Does the Choice of Treatment of Diabetes Mellitus Change Natural Course of Alzheimer Disease?
Received: 17-Aug-2017 / Accepted Date: 08-Jan-2018 / Published Date: 15-Jan-2018 DOI: 10.4172/2161-0460.1000415
Abstract
Alzheimer disease (AD) is common worldwide and almost every case has comorbidities. One of the most common comorbidities of AD is Diabetes mellitus (DM), with or without metabolic syndrome. Both diseases effect nerve tissue and successful treatment would improve the status of the patient. In patients with Alzheimer disease treatment of DM, the treatment could be harmful to the AD, because of that high insulin intake. This may lead to progression of AD. Insulin is considered the best treatment for DM, but insulin therapy could increase comorbidity with AD. No specific therapy for AD is known up to date, so because of that DM is one of the most important risk for AD, concomitant therapy for DM should be planned very carefully. All options of DM therapy should be considered, and different mechanisms of anti-diabetic drugs are preferable. Treatment of AD is more complex metabolic syndrome is present. Any inflammation causes local tissue damage, including brain tissue during AD. Release of interleukins, primarily TNF-α, IL-6, IL-1β in the presence of adipokine leptin, maintains chronic inflammatory status in local brain tissue. Thus, low doses of immunosuppressant therapy should be considered for treatment of AD in future. To delay apoptosis of nerve tissue cells, brain and nerve tissue defend against free oxygen radicals and improve metabolic status.
Keywords: Alzheimer disease; Diabetes mellitus; Treatment; Insulin
Introduction
Alzheimer’s disease characterized clinically by a progressive and gradual decline in cognitive function and neuropathologically, by the presence of neuronal threats, specific neuron loss, synapse loss and disturbance of cholinergic synapses. Most people with Alzheimer’s have the late-onset form of the disease, in which the symptoms become apparent in their mid-60s.
Genetic Basis of Metabolic Changes in Alzheimer's Diseases
The apolipoprotein E (APOE) gene is involved in late-onset Alzheimer’s. This gene has several forms. One of them, APOE ε4, increases a person’s risk of developing AD and is associated with an earlier age onset. Around 0.1% of the cases are familial forms of autosomal dominant inheritance, which has an onset before age 65 [1]. This form of the disease is known as onset familial Alzheimer’s disease. Most of the autosomal dominant familial AD can be attributed to mutations in one of three genes: those encoding amyloid precursor protein (APP) and presenilins 1 and 2 [2]. Most of the mutations in the APP and presenilin genes increase the production of a small protein called Aβ42, which is the main component of senile plaques [3]. Some of the mutations merely alter the ratio between Aβ42 and the other major forms particularly Aβ40 without increasing Aβ42 levels [4,5]. The major hallmarks of AD pathology are masses of the extracellular β-amyloid peptide (Aβ) and neurofibrillary tangles of the microtubule binding protein tau [6,7]. Aβ has crucial importance in the relation of AD as well as the crucial role of its pathogenesis. The neurotoxic potential of the Aβ peptide results from its biochemical properties, favoring aggregation into insoluble oligomers and protofibrils [7]. Etiological treatment of AD is not yet available, but also the underlying pathophysiological mechanisms, and hence risk factors, of Alzheimer’s disease remain uncertain [7]. Nevertheless, several potentially modifiable risk factors of dementia have been identified. One of them is hypertension, especially in mid-life, which creates a possibility of developing dementia [8]. Although less studied, obesity [9] and dyslipidemia [10] have also been recognized as possible modifiable risk factors for dementia. Risk of dementia also increases with diabetes [11]. These factors coexist under the heading of metabolic syndrome, which is the cluster of five cardiovascular risk factors including hypertension, abdominal obesity, high triglyceride level, low level of HDL cholesterol, and elevated fasting glycemia (impaired fasting glucose or diabetes) [12]. These risk factors trigger neuroinflammation and oxidative-nitrosative stress and consequently lead to reduction nitric oxide and enhance endothelin, Amyloid-β deposition, cerebral amyloid angiopathy and blood-brain barrier disruption.
