Research Article Open Access
Distribution and Characterization of Sex Hormones in Sediment and Removal Estimate by Sewage Treatment Plant in South Brazil
Karina Scurupa Machado1*, Julio Cesar R Azevedo1, Maria Cristina Borba Braga1, Paulo AL Ferreira2and Rubens Figueira21Department of Hydraulics and Sanitation, Federal University of Parana, Curitiba-PR, 81531-980, Brazil
2Oceanographic Institute, University of São Paulo, Oceanographic Square, 191, São Paulo-SP, 05508-120, Brazil
- *Corresponding Author:
- Karina Scurupa Machado
Department of Production Engineering
Federal University of Parana, Curitiba-PR, Brazil
81531-980
Tel: +55 41 995115715
E-mail: ksmachado@hotmail.com
Received date: February 10, 2016; Accepted date: March 21, 2017; Published date: March 28, 2017
Citation: Machado KS, Azevedo JCR, Braga MCB, Ferreira PAL, Figueira R (2017) Distribution and Characterization of Sex Hormones in Sediment and Removal Estimate by Sewage Treatment Plant in South Brazil. Environ Pollut Climate Change 1:115.
Copyright: © 2017 Machado KS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Environment Pollution and Climate Change
Abstract
Sex hormones are a group of endocrine disruptors excreted by humans and animals. These compounds have been detected in surface waters and sewage treatment plant (STP), all over the word. Due their physicochemical properties significant amount is deposited in the sediment of surface waters acting as reservoirs able to contaminate the water column. Although these compounds have origin in different sources, it is widely accepted that the main source of these contaminants is STP effluents. Despite toxicity and high input of this compound in the aquatic systems, little information is available on their concentration in the sediment and how these compounds are distributed in the environment. In this study, natural and synthetic female sex hormones (estrone - E1, 17β-estradiol - E2, 17α-Ethinylestradiol – EE and progesterone - PG) were monitored in the sediment of three rivers from the Iguaçu river basin, South Brazil. Also, a removal estimate of these compounds by the local STP was performed. The results showed significant concentrations of hormones, mainly E2, in the sediment samples and an inefficient removal by the STP, resulting in some cases, in the increasing of estrogens. An assessment of the sediment-water partition coefficient (Kd) showed high mobility in the environment for the estrogens, in contrast to the higher affinity for the sediment of the progesterone.
Keywords
Sex hormones; Sediment; Estrogens; Progestogens; Endocrine disruptor; Sewage
Introduction
Environmental complications caused by population overgrowth have been a major concern for governmental authorities and the society. The use of contraceptives is one of the main alternatives employed to effectively control the population growth [1]. However, its overuse became a complex matter due to the poor basic sanitation conditions of most countries, as the female sex hormones (FSHs) that constitute contraceptives are not efficiently removed in sewage treatment plants (STP) [2].
When these compounds are released into the environment, low levels of FSHs can induced to several problems to the aquatic fauna and human beings [3]. Caused conditions are related to their function as endocrine disruptors, which even in low concentrations can damage the functioning of endocrine systems of animal species. In this case, FSHs can induce fish and human feminization, mimicking the effects of natural hormones [4].
Among the female hormones, natural estrogens estrone (E1), 17β-estradiol (E2) and estriol (E3), synthetic estrogen 17α-ethinylestradiol (EE), and progesterone (PG) are the ones of main environmental concern due to their strength and amounts released. 17β-estradiol and estrone mean daily amounts excreted by average women during menstrual cycle is 3.5 and 8 μg, respectively, but can reach up to 259 and 600 μg, respectively, during gestation [5]. Therefore, FSHs are responsible for most of the endocrine disruptive effects in the environment [1,2,4].
The main source of FSHs contamination in aquatic systems is the release of untreated wastes and STPs water treatment inefficiency, followed by livestock [6,7]. The large amounts of FSHs present in wastes come mainly from human excretion of natural and contraceptive-originated hormones [8]. The levels of FSHs in contraceptives range from 30 to 300 μg per pill, and a fraction that can reach 80% of the ingested hormones is not absorbed by the organism and is eliminated in the urine [9].
Once released into the water, there are some mechanisms that determine the preferred routes of transport of FSHs in the environment and their elimination in STPs. These mechanisms are: FSHs biodegradation, sorption on suspended solids and bottom sediments, soil adsorption, volatilization and photolysis [1]. Sorption on suspended solid and its final sink to the bottom sediments tends to occur in the first 24 h of contact, and is faster for hormones with a higher octanol-water partition coefficient (Kow) and for smaller suspended particles [10]. According to Gomes et al. [11], the processes of sorption are dominant when log Kow is equal or greater than 3. Regarding grain size, its adsorption potential is higher in clay-sized particles and improves when associated with organic matter. In this scenario, its adsorption potential can reach values close to 20% [12].
The slightly hydrophobic nature of all FSHs, based on their Kow values (E2=3.94, E1=3.43, EE=4.15, PG=3.62) [13], drives the accumulation of significant amounts of female hormones in the sediments. This situation creates environmental reservoirs that control the bioavailability of FSHs and present a risk to the biota, as they can be released to the water column under specific environmental conditions [4]. Considering the inefficiency of STPs in removing FSHs, aquatic ecosystems around urban areas and water bodies that receive treated water from STPs are the most endangered, and studies assessing and reporting water quality in terms of female hormones are of great relevance, once these compounds can sorb to sediment and adversely affect aquatic fauna and flora and human life [1]. Given to the high propensity of sex hormones to be associated with wastewater, the assessment of these contaminants in areas under STPs influence is particularly important and although sex hormones have been detected in aquatic systems of worldwide, currently, there is limited data on the environmental behavior and fate of these compounds in different environmental media. Consequently, the exposure and risk associated with these chemicals are not adequately understood.
