Does Fibromyalgia Sit in a Chair? Symptomatic Relief with a Simulated Jogging Device
Received: 28-Mar-2017 / Accepted Date: 28-Apr-2017 / Published Date: 06-May-2017
Abstract
The symptoms of fibromyalgia are associated with physical inactivity such as excessive sitting. Physical inactivity itself produces increased oxidative stress and chronic inflammation, factors present in fibromyalgia. Therefore, increasing physical activity should benefit patients with fibromyalgia. Even in normal subjects, only about 10 to 20% of American adults comply with physical activity guidelines. We report effectiveness of daily use of a passive motion platform over a four-week period that releases endothelial nitric oxide as occurs during active exercise. The primary endpoint of this study was the Fibromyalgia Impact Questionnaire (FIQ). Improvement of FIQ with the motion platform was comparable to drug studies over the same time-period. Since the motion platform was not portable, patients needed to visit a fixed location within a hospital clinic to receive daily treatments and as time went by dropouts increased limiting its feasibility for longer-term studies. In this paper, we describe a portable, passive simulated jogging device that is low-cost and targeted to home use for future long-term evaluation as primary treatment or ancillary to drug therapies for fibromyalgia.
Keywords: Fibromyalgia; Symptomatic relief; Chronic pain; Inflammation
4845Introduction
Fibromyalgia affects up to 15 million Americans and 3.6% the world population. It is more prevalent in females than males (9:1 ratio), is diagnosed between ages of 20 to 50, and its incidence increases with age. Its major symptom is chronic widespread pain with multiple tender points. Fatigue, morning stiffness, and poor sleep quality are associated symptoms [1]. The cause and etiology are unknown but evidence has been reported increased oxidative stress, chronic inflammation and decreased blood flow play a role [2]. Pain appears to be centrally mediated with inappropriate heightened pain processing [3]. The root cause for fibromyalgia is an enigma.
Treatment of fibromyalgia with whole body periodic acceleration
In 2004, we published a small study dealing with symptomatic treatment of fibromyalgia using a non-invasive, passive exercise technology called whole body periodic acceleration (WBPA) which increases release of nitric oxide into the circulation through increased shear stress (friction) to endothelial cells [4]. Both endothelial derived nitric oxide produced during exercise as well as nitric oxide derived WBPA increase cerebral blood flow, suppress oxidative stress and reduce inflammation, contributory factors to symptoms of fibromyalgia [5-10]. Although exercise is highly recommended by most authorities for symptomatic relief of fibromyalgia, adherence is low [11-14]. Therefore, WBPA that effortlessly increases physical activity might serve as a complimentary means to manage fibromyalgia symptoms.
Methods (2004)
WBPA was administered by a motorized, motion platform onto which a mattress was placed. The motion platform created sinusoidal, repetitive head to foot movements at 140 cycles per minute and 0.23 g. The device was 222 cm long, 77.5 cm wide, weighed 211 kg and included a footboard for strapping feet in sandals to secure coupling to the motion platform.
Documentation of nitric oxide stimulation during WBPA was obtained by recording characteristic alterations of the arterial waveform displayed from a finger photoelectric plethysmograph. Descent of the dicrotic notch of this waveform down its diastolic limb is specific for nitric oxide release into the circulation [15,16]. During administration of WBPA, motion artifacts obscured visualization of the dicrotic notch making it necessary to carry out ensemble averaging of several heart beats in conjunction with an electrocardiograph trigger pulse to obtain a single averaged pulse for measurements [8].
Protocol and effectiveness of WBPA in fibromyalgia (2004)
This study consisted of fifteen, daily 45 min applications of WBPA five times a week with the outcomes measured as display of descent of the dicrotic notch and response to the acute version of the SF36 health-related quality of life questionnaire. All eight parameters assessing the quality of Life in the fibromyalgia study group were lower than a US normative population values at baseline. There were statistically significant improvements over baseline in scores for Role Physical, Bodily Pain, and Vitality after 15 treatments. The Vitality score reached a normal value at the end of the treatments. The other five categories of this questionnaire did not significantly change. Such beneficial effects of WBPA dissipated within weeks after cessation of treatment.
