Review Article Open Access
Early, Mid, and Late-Latency Auditory Evoked Potentials During Pregnancy: Two Cases
| Samuel R Atcherson1* and Sarah W Kennett2 | |
| 1University of Arkansas at Little Rock, USA | |
| 2University of Arkansas for Medical Sciences, USA | |
| Corresponding Author : | Samuel R Atcherson Department of Audiology and Speech Pathology University of Arkansas at Little Rock 2801 S. University Avenue, Little Rock, AR, USA Tel: 1-501-569-3155 Fax: 1-501-569-3157 Email:sratchrson@ualr.edu |
| Received July 18, 2014; Accepted November 27, 2014; Published November 28, 2014 | |
| Citation: Atcherson SR, Kennett SW (2014) Early, Mid, and Late-Latency Auditory Evoked Potentials During Pregnancy: Two Cases. J Preg Child Health 1:121. doi: 10.4172/2376-127X.1000121 | |
| Copyright: ©2014 Atcherson SR, et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Objective: There are various known hormone-related effects on audiometric thresholds and other auditory measures; however, research is scarce and mixed on the effect of pregnancy on the early-, mid-, and late-latency auditory evoked responses in a longitudinal fashion. Of clinical importance is to know whether electrophysiologic measures during pregnancy may further alter the results of, or mask, an already existing auditory disorder, which could complicate interpretation.
Study design: We briefly review the literature on the effect of pregnancy on auditory evoked potentials. In addition, we take the non-medical, allied health perspective and describe electrophysiological data collected in a longitudinal manner throughout the course of two healthy pregnancies (non-otosclerotic and non-preeclamptic) and 1-month post-partum in one participant with normal hearing and in another with stable mild-to-moderately severe sensorineural hearing loss.
Results: Early- and late-latency responses had little to no change during pregnancy for both participants; however, middle-latency responses were quite varied for the participant with normal hearing.
Conclusions: Auditory evoked potentials during pregnancy deserve further investigation, particularly in a longitudinal manner.
| Keywords |
| Auditory evoked potential; Hearing; Pregnancy; Longitudinal |
| Introduction |
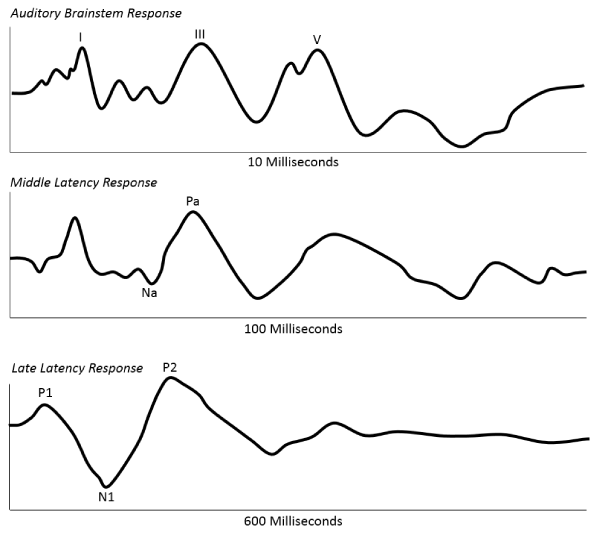
| The hormonal changes of estrogen (estradiol) and progesterone in women involving the menstrual cycle, pregnancy, and menopause have been known for some time. These hormonal changes have shown not only audiologic differences between men and women [1,2], but also differences between women on oral contraceptives and those who are not [1,3,4]. There are numerous explanations for these effects of hormonal changes, but they are generally thought to affect the auditory neurologic and vascular systems to impact both hearing and auditory processing [5]. With pregnancy, a decrease in estrogen is thought to decrease metabolism rates [6], which in turn may impact the availability of neurotransmitters at the synapse and influence neural conduction time [7]. Specifically, the neural effect of both estrogen and progesterone may be influenced by alterations of the inhibitory neurotransmitter gamma amino butyric acid (GABA) and the excitatory neurotransmitter acetylcholine [Ach] within the peripheral and central auditory systems [8,9]. For the purposes of this short review, we will focus on the reported effects of the normal menstrual cycle, menopause, pregnancy on commonly used auditory evoked potentials—auditory brainstem response (ABR), middle latency response (MLR), and late latency response (LLR)—which are measures of progressively higher levels in the auditory system. Figure 1 shows representative waveforms for ABR (waves I, III, and V), MLR (waves Na and Pa), and LLR (waves N1 and P2). We later describe, longitudinally, the results of these three auditory evoked potentials in two healthy pregnant mothers, one with normal hearing and one stable mild-tomoderately severe sensorineural hearing loss. |
| Effects of the menstrual cycle |
| The menstrual cycle is a series of recurring events over an average 28-day period involving the reproductive system in women from late puberty through about the fifth decade of life when menopause usually begins. The menstrual cycle (sometimes called the ovarian cycle) is generally divided up into three main phases, menstrual, follicular, and luteal with a brief ovulation phase that divides the follicular and luteal phases. During the menstrual cycle estradiol rises at the beginning of the menses to a peak of 0.7 ng/ml. just before ovulation and falls back down to pre-menstrual levels. Progesterone rises immediately around ovulation coming to a peak of about 8 ng/ml. by the mid-luteal phase before falling back to pre-menstrual levels. Upon termination of the luteal phase, the endometrial lining is shed (i.e., menstrual bleeding). The effects of the menstrual cycle in the audiological and short-latency evoked potential literature are quite mixed. Cox [10] found no changes nor differences at audiometric test frequencies of 500, 1000, and 2000 Hz between groups of women with balanced and unbalanced hormones during their menstrual cycles, yet Baker and Weiler [1] found that the first half of the cycle had lower (better) thresholds than the second half. Also, Fagen and Church [11] reported