In this context, this study focused on the distribution and characterization of female sex hormones in the sediments of three rivers from the Iguaçu River water basin (South Brazil). It is an urban area and contains a sewage treatment plant named Atuba Sul, which releases treated water in the studied area. Therefore, considering the influence of this STP on the FSHs concentrations, an estimate of the removal efficiency of these compounds was also performed. This data could be useful to further evaluate the potential ecological risk of these hormones in the rivers.
Materials and Methods
Study area
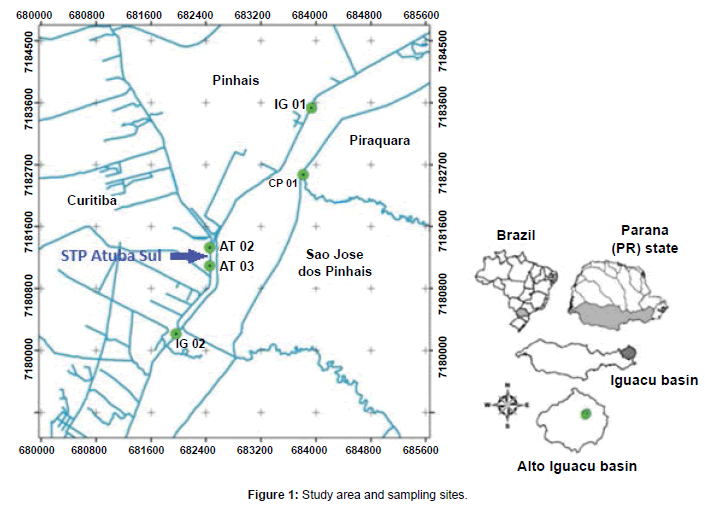
The study area (Figure 1) is located in the Alto Iguaçu water basin (Curitiba city, Parana-PR state, Brazil), a 3,000 km² drainage area. The springs of this basin are part of the Serra do Mar mountain range; its main river is Iguazu River, which runs for 90 km until the Curitiba Metropolitan Region limits. The population of this metropolitan area is of approximately 3 million inhabitants, concentrating 25% of the total population and 30% of the urban population of the Parana state, with low rates of sewage treatment [14].
This study monitored three rivers of the upper Alto Iguaçu basin: Iguaçu River (1), with preserved areas and good prospects for urban water supply; Atuba River (2), located in a region with intense human occupation and profound sewage pollution; and Paralelo Canal (3) (known as an overflow channel), a 20 km long canal parallel to Iguaçu River. This canal was initially made aiming at flood control, but now it is mainly used for urban water supply given that it prevents pollution of the water coming from the upper Iguaçu River. Even though it is considered a spring discharge region, the study area suffers deep implications on its water quality due to the irregular occupation of its floodplains.
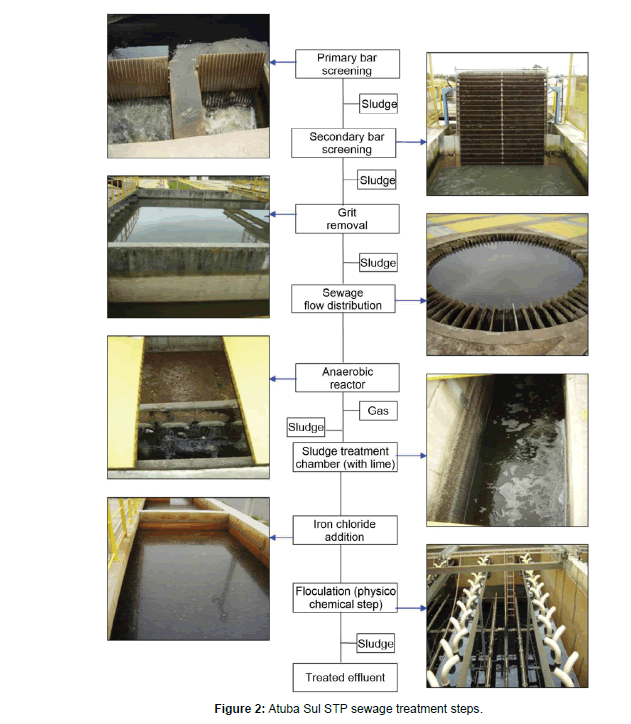
The Atuba Sul STP was built at the base level of the drainage basin. It releases treated water in the Atuba River with a mean flow rate of 700.5 Ls-1, mean effluent concentration of 70.1 mgL-1 and 68% efficiency of its anaerobic fluidized bed reactor (AFBR). Figure 2 presents the process of each sewage treatment step.
Sampling
The sampling sites are presented in Figure 1 and each was selected for a distinct characteristic. IG-01 (25º27’14’’ S, 49º10’17’’ W) is an upstream site located in the most preserved areas of the Iguaçu River; IG- 02 (25º29’01’’ S, 49º11’22’’ W) is a downstream site after the confluence of the Iguaçu and Atuba rivers. CP-01 (25º27’56” S, 49º10’17” W) is located in the Paralelo Canal; and AT-02 (25º28’17’’ S, 49º11’06’’ W) and AT-03 (25º28’21’’ S, 49º11’06’’ W) are in the upstream and downstream of the Atuba Sul STP, the most densely occupied region.