Farrar’s Study for FDA approval to market the motion platform (2017)
Based on the 2004 study, we asked permission of FDA to market the motion platform. They requested that a clinical study of WBPA be submitted of its effectiveness in ameliorating pain in fibromyalgia [17]. Dr John Farrar and his associates at the Pain Clinic, University of Pennsylvania School of Medicine were engaged carry out this clinical study which consisted of a comparison among WBPA, Craniosacral therapy [18,19] and a control group of Intention to Treat (ITT) [17]. Unpublished results from this study such as changes from baseline of four weeks of treatment with the Fibromyalgia Impact Questionnaire (FIQ) [20] as the primary endpoint as well as enrollment measures are reported below. Farrar’s group plan to report full details of this study in another publication [17].
Using Farrar’s unpublished data, we confirmed our initial 2004 report that WBPA had beneficial effects in fibromyalgia [4,17]. Further, we describe enrollment issues as well as difficulties in maintaining patient compliance for long-term treatment and follow-up with a non-portable device located within a clinical facility among others that limited commercialization of this device. The purpose of the current paper is to address effectiveness of the motion platform as well as its limitations in fibromyalgia treatment that prompted invention of portable device, the Gentle Jogger® (Sackner Wellness Products LLC, Miami, FL) that produces similar effects to the motion platform.
Recruitment for Farrar’s Study
Subjects ages 18 to 64 with complaints of fibromyalgia were recruited from local newspaper/magazine advertisements and contact with local fibromyalgia organizations in the Philadelphia, Pennsylvania area. Prospective participants were told they would have a 2/3 chance of getting an active treatment immediately and 1/3 chance of being asked to wait 12 weeks to receive the treatment. All patients who agreed to participate signed informed consent forms and underwent an examination at the Pain Center. All patients underwent a documented tender point exam conducted by a trained examiner to establish if they met the American College of Rheumatology (ACR) 1990 criteria for fibromyalgia. For the duration of the study, subjects were instructed to continue their current medical therapy, including a stable dose of all pain and anti-inflammatory medications, and current activity levels. They were instructed not to start any new therapy during the trial including new medications or any behavioral, exercise or physical therapy programs.
This project was originally approved and funded on April 1, 2004 and the first randomized patient was enrolled in September 2004. The last patient completed the study at the end of August 2007. Over the period of enrollment, 550 patients who thought that they had fibromyalgia contacted the Pain Center for potential enrollment. Of these, 87 were examined and 65 agreed to participate in the trial.
Randomization for Farrar’s Study (2017)
After baseline data were collected, all enrolled patients were randomized to one of three groups: 1) WBPA daily for four weeks, 2) craniosacral therapy three times a week for four weeks or 3) ITT delayed treatment, where treatment would be offered after 12 weeks of observation. Randomization was accomplished using sealed numbered envelopes containing the assignment generated by a random number generator processed by an uninvolved research coordinator.
Baseline data were collected for at least 3 days prior to the initiation of therapy. At each visit, a brief questionnaire was administered about the patient’s general and specific conditions, and weekly data collected for the primary outcome. Delayed treatment patients were called by telephone weekly to collect the same data. A second complete set of assessment forms was collected and a tender point examination was conducted at clinic visit week 4 (end of therapy). An a priori primary outcome was specified as the change over time in the Fibromyalgia Impact Questionnaire (FIQ) total score at week 4. The primary analysis compared all two group combinations of each of the two treatment groups versus the delayed treatment group.
All the primary and secondary outcome measures were collected at baseline and then at various points along the time line of the study. Only the primary outcome measure, the Fibromyalgia Impact Questionnaire (FIQ), is reported in current paper. Secondary outcome measures will be reported by Farrar and associates in another paper [17]. Of 65 agreed participants with fibromyalgia, 57 ultimately completed more than the initial visit and are included in our intent to treat analysis using the conservative baseline observation carry forward approach to missing data with 19 randomized to one of three groups, WBPA, cranio-sacral therapy, and the delayed treatment group.
Primary outcome of Farrar’s Study (2017)
The Fibromyalgia Impact Questionnaire (FIQ) was selected as the primary endpoint of the study. It is a disease-specific measure that assesses subjects’ perception of their physical function, pain, fatigue, stiffness, depression, anxiety, and overall well-being. The 10 item FIQ, ranging from 0 (best) to 100 (worst), has been widely accepted to describe the severity of fibromyalgia in study participants and to evaluate the effect of treatments.
Protocol and participation in Farrar’s Study (2017)
Patients who received WBPA were treated in sessions lasting 45 minutes, 5 days a week for the 4 weeks, totaling 20 treatments. Patients who received Craniosacral Therapy (CST) for 45 minutes per session by a single trained practitioner 3 days a week for a total of 12 sessions over 4 weeks, marked by light manual contact to the bones of the cranium which are purported to affect energy flow and physiological function. The intention to treat (ITT) control group received no treatment for the initial 12 weeks following which they could receive one of the two treatment types, if they wished to do so.
