1933.
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, et al (1975) Clinical staging of chronic lymphocytic leukemia. Blood 46: 219-234.
- Autrup JL, Hokland P, Pedersen LH, Autrup H (2002) Effect of glutathione S-transferases on the survival of patients with acute myeloid leukaemia. Eur J Pharmacol 438: 15-18.
- Knapp GR, Miller III CM (1992) Tests of statistical significance. In Middle East Edition. Chemical epidemiology and biostatistics The National Medical series for independent study from Williams and Wilkins Baltimore, Hong Kong, London and Sydency, pp: 255-312.
- Biswas S, Zhao X, Mone AP, Mo X, Vargo M, et al (2010) Arsenic trioxide and ascorbic acid demonstrate promising activity againstprimary human CLL cells in vitro. Leuk Res 34: 925-931.
- Binet JL, Caligaris-Cappio F, Catovsky D, Cheson B, Davis T (2006) Prespectives on the use of new diagnostic tools in the treatment of chronic lymphocytic leukemia. Blood 107: 859-861.
- Rossi D, Zucchetto A, Rossi FM, Capllo D, Cerri M, et al. (2008) CD49d expression is an independent risk factor of progressive disease in early stage chronic lymphocytic leukemia. Haematologica 93: 1575-1579.
- Shanafelt TD, Geyer SM, Bone ND, Tschumper RC, Witzig TE, et al. (2008) CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: A prognostic parameter with therapeutic potential. Br J Haematol 140: 537-546.
- Uzay A, ToptaÅŸ T, Kaygusuz I, Demiralp EE, TuÄŸlular TF, et al. (2012) The Prognostic Value of CD49d Expression in Turkish Patients with Chronic Lymphocytic Leukemia. Turk J Hematol 29: 354-360.
- Gattei V, Bulian P, Del Principe MI, Zucchetto A, Maurillo L, et al. (2008) Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood 111: 865-873.
- Majid A, Lin TT, Best G, Fishlock K, Hewamanab S, et al. (2011) CD49d is an independent prognostic marker that is associated with CXCR4 expression in CLL. Leuk Res 35: 750-756.
- Rossi D, Bodoni CL, Zucchetto A, Rasi S, De Paoli L, et al. (2010) Low CD49d expression and long telomere identify a chronic lymphocytic leukemia subset with highly favourable outcome. Am J Hematol 85: 619-622.
- Shanafelt TD, Hanson C, Dewald GW, Witzig TE, LaPlant B, et al. (2008) Karyotype evolution on fluorescent in situ hybridization analysis is associated with short survival in patients with chronic lymphocytic leukemia and is related to CD49d expression. J Clin Oncol 26: e5-e6.
- Zucchetto A, Bomben R, Dal Bo M, Bulian P, Benedetti D, et al. (2006) CD49d in B-cell chronic lymphocytic leukemia: correlated expression with CD38 and prognostic relevance. Leukemia 20: 523-525.
- Zucchetto A, Bomben R, Dal Bo M, Sonego P, Nanni P, et al. (2006) A scoring system based on the expression of six surface molecules allows the identification of three prognostic risk groups in B-cell chronic lymphocytic leukemia. J Cell Physiol 207: 354-363.
- Seiler T, Dohner H, Stingelbauer S (2006) Risk stratification in chronic lymphocytic leukemia. Semin Oncol 33: 186-194.
- Gooden CE, Jones P, Bates R, Shallenberger WM, Surti U, et al. (2018) CD49d shows superior performance characteristics for flow cytometric prognostic testing in chronic lymphocytic leukemia/Small Lymphocytic Lymphoma. Cytometry Part B Clin Cytom 94: 129-135.
- Hendy OM, El Shafie MA, Allam MM, Motalib TA, Khalaf FA, et al. (2016) The diagnostic and prognostic value of CD38 and CD49d expressions in chronic lymphocytic leukemia. Egypt J Haematol 41: 70-76.
- Buggins AG, Levi A, Gohil S, Fishlock K, Patten PE, et al. (2011) Evidence for a macromolecular complex in poor prognosis CLL that contains CD38, CD49d, CD44 and MMP-9. Br J Haematol 154: 216-222
- Till KJ, Lin K, Zuzel M, Cawley JC (2002) The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 99: 2977-2984.
- Pasikowska M, Walsby E, Apollonio B, Cuthill K, Phillips E, et al. (2016) Phenotype and immune function of lymph node and peripheral blood CLL cells are linked to transendothelial migration. Blood 128: 563-573
- Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer, et al. (2003) ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 348: 1764-1775.
- Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, et al. (2009) CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res 69: 4001-4009.
- Kamiguti AS, Lee ES, Till KJ, Harris R, Glenn MA, et al. (2004) The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. Br J Haematol 125: 128-140.
- Cro L, Morabito F, Zucal N, Fabris S, Lionetti M, et al. (2009) CD26 expression in mature B-cell neoplasia: it’s possible role as a new prognostic marker in B-CLL. Hematol Oncol 27: 140-147.
- Zucchetto A, Sonego P, Degan M, Bomben R, Dal Bo M, et al. (2005) Signature of B-CLL with different prognosis by shrunken centroids of surface antigen expression profiling. J Cell Physiol 204: 113-123.
- Zucchetto A, Sonego P, Degan M, Bomben R, Dal Bo M, et al. (2005) Surface-antigen expression profiling (SEP) in B-cell chronic lymphocytic leukemia (B-CLL): Identification of markers with prognostic relevance. J Immunol Methods 305: 20-32.
- Ransohoff RM (2007) Natalizumab for multiple sclerosis. N Engl J Med 356: 2622-2629.
- Pittner BT, Shanafelt TD, Kay NE, Jelinek DF (2005) CD38 expression levels in chronic lymphocytic leukemia B cells are associated with activation marker expression and differential responses to interferon stimulation. Leukemia 19: 2264-2272.
Citation: Abdel-Aziz AF, Yahya RS, Abdel-Messih HM, Ata AM (2019) Diagnostic Value of CD49d Expression in Patients with Chronic Lymphocytic Leukemia. Biochem Physiol 8:249. DOI: 10.4172/2168-9652.1000249
Copyright: © 2019 Abdel-Aziz AF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4602
- [From(publication date): 0-2019 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 3640
- PDF downloads: 962