Sediment samples were collected in 2011, in 5 different sampling expeditions during all year (covering the four season, temperature ranging of 10°C to 25°C), in the river banks and bed, in areas with predominance of silt and clay. To collect the samples it was used a Petersen sediment grabber. The sampling location of sediment samples is shown in the Figure 1. Three samples were collected from each site and mixed, resulting in the final sample used for the analysis. Sludge samples were collected directly at the end of the anaerobic reactor, lime addition and physicochemical steps (Figure 2) with a shovel. After collected, all samples were placed into plastic bags, frozen, lyophilized and disaggregated in a porcelain mortar.
FSHs analysis
FSHs in sediment and sludge were analyzed according to the analytical procedures described in Lopez de Alda [15]. In short, 60 mL methanol-acetonitrile (1:1) were added to 20 g of the sample in two steps (30+30 mL), extraction in ultrasonic bath (5 min for each step), extract filtering and drying in a rotary evaporator. For the extract clean- up, it was used a solid phase extraction cartridge (Stracta, model C18) (1.0 g, 6 mL), at a flow of 8-10 mL min-1. The FSHs were eluted with 10 mL acetonitrile and the extract was then reduced to 0.5 mL under nitrogen flux for chromatography injection.
The determination of the FSHs was performed in a liquid chromatographer (Shimadzu), equipped with a peristaltic pump (model LC 20AT), a degasser (model DGU-20A) and UV diode array detector (model SPD M20A). The injection volume was 20 μL and the analytical column was one of octadecylsilane (ODS C8, 4.6 mm × 15 cm, Shimadzu). The mobile phase was acetonitrile and water 50% for the estrogens, and 90% for progesterone, with a flux of 1.4 mL min-1. The wavelength used to detect the estrogens was 280 nm, while progesterone was detected at 241 nm. The retention time of the compounds was 7.56 min for E2, 8.88 min for EE, 10.31 min for E1 and 4.60 min for PG.
The selectivity efficiency of the method, i.e., the capacity of detecting and quantifying the FSH in the presence of numerous others compounds with similar properties, was evaluated with the comparison of the UV absorption spectra of the FSHs in the sample and in a solution of known FSHs levels. The high degree of similarity between those spectra proves the absence of contaminants. Moreover, recovery rate tests were performed in samples free of the compounds of interest. The recovery rates were higher than 75% for all hormones.
Results and Discussion
Levels and spatial distribution of FSHs in sediment
Table 1 presents the results of the FSHs levels at all sampling stations. E2 was the hormone with the highest level and frequency of occurrence, varying between <2.50 to 137.91 μg kg-1. E2 is the main estrogen produced by the human body and exerts a fundamental role in the regulation of the menstrual cycle, and is commonly used in the fabrication of contraceptives. Therefore, it has both a natural and a synthetic source, its detection in water bodies can be related to sewage contamination. This is consistent with the characteristics of the study area, as the Curitiba metropolitan region has low sewage collection rates [14]. Significant amounts of E2 are released every day and incorporated into sewage all over the world [4,5] and thus occurs in higher concentrations in human impacted environments when compared to other hormones.
| Hormone | Sampling | Sampling site | ||||
|---|---|---|---|---|---|---|
| IG-01 | IG-02 | AT-02 | AT-03 | CP-01 | ||
| 17ÃÂ?-estradiol (E2) | February | 4.75 | 94.91 | 66.40 | 50.22 | 8.90 |
| April | - | 34.96 | 105.44 | 56.38 | - | |
| June | 9.63 | 53.16 | 34.65 | 11.75 | 17.56 | |
| August | 18.52 | 21.30 | 137.91 | 69.4 | 37.03 | |
| October | 55.17 | 42.77 | 125.03 | 51.53 | - | |
| 17a-ethinylestradiol (EE) | February | 4.89 | 33.72 | 5.55 | 32.65 | 3.64 |
| April | - | 10.96 | 15.01 | 14.62 | - | |
| June | 7.08 | 13.54 | 30.57 | 10.40 | 9.48 | |
| August | 3.63 | ND | 35.60 | 15.59 | ND | |
| October | 14.27 | 24.61 | 18.25 | 21.71 | - | |
| Estrone (E1) | February | ND | 11.18 | ND | 14.33 | 12.43 |
| April | - | 25.47 | 16.16 | 28.59 | - | |
| June | 3.04 | 6.90 | ND | 4.05 | ND | |
| August | ND | ND | 18.47 | 42.18 | 3.85 | |
| October | 6.63 | 5.68 | 9.52 | ND | - | |
| Progesterone (PG) | February | 32.90 | 27.72 | 21.51 | 24.11 | 31.41 |
| April | - | 22.61 | 33.86 | 88.10 | - | |
| June | 25.59 | 34.45 | 17.62 | 64.44 | 21.93 | |
| August | 18.91 | 23.28 | 22.43 | 90.92 | ND | |
| October | ND | 27.57 | 52.91 | 50.03 | - | |
ND: Not Detected (estrogens<2.50 µg kg-1, progesterone<1.50 µg kg-1).