Fifty-seven patients were enrolled in the study, with 19 in each of the three treatment groups. The majority were women (54/57) and Caucasian (47/57), ranging in age from 24 to 64. There was a clinically unimportant, but statistically significant, difference in age among the groups with an average age of 49 years in the craniosacral therapy group and ITT control groups and 54 years in the WBPA group. There were no statistically significant differences in the baseline values for any of the symptom questionnaires. Over the course of the study, patients dropped out from each group. For the primary endpoint, seven subjects dropped out from each of the active treatment groups and 10 dropped out from the ITT control group. The most common stated reason was due to the time commitment to receive therapy.
Results
Primary outcome
The mean pre-treatment value for FIQ in the ITT group was 57.3, WBPA 61.5, and craniosacral therapy 53.9. There were no significant differences for baseline values of FIQ. The change in FIQ between baseline and week 4 for ITT was -0.9 (p>0.05), WPBA –10.7 (p<0.02), and craniosacral therapy –7.1 (p>0.05). Improvement over baseline FIQ with WBPA at four weeks was significant (17%) but not for the ITT control or craniosacral therapy groups. Secondary outcome measures and follow-up between 4 and 12 weeks without treatment will be reported by Farrar’s group in another paper.
Discussion
Pre-treatment and four week FIQ scores in patients with fibromyalgia after administration of drugs from other investigations and WBPA are listed in Table 1. In the short term, WBPA compares favorably with drug therapies of fibromyalgia. For long-term outcome results, it was not possible to use WPBA for testing because clinic travel five days a week becomes too onerous with missing appointments more frequent and data collection less reliable. This is not a factor in drug studies which generally require weekly, monthly or semi-annual evaluations for long-term studies while patients self-medicate at home.
| Sackner (2017) | |||||||
| WBPA (19 subjects) | ITT (19 subjects) | ||||||
| FIQ BL | FIQ 4 WK | Change | P value | FIQ BL | FIQ 4 WK | Change | P value |
| 62 | 51 | -11 | <0.02* | 57 | 56 | -1 | >0.05 |
| Arnold (2004) [32] | |||||||
| Duloxetine (103 subjects) | Placebo (101 subjects) | ||||||
| FIQ BL | FIQ 4 WK | Change | P value | FIQ BL | FIQ 4 WK | Change | P value |
| 49 | 38 | -11 | <0.027* | 50 | 45 | -5 | >0.05 |
| Patkar (2007) [33] | |||||||
| Paroxetine (58 subjects) | Placebo (58 subjects) | ||||||
| FIQ BL | FIQ 4 WK | Change | P value | FIQ BL | FIW 4 WK | Change | P value |
| 53 | 43 | -10 | <0.05* | 49 | 44 | -5 | >0.05 |
| North (2015) [34] | |||||||
| Gabapentin (17 subjects): No Control: Study analyzed as differences from baseline | |||||||
| FIQ BL | FIQ 4 WK | Change | P Value | ||||
| 70 | 42 | -28 | <0.02** | ||||
| *Statistical difference between active treatment and ITT or placebo; **Statistical difference between active treatment and baseline |
|||||||
Table 1: Fibromyalgia impact questionnaire.
Since our 2004 paper [4] in which we postulated that suppression of oxidative stress and chronic inflammation by endothelial nitric oxide might account for benefits of WPBA as well as the benefits of exercise in fibromyalgia, more recent observations have corroborated this assertion [21,22]. Furthermore, antioxidant properties of WBPA due to increased endothelial derived nitric oxide into the circulation have been demonstrated in animal models [10]. Oxidative stress and chronic inflammation are not causative factors of fibromyalgia, whose etiology remains elusive, but are strongly associated with fibromyalgia.
Studies of brain imaging with functional MRI have shown that fibromyalgia is associated with abnormal cerebral activity and connectivity among brain regions. A long-term physical exercise program appeared to improve faulty connectivity but correlation with symptomatic relief of fibromyalgia has not been established. Nevertheless, these studies as well as many others suggest that the seat of fibromyalgia symptoms is within the brain [23,24]. Therefore, for anti-oxidants and anti-inflammatory agents to be beneficial, they must either originate in the brain or cross the blood-brain barrier. WPBA meets these criteria by increasing endothelial nitric oxide, Brain Derived Neurotrophic Factor (BDNF), and Glial Derived Neurotrophic Factor (GDNF) within the brain [25].