The Diabetes Mellitus (DM) in Alzheimer's Disease (AD)
Individuals with the metabolic syndrome are generally characterized by central obesity accompanied by low-grade inflammation (i.e., higher plasma concentrations of C-reactive protein, interleukin IL-6 and other inflammatory markers) and insulin resistance. Insulin resistance is caused by impairment of protein synthesis including special protein glut-4 transporter, which is essential for transporting of glucose through cell membrane. So, they have a higher chance for developing CVD and/or T2DM [13,14]. AD appearance in elder ages is related to a gene on chromosome 19 coding for the cholesterol transporter protein apolipoprotein E (apoE). ApoE gene has several alleles, but those with highest frequencies are apoE-ε2, apoE- ε3 and apoE-ε4 [15]. Inheritance of apoE-ε4 is related with higher risk of developing AD in an autosomal-dominant fashion (i.e., inheritance one copy apoE-ε4 results in a 50% chance of developing AD). There are links postulated to cardiovascular risk factors, e.g. high level of cholesterol, hypertension and obesity as well as T2DM, leading to environmental factors playing a key role in AD appearence [16,17].
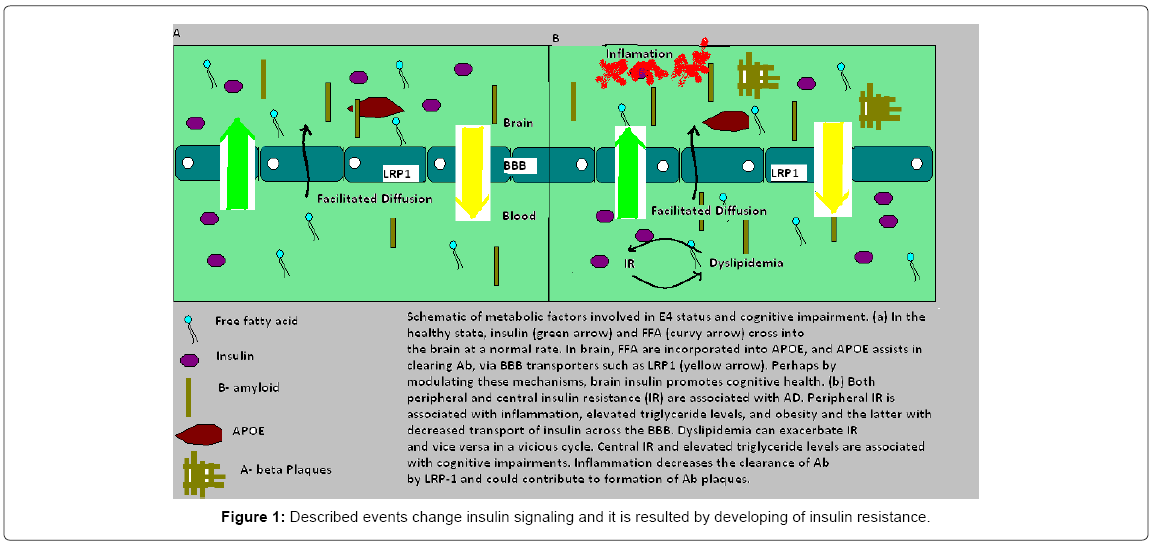
Transport systems in the brain endothelium through blood-brain barrier (BBB) mediate delivery of glucose and other nutrients from blood to brain, as well as the clearance of toxic metabolic and-products from the CNS to the circulation (Shema 1). Both AD and MCI subjects showed increased, insulin receptor substrate 1, a candidate biomarker of brain insulin resistance and GSK-3β, a kinase targeting tau phosphorylation [18,19]. All regions of the brain (InsRb) are covered by insulin receptors and in some areas with high density of receptors: in the hippocampus, entorhinal cortex and olfactory bulb [20] and periphery (InsRp). α-subunits are smaller in InsRb, glycosylation and insulin- binding are specifics (absence of negative co-operativity in InsRb) [21]. The reaction that happens within the brain starts when insulin binds to the α-subunit of the insulin receptor and that activates tyrosine kinase phosphorylation of the β-subunit of the receptor and leads to activation of several second-messenger transduction pathways. Obese patients have activation of the immune response mediated by specific signaling pathways, with Jun N-terminal kinase and IkappaB kinase beta/nuclear factor kappa-light-chain-enhancer of activated B cells and were studied very often. Described events change insulin signaling and it is resulted by developing of insulin resistance (Figure 1) [22].
Insulin plays an important role of neuronal survival and has a role as the growth factor. It is known that insulin activate IGF-R (Insulin like growth factor receptors) [12]. Insulin receptors have dendritic distribution, because of the direct role of insulin in the brain. Lack of insulin decreases the number of these receptors and synapse density in the same time. Number of synapses and their density has a crucial role in brain function and thus the insulin’s regulatory role of brain function.