Table 1: Female sex hormone concentrations (in µg kg-1) in the sediment samples from the Iguaçu River basin (S Brazil).
| Region | E2 | EE | E1 | PG | Source |
|---|---|---|---|---|---|
| Spain | <1.0 | 22.82 | 11.88 | <1.0 | Petrovice et al. [18] |
| nd | 4.16-22.8 | 1.32-11.9 | 0.08-6.82 | Lopez deAlda et al. [15] | |
| United States | - | - | - | 0.09-48.8 | Jenkins et al. [19] |
| Australia | 0.22-2.48 | <0.05-0.50 | 0.16-1.17 | - | Braga et al. [20] |
| United Kingdom | 0.03-1.20 | <0.04 | 0.40-3.30 | - | Labadie and Hill [21] |
| - | - | 28.8 | - | Labadie et al. [22] | |
| Argentina | 0.5-13 | 3.0-12 | 5.8-25 | - | Peres and Escandar [23] |
| Bazil | <2.5-137.91 | <3.0-36.60 | <2.5-42.18 | <1.5-90.92 | This study |
Table 2: Comparison of the levels of female sex hormones (in µg kg-1) in sediment with other studies from the literature (highest values in bold).
For E1, its concentration ranges between <2.50 to 42.18 μg kg-1. This hormone has no synthetic source, it is originated only in the human body, and is 12 times less physiologically active than E2. Lower levels were also observed for EE (<3.00–35.60 μg kg-1) and PG (<1.50–90.92 μg kg-1). EE has a synthetic origin and its absorption by the human body is approximately 15%, the remaining portion is excreted in the urine [16], while PG is a hormone directly related to pregnancy. Even though that hormone is found throughout all ovarian cycle, it is only during pregnancy that its highest levels are produced [17].
Regarding spatial distribution of FSHs, the highest concentrations were observed in AT-02 (E2 and EE) and AT-03 (E1 and PG), upstream and downstream of the Atuba Sul STP, respectively. This area is located in a region with intense human occupation and low levels of sanitation. Therefore, FSHs concentrations found at AT-02 have as the main origin as from untreated sewage, discharged by the irregular urban occupation in the Atuba river edges. Additionally, site AT-02 is close to the discharge area of solid wastes from the pretreatment stage (sand+organic matter step) of Atuba Sul STP. Site AT-03, on the other hand, is located in an area under direct influence of the effluent discharge from the STP. This difference in the location between AT-02 and AT-03 sites is clearly reflected in the FSHs concentration and composition. The potential of STP´s treatment to change the FSHs composition by converting each other’s is better discussed in the sequence of this study.
The results of this study were then compared with reported values in the literature (Table 2). This comparison showed that the levels of FSHs found in this monitoring were ten times higher for E2 and almost two times higher for E1, EE and PG (comparison between maximum values observed in this study and in the literature).
Apart from AT-02 and AT-03, the observed levels of E1, EE and PG in all sampling sites were compatible with those found in the literature. Regarding E2, hormone that showed the highest levels in this study, all monitored sites showed levels higher than those from the researched literature.
This difference between the observed and literature data is probably related to the features of the water basin of interest in this study, such as the large input of effluent from Atuba Sul STP (considering the low removal of FSHs by the STPs) [13,24,25], presence of myriad of clandestine discharge sites and the continuous increase in the contraceptive use. All these factors justify the need for a constant monitoring of hormones in the aquatic systems.
The levels of E1 vary between 0.16 and 28.80 μg kg-1 in the literature. According to some authors, the reason behind this difference is the conversion of E2 into E1 in high-O2 environments, resulting in lower levels of E2 (0.03–13.00 μg kg-1) [15,18,21]. In the present study, E2 levels (4.75-137.91 μg kg-1) were greater than E1 (3.04-42.18 μg kg-1), indicating that E2 oxidation was not significant. This may be the cause of the dominance of E2 in the Alto Iguaçu water basin. Conversion of hormones is deeply connected with the limnological conditions of the water body.
Many studies evaluate the occurrence of FSHs in marine environments that receive urban wastes. Hashimoto et al. [26] detected 1.22-14.40 μg kg-1 of E1 and levels lower than 0.26 μg kg-1 for E2 and EE in Tokyo Bay (Japan). Houtman et al. [27] observed levels <0.37 μg kg-1 for E1, <0.17 for μg kg-1 E2 and <0.55 μg kg-1 for EE in Zierikzee Port (Netherlands). Zang et al. [28] found that the concentrations of E1, E2 and EE were below 7.38, 2.35 and 2.18 μg kg-1, respectively, in Xiamen Bay (China). It´s mean that, even in marine environments, the levels of E1 are higher than those of E2 and EE.
In situ sediment-water partition coefficient (Kd)
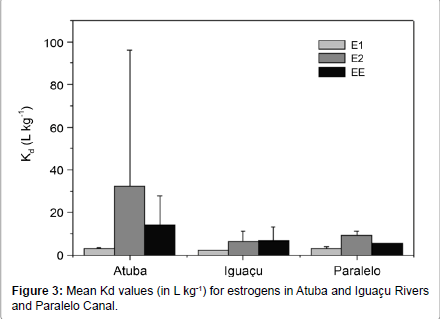
The partition coefficient (Kd) was calculated in all sampling sites in order to provide a better understanding of the behavior of FSHs in the aquatic environment. The calculated values varied between 1.54- 536.00 L kg-1 (Figure 3), and the higher values were found for PG (mean=251.84 L kg-1, range=80.10-536.97 L kg-1). These results showed the strong affinity of progesterone with the sediment, with low mobility in the environment. The lower values of Kd (mean=20.70 L kg-1, range=1.65-201.21 Lkg-1) for the estrogens mark their ease of transport in the water bodies when compared to PG.