The basis for increased oxidative stress and chronic inflammation accompanying physical inactivity in general [26] as well as in patients with fibromyalgia has been investigated in the al-Andalus Project [27,28]. This project was designed to discriminate between presence or absence of fibromyalgia in patients by determining their ability to perform a specific set of physical fitness tests, including the arm curl, handgrip strength, 30-s chair stand, back scratch and 6-minute walk. 566 fibromyalgia women (average age 51.9 years) and 249 healthy control women (average age 49.3 years), along with 24 fibromyalgia men (average age 47 years) and 56 healthy control men (average age 49.7 years) in southern Spain were enrolled in this study. The body composition of all participants was determined using a bioelectric impedance device, and the cardiorespiratory fitness evaluated by the 6- minute walk test. With exception of muscle mass, all body composition parameters differed significantly between women with fibromyalgia and healthy controls. Obesity was more prevalent among women with fibromyalgia in comparison with healthy control women. No differences were found in either body composition or weight status between fibromyalgia men and healthy men although men with fibromyalgia had lower cardiorespiratory fitness.
It has become apparent that excessive sitting is hazardous to health. Only 10 to 20% of American adults walk briskly or jog slowly 30 minutes daily as recommended by the American Heart Association. They sit about 5 hours daily watching television and an additional 8 hours a day operating a computer, riding in a vehicle and eating. The lack of aerobic exercise and excessive physical inactivity are independent risks for shortened lifespan, heart disease, high blood pressure, and other chronic diseases and conditions such as Type 2 diabetes, obesity, cancer, and dementia. These conditions have their basis in increased oxidative stress and chronic inflammation.
Patients with fibromyalgia are no exception to a sedentary lifestyle which is worldwide in scope. Segura-Jimenez and associates [27,28] in southern Spain have extensively studied the amount of time spent in sedentary behavior in patients with fibromyalgia compared with controls using accelerometers. Such behavior mostly involves sitting. Fibromyalgia patients take fewer steps/day compared with the control subjects. Only 21% of patients with fibromyalgia and 46% of the control subjects adhere to recommendations for 150 minutes/week of moderate-to-vigorous physical activity. Only 16% of these patients walked at least 10,000 steps/day compared with 45% of control subjects. Women who constituted most of the patients with fibromyalgia spent a far greater time in sedentary behaviors and were less physically active than their age-matched controls.
Medication do not appear to be highly effective in preventing or treating this “sitting disease” which is lifestyle related, associated with ill heath, not specific to patients with fibromyalgia but a common occurrence. Prevention and treatment are obvious: sit less and increase physical activity! Motivational programs to accomplish this goal in the workplace and home have generally failed because compliance is difficult to attain for most individuals who have a lifelong habit of sitting for long durations while watching television and/or a computer screen, eating, and during vehicular transportation. Although its etiology is unknown, one might consider that fibromyalgia sits in a chair!
Wellness products are needed in the workplace and home that boost physical activity while allowing an individual to sit since changing this habit is so difficult to accomplish. These products should not require intense mental concentration or physical exertion thereby allowing most activities of daily living without resorting to multitasking. A major regulatory breakthrough occurred in 2016 when FDA decided not to regulated low risk, non-invasive wellness devices thereby facilitating early, less costly market penetration [29]. FDA recommended that such a wellness product have the following major intended uses: In conjunction with a healthy lifestyle, Gentle Jogger should help prevent and aid in living well with heart disease, diabetes Type 2, and high blood pressure. In addition, FDA allowed the following general wellness claims: to improve a.) Physical fitness, b.) relaxation, c.) Mental acuity, d.) General mobility and e.) Specific body function or structure such as swelling of ankles or legs, stiffness, diminished flexibility. It should be noted that FDA requires that devices claiming to treat fibromyalgia are regulated by FDA but general wellness claims listed above that commonly occur in patients with fibromyalgia can be stated without mention of fibromyalgia.