Influence of Insulin to Microenviromental Changes in Brain in Alzheimer Disease
Hyperglycemia and glucose metabolites may increase destruction of the brain and its vasculature. Neuronal function and survival is influenced by toxic levels of insulin, and elevation of peripheral insulin level acutely increases in cerebrospinal fluid (CSF) concentration. Synaptic plasticity, learning and memory could be directly modulated by insulin, and disorder in insulin signaling pathways in the periphery and in the brain have recently been included in Alzheimer’s disease and brain aging. Metabolism of beta-amyloid and tau, the building blocks of amyloid plaques and neurofibrillary tangles, the neuropathological hallmarks of Alzheimer’s disease, also being regulated by insulin [23]. Promoting glucose uptake in glial cells and specific neural circuits, including the hippocampal circuit is insignificantly controlled by CNS insulin [24]. Synaptogenesis and synaptic remodeling are promoted by the cascade of signals triggered by activated insulin receptor [25]. Neuronal survival, by protecting against apoptosis, hypoxic stress, and nitric oxide toxicity, is also influenced by insulin signaling [26]. Accelerated apoptosis is one of the most important factors for AD development [27] Structural brain changes including whole-brain [28] and medial temporal atrophy [29] are linked to dysfunctional insulin signaling. AD and type 2 diabetes mellitus are two of the most common diseases in elder population. It is very important to more precisely define the role of insulin in brain aging and appearance of AD. Its known that diabetes mellitus (DM) is a risk factor for non-genetic AD. Recent studies in humans have shown that cell signaling related to insulin is disrupted in the AD brain [30]. Tau phosphorylation and formation of neurofibrillary tangles NFT could be impaired by diabetes. Physiologically tau promotes the assembly and stabilization of microtubules, hyperphosphorylation and disrupts microtubules [31]. Importantly, in the human diabetic brain tau phosphorylation is increased at the same sites in AD [32]. Diabetes could increase tau-phosphorylation, leading to the development of NFT. Glucose uptake from tissue is controlled by insulin that binds to specific receptors expressed on cell membranes and triggers the phosphorylation of cellular substrates. Insulin resistance and diabetes type 2 are coming as a result of switch from a tyrosine phosphorylation to a serine phosphorylation of family of proteins known as the insulin receptor substrate (IRS).
Local Inflammatory Response in Development of AD
For pathogenesis of obesity and type 2 diabetes, the key component is excessive nutrient intake.
Cells of white fat tissue (macrophages and adipocytes) produce specific reactions to molecules such as reactive oxygen species, free fatty acids and advanced glycation products, in the production of TNF-α, IL- 1β, IL-6, CCL2 and adipokins such as leptin [33]. Consequently, leptin induces immune cells to uptake glucose and enhance their metabolism.
Insulin resistance may occur as a phosphorylation substrate receptor insulin receptor-1 reaction; induced by cytokine TNF-α and IL-1β [34], while NLRP3 (Nod-like receptor family, Pyrine domain containing 3) is able to activated isceleni amyloid, which is deposited in the pancreas [35,36].
Abnormal removal of amyloid beta peptide is correlated with peripheral hyperinsulinemia and GSK3beta cdk5 activity stimulates tau hyperphosphorylation. This reaction leads to cell cascades that induce cognitive decline and neurodegenerative phenotyping. Chronic peripheral hyperinsulinemia decreases the transportation of insulin thru the BBB (Blood Brain Barrier). Insulin signaling in the brain becomes lower; all of the insulin activity is altering, including anti-apoptotic effect. Some medicines used in mellitus type II reduce the cognitive impairment associated with AD [37].
Oxidative stress and glucose are the two most common phenomenon associated with biological aging and high percentage associated with pathogenesis of AD. Chronic hyperglycemia and chronic hyperinsulinemia, first induces production of advanced iron endokvant (AGEs), which causes excessive production of reactive oxygen species (ROS).
Patients with AD have lower insulin levels in the blood than the insulin levels in the cerebrospinal fluid. In etiological roles of diabetes and obesity, inflammation plays key role.
However, it is well known that some medicines can directly affect the plasma concentrations of inflammatory cytokines. Rosiglitazone significantly decreases levels of IL-6, IL-18 and TNF-alpha, where metformin does not [38]. Glimepiride significantly reduces TNF- alpha, IL-1, IL-6, PrP82-146 (prion desease), amyloid-β (Aβ) 42 (AD) and α-synuclein (αSN, PD) [39]. Vildagliptin also reduces plasma levels of IL-6, CRP and TNF-alpha, even more than glimepiride [40]. Knowledge of these facts, is very important to manage and choice the best therapeutical options for DM patients.