According to other studies, there are a wide variety of Kd values for FSHs. Peck et al. [29] observed values ranging between 4 and 74 Lkg-1, Houthaus et al. [10] found Kd between 4 and 260 Lkg-1; Petrovic et al. [18], between 128 and 479 L kg-1; and Carballa et al. [30], between 400 and 700 Lkg-1. Therefore, when this selection of values is compared to the range found in the Alto Iguaçu water basin (1.54-536 Lkg-1), the presented values are in accordance with the literature. The variation of the estrogen´s Kd in Iguaçu, Atuba Rivers and Paralelo Canal is presented in Figure 3. The variation of Kd values is probably linked to limnological conditions/water quality and sanitation level, that significantly vary among the three rivers. Iguaçu river shores consist in preserved areas and good prospects for urban water supply, which mean good limnological conditions that resulted in the lowest Kd values. Despite of the handling of the Canal Paralelo to avoid pollution, this river receives significant sewage pollution from Itaqui river upstream site sampling, resulting in the reduction of its water quality. Atuba River, in contrast, is located in a region with intense human occupation and profound sewage pollution, which result in low levels of water quality. Limnological parameters of these three rivers that confirm the above information are reported at.
Among the rivers, it can be seen that Atuba River was the one with the environmental conditions most favorable to the adsorption of estrogens in the suspended solid phase. There are several factors that can influence the Kd values, resulted from the environmental conditions in a given aquatic system [26]. These can be related to pH, salinity, ionic strength, total organic carbon (TOC) content and particle size distribution [20]. Due the importance of TOC in the FSHs final deposition in sediments [28,31,32] and in order to evaluate the strength of such adsorption, the Kd coefficient was expressed in the form of the hormone partition coefficient (Koc), concerning the fraction associated with organic carbon. The observed means of Koc were 2.94 ± 0.53 L kg-1 (E1), 2.60 ± 0.50 L kg-1 (E2), 2.49 ± 0.37 L kg-1 (EE) and 3.89 ± 0.53 L kg-1 (PG) and follow the order PG>E1>E2>EE regarding hormone adsorption to the sediment.
According to this sequence, PG and E1 presented the highest adsorption strength to the organic fraction of the sediment. This partition contrast between organic and dissolved fraction for FSHs is caused by the hydrophobic properties of each hormone, which is measured by the Kow (organic/water partition) coefficient (E2=3.94, E1=3.43, EE=4.15, PG=3.62) [13], associated with the physicochemical and limnological condition of the aquatic body.
The Pearson linear correlation index between the FSHs and COT in sediment was 0.75 (p<0.005) and showed that the increase in COT content leads to higher levels of FSHs. Meanwhile, the adsorption of hormones to the sediment particles presents low electrostatic force as it was not observed a positive linear correlation between Koc and TOC content. This may be related to the presence of others more hydrophobic substances in the study area. Moreover, Lai et al. [31], Robinson et al. [32] and Bowman et al. [33] determined strong and significant linear correlations (above 0.98, p<0.001) between Koc and COT, which indicated that strong electrostatic forces can also bind FSHs to the organic fraction of the sediments.
Estimative of FSHs removal efficiency at the Atuba Sul STP
Considering the Atuba Sul STP one of the main sources of hormones to the sampling sites, a quantification of hormones before and after sewage treatment in sediment samples and after the anaerobic reactor, lime and physicochemical steps in sludge samples was made in order to estimate their removal efficiency during the sewage processing. The results (Table 3) showed that there was a reduction of 6-48% and of 26- 63% in the levels of EE and PG, respectively. However, it was observed an increase of 25-45% and of 8-26% in the concentrations of E1 and E2, both natural estrogens, which is explained further below.
| Sampling | Water (µg L-1) | Sludge (µg kg-1) | |||||
|---|---|---|---|---|---|---|---|
| Affluent | Effluent | Efficiency (%) |
S1 | S2 | S3 | ||
| E2 | 01 | 7.61 | 4.41 | -42 | - | - | - |
| 02 | 8.66 | 9.77 | +13 | - | - | - | |
| 03 | 1.97 | 2.66 | +26 | 126.66 | 13.03 | ND | |
| 04 | 1.83 | 2.27 | +24 | - | - | - | |
| 05 | 1.61 | 1.74 | +8 | - | - | - | |
| EE | 01 | 8.66 | 4.53 | -48 | - | - | - |
| 02 | 3.56 | 2.94 | -17 | - | - | - | |
| 03 | 0.67 | 0.60 | -10 | 133.10 | 89.36 | ND | |
| 04 | 1.41 | 1.06 | -25 | - | - | - | |
| 05 | 0.51 | 0.48 | -6 | - | - | - | |
| E1 | 01 | ND | ND | 0 | - | - | - |
| 02 | ND | 0.25 | +25 | - | - | - | |
| 03 | ND | 0.45 | +45 | - | - | - | |
| 04 | 0.63 | 0.90 | +43 | ND | ND | ND | |
| 05 | ND | ND | 0 | - | - | - | |
| PG | 01 | 0.32 | 0.12 | -63 | - | - | - |
| 02 | 0.50 | 0.37 | -26 | - | - | - | |
| 03 | 0.17 | 0.10 | -41 | 2,230.89 | 229.30 | 59.67 | |
ND: Not Detected (<2.5 µg L-1).