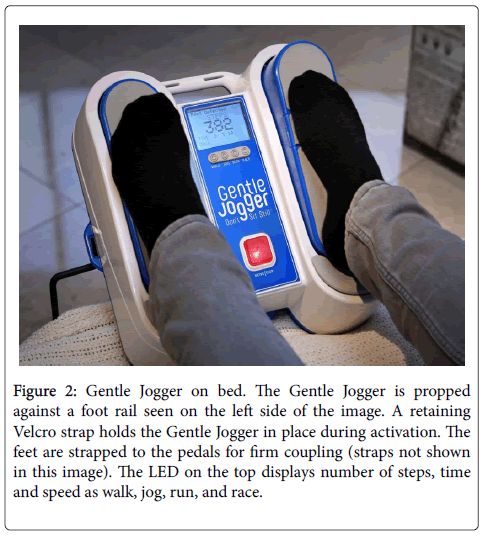
Because of the disadvantages and expense of the motion platform used in Farrar’s [17] study of fibromyalgia described above, we introduce a new, patented “Passive Simulated Jogging Device” in this paper as potential treatment for symptoms of fibromyalgia. It has the advantages of non-regulated wellness device with portability, low cost and self-operation in the home and/or workplace environment. This device incorporates computer controlled, motorized movements of foot pedals placed within a chassis to repetitively tap against a semirigid surface as a simulation for jogging or running on the ground but sitting. It weighs about 10.5 lb or 4.5 Kg and can be placed on the floor for administration while sitting or with optional hardware secured to the footplate of a bed. The foot pedals of the Gentle Jogger® rapidly and repetitively lift the forefeet upward about 1” followed by downward tapping against a semi-rigid surface to simulate the effect of jogging against the ground. The Gentle Jogger alternates right and left movements of the two pedals (Figures 1 and 2). The passive process increases levels of the same beneficial substances as exercise through increased pulsatile shear stress because of a combination of repetitive, rapid movements of the legs and downward tapping of the feet against a semi-rigid surface that stimulate genes with the inner lining of blood vessels. It has not yet been tested in fibromyalgia but has already been shown to increase endothelial nitric oxide from analysis of the finger pulse waveform and fall in skin temperature as depicted by thermal imaging comparable to active exercise [30]. The Gentle Jogger used 2 hours daily within a single time interval or in multiple intervals of time such as four-30 minute sessions, has potential to dramatically benefit health [31]. Although 2 hours daily might seem a long time for boosting activity, individuals are not distracted while watching television that averages 5 hours a day at home or an additional 5 hours a day in front of a computer screen by office workers since multitasking is not an issue.
Figure 2: Gentle Jogger on bed. The Gentle Jogger is propped against a foot rail seen on the left side of the image. A retaining Velcro strap holds the Gentle Jogger in place during activation. The feet are strapped to the pedals for firm coupling (straps not shown in this image). The LED on the top displays number of steps, time and speed as walk, jog, run, and race.
References
- Menzies V (2016) Fibromyalgia syndrome: current considerations in symptom management. Am J Nurs 116: 24-32.
- La RM, Rus A, Molina F Del Moral ML (2013) Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin Exp Rheumatol 31: S121-S127.
- Boomershine CS (2015) Fibromyalgia: the prototypical central sensitivity syndrome. Curr Rheumatol Rev 11: 131-145.
- Sackner MA, Gummels EM, Adams JA (2004) Say NO to fibromyalgia and chronic fatigue syndrome: an alternative and complementary therapy to aerobic exercise. Med Hypotheses 63: 118-123.
- Adams JA, Mangino MJ, Bassuk J, Kurlansky P, Sackner MA (2001) Regional blood flow during periodic acceleration. Crit Care Med 29: 1983-1988.
- Ogoh S, Ainslie PN (1985) Cerebral blood flow during exercise: mechanisms of regulation. J ApplPhysiol 107: 1370-1380.
- Phillips SA, Mahmoud AM, Brown MD, Haus JM (2015) Exercise interventions and peripheral arterial function: implications for cardio-metabolic disease. Prog Cardiovasc Dis 57: 521-534.
- Sackner MA, Gummels E, Adams JA (2005) Nitric oxide is released into circulation with whole-body, periodic acceleration. Chest 127: 30-39.
- Sackner MA, Gummels E, Adams JA (2005) Effect of moderate-intensity exercise, whole-body periodic acceleration, and passive cycling on nitric oxide release into circulation. Chest 128: 2794-2803.
- Uryash A, Bassuk J, Kurlansky P, Altamirano F, Lopez JR (2015) Antioxidant properties of whole body periodic acceleration (pGz). PLoS One 10: e0131392
- Ang DC, Kaleth AS, Bigatti S, Mazzuca SA, Jensen MP et al. (2013) Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized-controlled trial. Clin J Pain29: 296-304.
- Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB (2016) Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci 6.