Role of Insulin in Overall Degradational Process in Pathogenesis of AD
Insulin and amyloid-beta (Abeta) have a common, primary depurative mechanism - Insulin-Degrading Enzyme (IDE). IDE has more affinity for insulin than for Abeta and brain hyperinsulinism and is able to affect Abeta’s main clearance mechanism. Intracellular accumulation of amyloid precursor protein is modulated by insulin. IDE degrades both insulin and amylin, but also peptides related to pathology of DM2, together with amyloid-beta in the AD brain. Bigger selectiveness of IDE for insulin may cause hyperinsulinemia elevate amyloid beta [41]. Brain aging could be accelerated by both hyperglycemia and hyperinsulinemia, but also by inducing tau hyperphosphorylation and amyloid oligomerization, as well as by leading to widespread brain microangiopathy. Patients that suffer from diabetes are more prone to develop extended and earlier-than-normal leukoaraiosis (White Matter High-Intensity Lesions - WMHL). WMHL is also noticed in brain scans of elderly people and it is associated with higher risk for executive dysfunction, cognitive impairment and dementia [42].
In cerebrovascular amyloid angiopathy, which is associated with AD, an increase of expression of RAGE (receptor for advanced glycation end-products)? Important role in pathogenesis of AD and chronic complications of DM play carbohydrate-derived advanced glycation end-products (AGEs) [43]. Post-mortem analyzed samples of AD with diabetes compared to AD without diabetes or non-demented controls have shown their significant increase [44]. RAGE is specific cell surface receptor for amyloid β and it results with easier neuronal damage [45,46].
Lower levels of insulin in the cerebrospinal fluid is present in patients with AD and post-mortem analyses have shown fewer insulin receptors and downstream signaling activity in the AD brain compared to normal controls [47,48]. Insulin influences the metabolism of beta- amyloid peptides through GSK3α and GSK3β and the phosphorylation of Tau [28,49]. As Insulin-Degrading Enzyme has more affinity for insulin, it breaks down insulin’s extracellular concentrations. Insulin resistance can result with excessive insulin, competition with amyloid β for binding site of IDE and consequently accumulation of amyloid β in the central nervous system (CNS) [50]. Specific dietary system and inability for taking medicine result with worse glucose control at AD patients. Poor cognitive function increases risk of hypoglycemia in patients with type 2 diabetes [51]. Insulin resistance could be induced by disturbed insulin receptor and IGF-1 receptor signaling caused by proinflammatory cytokines [51].
Deleterious effects of HFD on learning and memory, and long- term potentiation of CA1 hippocampus fibrillary acidic protein fungus, a mammalian target of rapamycin and a vascular endothelial growth factor may be reversible in these cases by using a glucagon-like peptide 1, which stimulates insulin [52]. This is the future of AD treatment [53]. AD may occur in IRBS, and the reason to support this fact is that IRBS is associated with anatomical, behavioral and molecular changes. Insulin applied directly in brain, is capable to enhance cognitive selected parameters. The idea is in accordance with the hypothesis of AD pathogenic harmful signals, and it is capable to consolidate the pleiotropic effects of agents that are toxic to the insulin production/secretion (e.g. Cells of the pancreas) and IRBs caused by different mechanisms in AD. In accordance with that, innovative therapy and prevention of AD, diabetic complications are usually associated with metabolic and hormonal changes, and suggest new therapeutic approach for AD [41].
It is very clear that excessive insulin invokes synchronous increases in levels of Abeta and inflammatory agents, effects that are exacerbated by age and obesity. This constellation of events may have deleterious effects on memory. Therapeutic benefit for adults with age-related memory impairment and AD could be achieved by treatments focused on preventing or correcting insulin abnormalities [54].
Discussion
AD often coexists with other diseases, referred as metabolic syndrome (hypertension, diabetes mellitus, abdominal obesity, hypertriglyceridemia, low high density lipoprotein (HDL) levels, cardiovascular disease [55,56]. These and some other factors as vitamin B12/folate deficiency, depression, and traumatic brain injury synergistically promote diverse pathological mechanisms including cerebral hypoperfusion and glucose hypometabolism. These risk factors trigger neuroinflammation, oxidative-nitrosative stress; decrease NO, and endothelin increase, Amyloid-β deposition, cerebral amyloid angiopathy, and blood-brain barrier disruption. Inflammation trigger long term damage, involving fatty acids, DNA, mitochondria and results in ATP hypometabolism, amyloid B deposition, endothelial dysfunction and blood-brain barrier disruption [57].