Table 3: Levels of FSHs in water (in µg L-1)before and after anaerobic sewage treatment and respective increase/decrease in level. Levels of FSHs in sludge (in µg kg-1) from the anaerobic reactor (S1), lime treatment (S2) and physicochemical step (S3) of sewage treatment.
In general, aerobic sewage treatments are more effective in removing hormones when compared to anaerobic ones. In a trickling filter plant, Ternes et al. [34] removed 64% of EE, while Johnson et al. [24], Khanal et al. [13] and Carballa et al. [35], having employed an activated sludge system, removed 74%, 64% and 0% of estrogens, respectively. In Brazil, Ghiselli [36] removed 13 to 17% of estrogens and 17.9% of progesterone using an activated sludge plant. According to Khanal et al. [13], the activated sludge system in sewage treatments is more effective regarding hormones removal due to the nitrification process, which occurs with residence times greater than 10 days [30]. On the other hand, Lee and Liu [37] calculated removal rates of 50% of E2 in a 7 days long anaerobic processing, Servos et al. [25] estimated removal of 68% of E2 and 66% of E1 in an anaerobic lagoon plant, while Carballa et al. [30] obtained 90% of EE removal in laboratory anaerobic digesters.
When compared to the previous reference studies, the results of this present monitoring (6-63% removal of FSHs, Table 3) are in accordance, and the reduction of the hormone concentration depends on the hormone of interest and the treatment process used in the STP. Nevertheless, the observed decrease in FSHs don´t necessarily implies hormone degradation. Gesel et al. [38] explains that the complete degradation of FSHs is only achieved with the destruction of the phenolic ring and consequent production of metabolites. The determination of such metabolites was not performed in this study.
Another relevant point is that, based on the redox conditions of the processing, may occurs conversion of one type of estrogens into another [39,40]. A typical example is the biological conversion of E2 into E1 and other metabolites with similar estrogenic potential [39]. Moreover, new active forms of hormones can be produced from their inactive forms during the STP processing. Estrogens are excreted as glucuronides and sulphates and during the sewage treatment, bacteria such as Escherichia coli (present in feces) produce enzymes glucuronidase and arylsulfatase, which causes the conversion of inactive into active forms of estrogen [13,41]. This conversion is complete and thermodynamically irreversible and results in an increase in the estrogenicity of those molecules [13,40,42].
Those reactions can be the cause behind the increase in the levels of estrogens after the sewage treatment (25-45% for E1, 8-26% for E2, Table 3). This may also occur before the sewage reaches the STP. Thus, the sewage collection network and the STPs can be considered as reactors that convert inactive forms (conjugated) into active forms (free) of estrogen [41]. Furthermore, the increase in natural estrogens can reach a level of 67% after the sewage entered the STP [43]. Carballa et al. [39] claim that this increase occurs mainly during the primary treatment, specifically in the sedimentation step, while D’Ascenzo et al. [41] consider that it may occurs in all steps of sewage treatment.
Regarding the sludge samples, it appears that, based on the results, no significant transference of hormones to the sludge occurs in the STP. Estrogen levels in sludge from the anaerobic treatment were 126.66 μg kg-1 (E2), 133.10 μg kg-1 (EE) and <2.50 μg kg-1 (E1), while the observed levels for the sludge from the lime treatment step were 13.03 μg kg-1 (E2), 89.36 μg kg-1 (EE) and<2.50 μg kg-1 (E1) (Table 3). Therefore, it was observed a decrease of 90% (E2) and 33% (EE) after lime was added. It must be considered that the decrease in estrogen levels after the lime step does not consider the natural degradation estrogens suffers in the 60-days-period that the sludge is stored during the processing. No detectable levels of estrogens were measured in the sludge that came from the physicochemical treatment.
It is expected that, based on the hydrophobic behavior of estrogens, the adsorption mechanisms of these compounds result in their effective removal from the water and sludge enrichment. However, as seen with the results of this study and with the researched literature, an effective removal does not always happen [30,36,37,39]. Lai et al. [31], investigating sediment-water partition of estrogens, suggest that the removal is not totally successful due to the competition of the available active sites in suspended matter particles with other more hydrophobic substances. Once all sites are saturated, removal potential becomes null. Moreover, FSHs adsorption in suspended matter only occurs when its concentration is above 3 g L-1 [16] and Holthaus et al. [10] claims that suspended solids are responsible for less than 1% of the total FSHs removal from the water.
Finally, regarding progesterone, its adsorption to sludge in the sewage treatment was clearly more intense, as its concentration in anaerobic sludge was 2,230.89 μg kg-1, while in the lime-treated sludge was about 229.30 μg kg-1. In the sludge originated from the physicochemical treatment, it was 59.67 126.66 μg kg-1 (Table 3). The molecular structure of progesterone presents some differences when compared to estrogens, mainly its functional group and molecular mass [39]. Those characteristics and the chemical conditions of the mean (such as dissolved oxygen and nitrate) are probably responsible by the greater affinity of PG to the solid fraction, which in turn causes both the sludge and the sediment to have higher PG levels. This phenomenon was also observed through the Kd values, higher for PG than for the estrogens in the sediment-water partition, and in the levels in sludge from the anaerobic treatment (Figure 3).
Conclusion
This study assessed the levels of FSHs in three water bodies in South Brazil (Atuba and Iguaçu Rivers and Paralelo Canal). The observed concentrations were significantly high, especially for 17β-estradiol, a hormone with both natural and synthetic origin, largely used in contraceptives, with levels ten times higher than those reported in the literature. The Atuba River presented the highest levels of hormones due to the presence of Atuba Sul sewage treatment plant.