- Giannotti E, Koutsikos K, Pigatto M, Rampudda ME, Doria A (2014) Medium-/long-term effects of a specific exercise protocol combined with patient education on spine mobility, chronic fatigue, pain, aerobic fitness and level of disability in fibromyalgia. Biomed Res Int 474029.
- Kelley GA, Kelley KS, Hootman JM, Jones DL (2010) Exercise and global well-being in community-dwelling adults with fibromyalgia: a systematic review with meta-analysis. BMC Public Health 10: 198.
- Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TL, et al. (1999) Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol 34: 2007-2014.
- Nier BA, Harrington LS, Carrier MJ, Weinberg PD (2008) Evidence for a specific influence of the nitrergic pathway on the peripheral pulse waveform in rabbits. Exp Physiol 93: 503-512.
- Farrar JT, Musser J, Chen L (2017) Efficacy of whole body periodic acceleration and cranio-sacral therapy for treatment of fibromyalgia (in preparation).
- Castro-Sanchez AM, Mataran-Penarrocha GA, Sanchez-Labraca N, Quesada-Rubio JM, Granero-Molina J (2011) A randomized controlled trial investigating the effects of craniosacral therapy on pain and heart rate variability in fibromyalgia patients. ClinRehabil 25: 25-35.
- Mataran-Penarrocha GA, Castro-Sanchez AM, Garcia GC, Moreno-Lorenzo C, Carreño TP, eta l. (2011) Influence of craniosacral therapy on anxiety, depression and quality of life in patients with fibromyalgia. Evid Based Complement Alternat Med 2011: 178769
- Bennett R (2005) The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 23: S154-S162.
- Neyal M, Yimenicioglu F, Aydeniz A Taskin A, Saglam S, et al. (2013) Plasma nitrite levels, total antioxidant status, total oxidant status, and oxidative streslgiaClinNeurolNeurosurg 115: 736-740.
- Toker A, Kucuksen S, Kucuk A, Cicekler H (2014) Serum ischemia-modified albumin and malondialdehyde levels and superoxide dismutase activity in patients with fibromyalgia. Clin Lab 60: 1609-1615.
- Flodin P, Martinsen S, Mannerkorpi K (2015) Normalization of aberrant resting state functional connectivity in fibromyalgia patients following a three month physical exercise therapy. Neuroimage Clin 9: 134-139.
- Lopez-Sola M, Woo CW, Pujol J, Deus J, Harrison BJ, et al. (2017) Towards a neurophysiological signature for fibromyalgia. Pain 158: 34-47.
- Adams JA, Uryash A, Bassuk J, Sackner MA, Kurlansky P (2014) Biological basis of neuroprotection and neurotherapeutic effects of Whole Body Periodic Acceleration (pGz). Med Hypotheses 82: 681-687.
- Laufs U, Wassmann S, Czech T (2005) Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. ArteriosclerThrombVascBiol 25: 809-814.
- Segura-Jimenez V, Alvarez-Gallardo IC, Estevez-Lopez F, Soriano-Maldonado A, Delgado-Fernández M, et al. (2015) Differences in sedentary time and physical activity between female patients with fibromyalgia and healthy controls: the al-Andalus project. Arthritis Rheumatol 67: 3047-3057.
- Segura-Jimenez V, Borges-Cosic M, Soriano-Maldonado A, Estévez-López F, Ãlvarez-Gallardo IC, et al. (2017) Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: the al-Andalus study. Scand J Med Sci Sports 27: 83-92.
- FDA (2016) General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff.
- Merla A, Mattei PA, Di Donato L, Romani GL (2010) Thermal imaging of cutaneous temperature modifications in runners during graded exercise. Ann Biomed Eng 38: 158-163.
- Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW (2015) Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J 36: 2643-2649.
- Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ (2004) A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 50: 2974-2984.
- Patkar AA, Masand PS, Krulewicz S, Mannelli P, Peindl K, et al. (2007) A randomized, controlled, trial of controlled release paroxetine in fibromyalgia. Am J Med 2007 120: 448-454.
- North JM, Hong KS, Rauck RL (2016) The effect of a novel form of extended-release gabapentin on pain and sleep in fibromyalgia subjects: an open-label pilot study. Pain Pract 16: 720-729.
Citation: Sackner MA, Adams JA (2017) Does Fibromyalgia Sit in a Chair? Symptomatic Relief with a Simulated Jogging Device. Fibrom Open Access 2: 117.
Copyright: © 2017 Sackner MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 12144
- [From(publication date): 0-2017 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 11215
- PDF downloads: 929