Beta secretase 1 and gamma secretase, as a part of the presenilin complex, have a pivotal role in forming and generating of amyloid B (successive cleavage of APP). Several amyloid B isoforms, difference between them is number of the terminal amino acids, Aβ [58] have a pivotal role in the pathogenesis of AD. Amyloid β favor aggregation into insoluble oligomers and protofibrils [59,60]. Big GWAS study (Genome Wide Association Study) showed the role of microglia in pathogenesis of AD. It’s shown that MetS causes dysregulation of mitochonondria, which results in defective NAD+ sirtuin pathway. Sirtuins are a family of seven proteins that are included in longevity and inflammation. One of them, SIRT3 is present in mitochondria and plays a role in metabolic adaptation. Its role is to deacetylate and to activate key metabolic enzymes and transcriptional regulators, utilizing NAD+ in the process. Improving of mitochondrial dysfuntion of sirtuin pathway is one of therapeutic strategies and results in decrease of neuroinflammation [59].
AD can be a result of missense gene mutations, which are involved in increased synthesis of the amyloid-beta protein precursor derivatives amyloid-beta. It leads to autosomal dominant familial AD but it’s not often. And, the main population of AD patients have a sporadic AD (sAD) of late onset, for witch is any evidence is caused by amyloid-beta precursor [60].
The prevalence of the metabolic syndrome, similar to that for cognitive disorders, increases dramatically with age. In younger population, it is often presented in individuals with APOE-ε4 [61]. However, metabolic syndrome is a risk factor for accelerated cognitive aging, mild cognitive impairment (MCI), vascular dementia and Alzheimer’s disease. These patients are literally described as a “metabolic-cognitive syndrome” [62-64] and it seems the prevalence of these cognitive disorders is higher in population of elders with metabolic syndrome and with elevated of inflammatory markers in the blood [62].
MetS and Alzheimer’s disease are not inflammatory diseases, but chronic inflammation that exists seems to be result of deregulation of the endocrine homeostasis of adipose tissue [65]. Adiponectin, expressed by adipose tissue, plays an important role in metabolic control of inflammatory response and may play a key role in a pathophysiology of neurodegeneration. Low grade adiponectin may be a surrogate marker of cognitive impairment and Alzheimer’s [66].
In recent literature, it has been reported that AD is more frequent in population with MetS then in population without (7.2% vs. 2.8%). It is interesting that women with MeS are more likely to get AD than men with MetS (8.3% vs. 1.9%) [67]. It also seems that diet, such as high consumption of vegetables and fruits, has a central place in preventing both these diseases.
New Possibilities for Treatment of AD Using Intranasal Insulin
All antidiabetic drugs may affect AD indirectly through effects on circulating concentrations of glucose, insulin, inflammatory markers and by generation of reactive oxygen species. Insulin resistance within the brain is most closely linked to AD, targeting insulin signaling in the brain as key.
When it comes to AD, insulin can be applied via intravenous and intranasal application. That is not usual, but a possibility of AD treatment. At present, only symptomatic interventions are available for treating AD. To optimize symptomatic treatment, a personalized therapy approach has been suggested.
In the study with insulin infusion rate (1.0 mU/kg/min) applied to nondemented older adults, insulin did result in an increased memory performance across all selected individuals [68]. However infusion of peripheral insulin is not an optional long-term therapy for AD, due to the marked hypoglycemia. When insulin is infused peripherally, therefore leaving us a clear pathway for intranasal application.
Recent prospective observational study of Isik AT, has evaluated elderly patients with type 2 DM with AD. After sixth-month evaluation, it revealed no difference between sitagliptin and non-sitagliptin groups in terms of weight, body mass index and HbA1c. Authors concluded that number of patients that required reduced insulin dose were significantly higher in the sitagliptin group and sitagliptin therapy was associated with an increase in the Mini-Mental State Examination (MMSE) scores. The main outcome revealed that besides its effects, similar to those of insulin and metformin in glycemic control, and in reducing need for insulin, 6 months sitagliptin therapy may also associated with improvement of cognitive function in elderly diabetic patients with and without AD [69].
Additionally, some studies using intravenous and intranasal insulin have shown that the effect of insulin on memory differs on the basis of APOE genotype.
Intranasal insulin administration seems to open the possibility for a safe, and at least in the short term, effective symptomatic intervention that delays loss of cognition in AD patients [70]. Another study involving individuals with AD or mild cognitive impairment found that 4 months of intranasal insulin (20 IU, 2x/day) preserved cognition as measured by ADAS-cog, improved delayed memory, and improved caregiver-rated functional ability [71].