Among the analyzed female sex hormones, progesterone presented the strongest affinity to the sediment and lower mobility due to its high partition coefficient compared to all estrogens. Stronger adsorption to the organic fraction of the sediments was also observed for progesterone, followed by estrone, based on the analysis of the organic partition coefficient. However, the absence of a strong linear correlation between the organic partition coefficient and the levels of total organic carbon pointed out that the electrostatic force in the adsorption process is weak, possibly caused by the presence of other more hydrophobic substances.
Considering the high concentrations of hormones found in the study area and their removal efficiency by the local sewage treatment plant, it is clear that the sewage treatment is ineffective in removing such substances. Even though some hormones such as 17α-ethinylestradiol and progesterone have up to 60% of their content removed, the treatment process can also result in the increase of 17β-estradiol and estrone through the conversion of inactive forms of hormones into more active ones with enzymes commonly found in the sewage itself.
Still concerning the sewage treatment, it was observed that those estrogens that are not removed during this process are not transferred to the sludge due to competition with more hydrophobic substances and their low levels in the sewage. Differently from the estrogens, most of the progesterone is transferred to the sludge, as its molecules have strong affinity to the solid fraction and high partition coefficients and the limnological conditions, such as low oxygen and nitrate levels, contribute to its transference.
Therefore, this work made a useful evaluation of the dynamics involving female sex hormones in aquatic systems, encompassing mechanisms of adsorption, transport patterns, trends and fate in the environment and its behavior through sewage treatment processing, relevant as sewage treatment stations and untreated sewage are the main sources of female sex hormones. This knowledge is important for a better understanding of hormone contamination in water bodies and monitoring their toxic effects on the ecosystem and human risks.
References
- Barel-Cohen K, Shore LS, Shemesh M, Wenzel A, Mueller J, et al. (2006) Monitoring of natural and synthetic hormones in a polluted river. J Environ Manage 78: 16-23.
- Belfroid AC, Van Der Horst A, Vethaak AD, Schafer GBJ, Wegener J, et al. (1999) Analysis and Occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands. Sci Total Environ 225: 101âÂ?Â?108.
- Jolly C, Katsiadaki L, Le Belle N, Mayer L, Dufour S (2006) Development of a stickleback kidney cell culture assay for the screening of androgenic and anti-androgenic endocrine disrupters. Aquat Toxicol 79: 158âÂ?Â?166.
- Matic I, Gruji´ S, Jaukovi´ Z, Lausevi M (2014) Trace analysis of selected hormones and sterols in river sediments by liquid chromatography-atmospheric pressure chemical ionizationâÂ?Â?tandem mass spectrometry. J Chromatogr A 1364: 117âÂ?Â?127.
- Ying G, Kookana RS, Ru Y (2002) Occurrence and fate of hormone steroids in the environment. Environ International 28: 545âÂ?Â?551.
- Raman DR, Williams E, Layton AC, Burns R, Easter JP, et al. (2004) Estrogen content of dairy and swine wastes. Environ Sci Technol 38: 3567-3573.
- Zheng W, Yates SRE, Bradford SA (2008) Analysis of steroid hormones in a typical dairy waste disposal system. Environ Sci Technol 42: 530âÂ?Â?535.
- Ponezi D, Claudino E (2006) Drugs in environmental matrices âÂ?Â? Review. Pluridisciplinary Center for Chemical, Biological and Agricultural Research, State University of Campina, Brazil.
- Champe PC, Harvey RA, Ferrier DR (2006) Illustrated Biochemistry, 3rd Edition, Artmed Publishing House.
- Holthaus KIE, Johnson SC, Jurgens MD, Williams RJ, Smith JJL, et al. (2002) The potential for estradiol and ethinylestradiol to sorb to suspended and bed sediments in some English rivers. Environ Toxicol Chem 21: 2526-2535.
- Gomes RL, Avcioglu E, Scrimshaw MD, Lester JN (2004) Steroid estrogen determination in sediment and sewage sludge: A critique of sample preparation and chromatographic/mass spectrometry considerations, incorporating a case in method development. Trends Anal Chem 23: 737-743.
- Schäfer AI, Nghiem LD, Waite TD (2003) Removal of natural hormone estrone from aqueous solutions using nanofiltration and reverse osmosis. Environ Sci Technol 37: 82-188.
- Khanal SK, Xie B, Thompson ML, Sung S, Ong SK, et al. (2006) Fate, transport and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Tecnol 40: 6547-6556.
- Azevedo JCR, Pagioro TA, Dias LN, Wosiack ACE, Motter E (2007) Water monitoring quality of some tributaries of the Iguaçu River in the metropolitan region of Curitiba âÂ?Â? PR. Inn Annals of the XI Brazilian Congress of Limnology.
- López De Alda M, Gil A, Paz E, Barceló D (2002) Occurrence and analysis of estrogens and progestogens in river sediments by liquid chromatography-electrospray-mass spectrometry. Analyst 127: 1299âÂ?Â?1304.
- Johnson E, Williams A (2004) Model to estimate influent and effluent concentrations of estradiol, estrone and ethinylestradiol at sewage treatment works. Environ Sci Technol 38: 3649-3658
- Goldefien A (1995) Basic and clinical pharmacology. 6th Edn, Norwalk Ed. Appleton e Lange: Prentice-Hall International 1: 608-636.