The current hypothesis is that intranasal insulin is directly influencing cognition by acting on neuronal IRs to overcome resistance, but this has not been shown directly. Recent evidence has shown that intranasal insulin may be working through indirect pathways to influence cognition in terms of increasing regional cerebral blood flow and cognition in T2D patients [72]. Insulin sensitizers, as an alternative therapy, increase insulin action in the brain. These agents are effectively used in patients with T2D. A common class of insulin-sensitizing drugs is thiazolidinediones, which includes rosiglitazone and pioglitazone.
Thiazolidinediones also have potent anti-inflammatory properties. Given the role of insulin resistance and inflammation in the pathogenesis of AD, these agents are being studied as a potential treatment for AD. One small study showed that persons receiving rosiglitazone 4 mg daily had improved memory and selective attention [73]. Another study with more than 500 patients with mild to moderate AD were randomized to 6 months of treatment with placebo or rosiglitazone 2, 4 or 8 mg, resulting in significant improvement on the Alzheimer’s Disease Assessment Scale-cognitive subscale in APOE-ε4-negative patients on 8 mg rosiglitazone, while persons with the APOE-ε4 allele showed no benefit [74].
Pioglitazone (AD4833) is an insulin sensitizer of the thiazolidinedione class of nuclear Peroxisome-Proliferator Activated Receptor γ (PPARγ) agonists. It binds to PPARγ, affecting gene transcription and reducing inflammation. Recent study revealed an expert opinion showing that Pioglitazone is safe and well tolerated as proved in phase II study in AD. So far, two large Phase III trials are ongoing, but there are no preliminary results yet on a possible beneficial effect on cognition in patients with AD [75].
Some studies suggest that DM might have a relatively strong protective effect against AD, whereas the condition deteriorate more severely when there is a concomitant insulin resistant brain state (IRBS). IRBS is associated with anatomical, behavioral, and molecular changes that justify the proposal that AD may be due to an IRBS. This is explored in the context of accumulating evidence that the IRBS need not be related to peripheral insulin resistance, and that administration of insulin directly to the brain improves selected cognitive parameters targeted in AD [76].
Conclusion
As is discussed above, overall involvement of insulin in pathogenesis of Alzheimer disease and expressed role of it in some degradation process in the brain and formation of amyloid suggests importance of knowledge of insulin level in plasma. Therefore, measurement of insulin levels in the blood should be considered when considering treatment of Alzheimer disease. The dominant outlook is that insulin is always the best choice in the treatment of DM with AD cannot be applied completely, because obviously oral hypoglycemic agents reducing progress of insulin resistance, cognitive impairment and AD in the end.
References
- Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer's disease. Lancet 368: 387-403
- Waring SC, Rosenberg RN (2008) Genome-wide association studies in Alzheimer disease. Arch Neurol 65: 329-334.
- Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399: A23-A31
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, et al. (1996) Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17: 1005-1013.
- Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, et al. (2007) FAD mutants unable to increase neurotoxic Aß 42 suggest that mutation effects on neurodegeneration may be independent of effects on Abeta. J Neurochem 101:674–681.
- Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24: 1121-1159.
- Gorelick PB (2004) Risk factors for vascular dementia and Alzheimer disease. Stroke 35: 2620-2622.
- Qiu C, Winblad B, Fratiglioni L (2005) The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4: 487-499.
- Gorospe EC, Dave JK (2007) The risk of dementia with increased body mass index. Age Ageing 36: 23-29.
- Reitz C, Tang MX, Luchsinger J, Mayeux R (2004) Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 61: 705-714.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5: 64-74.
- Fernanda G De Folice (2013) Alzheimer's disease and insulin resistance: Translating basic science into clinical applications. J Clin Invest 123:531-539.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman J, et al. (2009) Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640-1645.
- Tenenbaum A, Fisman EZ (2011) The metabolic syndrome… is dead: These reports are an exaggeration. Cardiovasc Diabetol .
- Ordovas JM, Litwack-Klein L, Wilson PW, Schaefer MM, Schaefer EJ (1987) Apolipoprotein E isoform phenotyping methodology and population frequency with identification of apoE1 and apoE5 isoforms. J Lipid Res 28: 371-380.
- Rogers J (1998) Candle and darkness: Current research in alzheimer's disease. Chicago: Bonus Books Inc.
- Raiha I, Kaprio J, Koskenvuo M, Rajala T, Sourander L (1998) Environmental differences in twin pairs discordant for Alzheimer's disease. J Neurol Neurosurg Psychiatry 65: 785-787.
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, KÃ¥reholt I, et al. (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62: 1556-1560.
- Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, et al. (2015) Alteration of mTOR signaling occurs early in the progression of alzheimer disease (AD): Analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133: 739-749.
- Adamo M, Raizada MK, LeRoith D (1989) Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol 3: 71-100.
- Werner H, Roberts CTJ, Raizada MK, Bondy CA, Adamo M, et al. (1993) Developmental regulation of the insulin and insulin-like growth factor receptors in the central nervous system. In: Zagon IS, McLaughlin PJ, editors. Receptors in the Developing Nervous System. London: Chapman and Hall pp. 109-127.
- Karalis KP, Giannogonas P, Kodela E, Koutmani Y, Zoumakis M, et al. (2009) Mechanisms of obesity and related pathology: Linking immune responses to metabolic stress. FEBS J 276: 5747-5754.
- Biessels GJ, Kappelle LJ (2005) Increased risk of Alzheimer's disease in Type II diabetes: Insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans 33: 1041-1044.
- van der Heide LP, Ramakers GM, Smidt MP (2006) Insulin signaling in the central nervous system: Learning to survive. Prog Neurobiol 79: 205-221.
- Chiu SL, Chen CM, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58: 708-719.
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, et al. (1999) Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem 73: 2037-2046.
- Hasanbasic S, Jahic A, Karahmet E, Sejranic A, Prnjavorac B (2016) The role of cysteine protease in alzheimer disease. Mater Sociomed 28: 235-238.
- Araki Y, Nomura M, Tanaka H, Yamamoto H, Yamamoto T, et al. (1994) MRI of the brain in diabetes mellitus. Neuroradiology 36: 101-103.
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, et al. (2003) Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46: 1604-1610.
- Sato N, Takeda S, Uchio-Yamada K, Ueda H, Fujisawa T, et al. (2011) Role of insulin signaling in the interaction between alzheimer disease and diabetes mellitus: A missing link to therapeutic potential. Current Aging Science 4: 118–127.
- Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118: 53-69.
- Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2011) Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol 225: 54-62.
- Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118: 53-69.
- Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793-1801.
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, et al. (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14: 812-820.
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, et al. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11: 897-904.
- Neumann KF, Rojo L, Navarrete LP, FarÃas G, Reyes P, et al. (2008) Insulin resistance and Alzheimer's disease: molecular links & clinical implications. Curr Alzheimer Res 5: 438-547.
- Kim HJ, Kang ES, Kim DJ, Kim SH, Ahn CW, et al. (2007) Effects of rosiglitazone and metformin on inflammatory markers and adipokines: Decrease in interleukin-18 is an independent factor for the improvement of homeostasis model assessment-beta in type 2 diabetes mellitus. Clin Endocrinol (Oxf) 66: 282-289
- Ingham V, Williams A, Bate C (2014) Glimepiride reduces CD14 expression and cytokine secretion from macrophages. J Neuroinflammation 11: 115.
- Derosa G, Bonaventura A, Bianchi L, Romano D, Fogari E, et al. (2014) Maffioli P.Comparison of vildagliptin and glimepiride: Effects on glycaemic control, fat tolerance and inflammatory markers in people with type 2 diabetes. Diabet Med 31: 1515-1523.
- Qiu WQ, Folstein MF (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging 27: 190-198.
- Roriz-Filho J, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, et al. (2009) (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta 1792: 432-443.
- Valente T, Gella A, Fern`andez-Busquets X, Unzeta M, Durany N, et al. (2010) Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiology of Disease 37: 67-76.
- R Businaro, S Leone, C Fabrizi (2006) S100B protects LAN- 5 neuroblastoma cells against Aß amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Aß amyloid neurotoxicity at high doses. Journal of Neuroscience Research 83: 897-906.
- Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M (2005) Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer’s disease. Medical Hypotheses 64: 1205-1207.
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, et al. (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: Relationship to severity of dementia and apolipoprotein E genotype. Neurology 50: 164-168.
- Phiel CJ, Wilson CA, Lee VM, Klein PS (2003) GSK-3alpha regulates production of Alzheimer's disease         amyloid-beta peptides. Nature 423: 435-439.
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, et al. (2004) Role for neuronal insulin resistance in neurodegenerative diseases. JC Proc Natl Acad Sci U S A 101: 3100-3115.
- Craft S, Cholerton B, Baker LD (2013) Insulin and Alzheimer's disease: Untangling the web. J Alzheimers Dis 33 Suppl 1: S263-275.
- Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, et al. (2012) Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: Posthoc epidemiologicanalysis of the ACCORD trial. Diabetes Care 35: 787-793.
- Â Lennox R, Porter DW, Flatt PR, Holscher C, Irwin N, et al. (2014) Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology. 86: 22-30.