- Petrovicè M, Eljarrat E, Alda JL, Barcelo ÃÂ?D (2001) Analysis and environmental levels of endocrine disrupting compounds in freshwater sediments. Trends Anal Chem 20: 234-245.
- Jenkins R, Wilson EM, Angus R, Howel WM, Kirk M (2003) Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol Sci 73: 53âÂ?Â?59.
- Braga O, Smythe GA, Schafer AI, Feitz AJ (2005) Steroid estrogens in ocean sediments. Chemosphere 61: 827âÂ?Â?833.
- Labadie PE, Hill E (2007) Analysis of estrogens in river sediments by liquid chromatographyâÂ?Â?electrospray ionization mass spectrometry: Comparison of tandem mass spectrometry and time-of-flight mass spectrometry. J Chromatogr 9: 174-181.
- Labadie P, Cundy AB, Stone K, Andrews M, Valbonesi S, et al. (2007) Evidence for the migration of steroidal estrogens through river bed sediments. Environ Sci Technol 41: 4299âÂ?Â?4304.
- Perez RL, Escandar GM (2016) Multivariate calibration-assisted high-performance liquid chromatography with dual UV and fluorimetric detection for the analysis of natural and synthetic sex hormones in environmental waters and sediments. Environ Pollut 209: 114-122
- Johnson AC, Belfroid A, Di Corcia A (2000) Estimating steroid oestrogen input into activated sludge treatment works and observation on their removal from the effluent. Sci Total Environ 256: 163- 173.
- Servos MR, Bennie DT, Burnison BK, Jorkovic A, Mcinnis R, et al. (2005) Distribution of estrogens, 17beta-estradiol and estrone, in Canadian municipal wastewater treatment plant. Sci Total Environ 336: 155-170.
- Hashimoto S, Akatsuka Y, Kurihara R, Matsuoka S, Nakatsukuri M, et al. (2005) Evaluation of the Ishikawa cell line bioassay for the detection of estrogenic substances from sediment extracts. Environ Toxicol Chem 24: 1587âÂ?Â?1593.
- Houtman CJ, Boij P, Jover E, Pascual Del Rio D, Swart K, et al. (2006) Estrogenic and dioxin-like compounds in sediment from Zierikzee harbour identified with CALUX assay-directed fractionation combined with one and two dimensional gas chromatography analyses. Chemosphere 65: 2244-2252.
- Zhang X, Li Q, Li G, Wang Z, Yan C (2004) Levels of estrogenic compounds in Xiamen Bay sediment, China. Marine Poll Bull 58: 1210âÂ?Â?1216.
- Peck M, Gibson RW, Kortenkamp A, Hill E (2004) Sediments are major sinks of steroidal estrogens in two United Kingdom Rivers. Environ Toxicol Chem 23: 945âÂ?Â?952.
- Carballa M, Manterole G, Larrea L, Ternes T, Omil F, et al. (2007) Fate of pharmaceutical and personal care products (PPCPs) during anaerobic digestion of sewage sludge. Water Res 41: 2139âÂ?Â?2150.
- Lai KM, Johnson KL, Scrimshaw MD, Lester JN (2000) Binding waterborne steroid estrogens to solid phases in river and estuarine systems. Environ Sci Technol 34: 3890-3894.
- Robinson B, Hui JP, Soo E, Hellou J (2009) Estrogenic compounds in seawater and sediment from halifax harbour, Nova Scotia, Canada. Environ Toxicol Chem 28: 18âÂ?Â?25.
- Bowman JC, Zhou JL, Readman JW (2002) Sediment-water interactions of natural oestrogens under estuarine conditions. Mar Chem 77: 263âÂ?Â?276.
- Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD et al. (1999) Behavior and occurrence of estrogens in municipal sewage treatment plants. Investigations in Germany, Canada and Brazil. Sci Total Environ 225: 81-90.
- Carballa M, Manterola G, Larrea L, Ternes T, Omil F, et al. (2007) Influence of ozone pre-treatment on sludge anaerobic digestion: Removal of pharmaceutical and personal care products. Chemosphere 67: 1444âÂ?Â?1452.
- Ghiselli G (2007) Endocrine disruptors in the environment. QuÃmica Nova 32: 127-132.
- Lee M, LIU A (2002) Degradation of 17ÃÂ?-estradiol and its metabolites by sewage bacteria. Water Air Soil Pollut 134: 353âÂ?Â?368.
- Gesell M, Hammer E, Specht M, Francke W, Schauer F (2001) Biotransformation of biphenyl by Paecilomyces lilacinus and characterization of ring cleavage products. Environ Microbiol 67: 1551-1557.
- Carballa M, Omil F, Lema JM, Lhompart M, Garcia-Jares C, et al. (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38: 2918âÂ?Â?2926.
- Hutchins SR, White M, Hudson F, Fine D (2007) Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates. Environ Sci Technol 41: 738-744.
- DâÂ?Â?ascenzo A, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, et al. (2003) Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci Total Environ 302: 199âÂ?Â?209.
- Tanaka T, Tamura T, Ishizaki Y, Kawasaki A, Kawasw T, et al. (2009) Enzymatic treatment of estrogens and estrogen glucuronide. J Environ Sci 21: 731âÂ?Â?735.
- Adler P, Steger-Hartmann T, Kalbfus W (2001) Distribution of natural and synthetic estrogenic steroid hormones in water samples fromsouthern and middle Germany. Acta Hydrochim Hydrobiol 29: 227-241.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 4190
- [From(publication date):
April-2017 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 3214
- PDF downloads : 976