- Holscher C (2014) Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’s and Parkinson’s diseases. Biochem Soc Trans 42: 593-599.
- Kuljiš RO, Salkovic-Petrišic M (2011) Dementia, diabetes, Alzheimer's disease, and insulin resistance in the brain: progress, dilemmas, new opportunities, and a hypothesis to tackle intersecting epidemics. J Alzheimers Dis 25: 29-41.
- Solfrizzi V, Scafato E, Capurso C, D'Introno A, Colacicco AM, et al. (2011) Italian longitudinal study on aging working group. metabolic syndrome, mild cognitive impairment, and progression to dementia. Neurobiol Aging 32: 1932-1941.
- Ricci G, Pirillo I, Tomassoni D, Sirignano A, Grappasonni I (2017) Metabolic syndrome, hypertension, and nervous system injury: Epidemiological correlates. Clin Exp Hypertens 39: 8-16.
- Daulatzai MA (2017) Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res 95: 943-972.
- Pugazhenthi S (2017) Metabolic syndrome and the cellular phase of alzheimer's disease. Prog Mol Biol Transl Sci 146: 243-258.
- De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV (2012) Alzheimer's disease. Subcell Biochem 65: 329-352.
- Salkovic-Petrisic M, Osmanovic J, Grünblatt E, Riederer P, Hoyer S (2009) Modeling sporadic alzheimer's disease: The insulin resistant brain state generates multiple long-term morphobiological abnormalities including hyperphosphorylated tau protein and amyloid-beta. J Alzheimers Dis 18: 729-750.
- Feng L, Chong MS, Lim WS, Lee TS, Collinson SL, et al. (2013) Metabolic syndrome and amnestic mild cognitive impairment: Singapore Longitudinal Ageing Study-2 findings. J Alzheimers Dis 34: 649-657.
- Yaffe K (2007) Metabolic syndrome and cognitive decline. Curr Alzheimer Res 4: 123-126.
- Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A et al. (2010) Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Res Rev 9: 399-417.
- Panza F, Frisardi V, Seripa D, Imbimbo BP, Sancarlo D, et al. (2011) Metabolic syndrome, mild cognitive impairment, and dementia. Curr Alzheimer Res 8: 492-509.
- Misiak B, Leszek J, Kiejna A (2012) Metabolic syndrome, mild cognitive impairment and Alzheimer's disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull 89: 144-149.
- Teixeira AL, Diniz BS, Campos AC, Miranda AS, Rocha NP, et al. (2013) Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer's disease. Neuromolecular Med 15: 115-121.
- Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hänninen T, et al. (2006) Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology 67: 843-847.
- Pistollato F, Cano SS, Elio I, Vergara MM, Giampieri F et al. (2015) The Use of Neuroimaging to Assess Associations Among Diet, Nutrients, Metabolic Syndrome, and Alzheimer's Disease. J Alzheimers Dis 48: 303-318.
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, et al. (2003) Insulin increases CSF Abeta42 levels in normal older adults. Neurology 60: 1899-1903.
- Isik AT, Soysal P, Yay A, Usarel C (2017) The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract 123: 192-198.
- RibariÄ S (2016) The rationale for insulin therapy in alzheimer's disease. Molecules 21.
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, et al. (2012) Intranasal insulin therapy for alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch Neurol 69: 29-38.
- Novak V, Milberg W, Hao Y, Munshi M, Novak P, et al. (2014) Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37: 751-759.
- Watson G, Cholerton BA, Reger MA, Baker LD, Plymate SR, et al. (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. Am J Geriatr Psychiatry 13: 950–958
- Risner M, Saunders A, Altman J, Ormandy GC, Craft S, et al. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate alzheimer’s disease. Pharmacogenomics J 6: 246-254.
- Galimberti D, Scarpini E (2017) Pioglitazone for the treatment of alzheimer's disease. Expert Opin Investig Drugs 26: 97-101.
- Kuljiš RO, Salkovic-Petrišic M (2011) Dementia, diabetes, Alzheimer's disease, and insulin resistance in the brain: Progress, dilemmas, new opportunities, and a hypothesis to tackle intersecting epidemics. J Alzheimers Dis 25: 29-41.
Citation: Karahmet E, Prnjavorac B, Sejranić A, Karahmet E, Mujaković A, et al. (2018) Does the Choice of Treatment of Diabetes Mellitus Change Natural Course of Alzheimer Disease? J Alzheimers Dis Parkinsonism 8: 415. DOI: 10.4172/2161-0460.1000415
Copyright: ©2018 Karahmet E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5921
- [From(publication date): 0-2018 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 4961
- PDF downloads: 960