no change in auditory brainstem responses (ABRs) during the menstrual cycle, while Elkind-Hirsch et al. [9] reported a significant increase in the latency of wave III and wave V peak latencies and in the I-V interpeak interval associated with the ovulation phase, indicating a temporary worsening of hearing thresholds. Yadav et al. [12] had results quite similar to Elkind-Hirsch et al. [9], both of which were drastically different from the results by Caruso et al. [4] who found that wave I and the wave I-V interval had the shortest latencies at the mid-cycle, ovulation phase compared to the early follicular and late luteal phases. Most recently, Al-Mana et al. [13] only found longer wave V latencies in the late follicular phase. Interestingly, they also found a significantly positive correlation of ABR changes with transient-evoked otoacoustic emission (TEOAE) changes showing a frequency shift in the same late follicular phase. In an unusual case report, Souaid and Rappaport [14] confirmed a case of fluctuating sensorineural hearing loss in a 45-year old patient as a result of hormonal changes during the menstrual cycle. During menstruation, the patient was found to have bilateral highfrequency sensorineural hearing loss. Additionally, ABRs were abnormal bilaterally presenting with prolonged absolute and interpeak latencies, despite normal magnetic resonance imaging (MRI). |
| Despite the mixed results in the above studies involving the peripheral and more caudal aspects of the auditory system, Yadav et al. [15] found some changes in the more rostral areas of the auditory system during four tested phases of the menstrual cycle using later auditory evoked potentials (MLR and LLR). Although they did not reach significance, there was a trend for most waves of the MLR to increase in latency during the mid-cycle phase. For the LLR, however, latencies increased for P2 and N2 during both the mid-cycle (ovulation phase) and pre-menstrual phases (late luteal), while waves P1 and N1 remained stable. |
| Effects of menopause |
| Menopause is used to describe a cessation of menstruation marked by a decline in the human ovaries. The biological marker of menopause is usually 1 full year from the date of the last menstrual period. However, hormonal changes can begin taking place before the last menstrual period, through active menopause, and during the post-menopausal time frame. In terms of audiologic effects, Wharton and Church [2] compared older post-menopausal women with their younger counterparts. They found that all absolute and relative ABR latencies were prolonged in the post-menopausal women despite hearing levels at 20 dB HL or less. Although the aging process is a strong factor, the study also included younger and older men. When compared to men, women had larger changes in latency. ABR amplitudes were also evaluated and were reportedly larger in younger women, but amplitudes were essentially the same for both groups of men. The hormonal change impact on post-menopausal women was attributed to a drop in core temperature and metabolism rates. Tandon et al. [16] went a step further and recorded not only ABR, but also MLR and LLR in 22 post-menopausal women. They confirmed similar findings as Wharton and Church [2] for the ABR. According to Tandon et al. [16], the MLR and LLR data could not be directly compared with other data in the literature because of differences in study methodologies. |
| Effects of pregnancy |
| Pregnancy is a major life event that is marked by a fertilized ovum implanting itself to the endometrial lining during the regular menstrual cycle while progesterone is rising. When pregnancy occurs, the mother will typically undergo several physiological changes as regulated by their hormones. With the implantation of the fertilized ovum, the endometrium will not be shed and a different set of hormonal circumstances (due to temporary cessation of the menstrual cycle) will take place in preparation for a growing fetus. During pregnancy, both estrogen and progesterone rise, but in significantly different amounts. One of the major estrogens, estrodiol, rises from less than 2 ng/ml. in the first few weeks of pregnancy to as high as 18 ng/ml. at the time of birth. On the other hand, progesterone rises from less than 25 ng/ml. to 150 ng/ml. in the same time frame. Thus, pregnancy and menstrual cycles reflect two different hormonal cycle patterns between estrogen and progesterone. In term of audiological observations, Schmidt et al [17] reported prospectively in 82 pregnant women that 33% experienced tinnitus, 24% experienced pressure in the ear, and 18% experienced some perceptual decrease in hearing sensitivity irrespective of the trimester in which the data was obtained. These self-reported experiences provide clues that the auditory system is impacted by the hormonal changes associated with pregnancy. In one of the first studies involving objective, electrophysiological measures, Tandon et al. [18] reported prolonged ABR interpeak latencies I-III, III-V, and I-V in pregnant women compared to non-pregnant controls, but no differences in absolute latencies. Almost a decade later, Egeli and Gürel [19] found that only wave I latency was statistically longer in pregnant than non-pregnant controls with no other absolute nor interwave latencies that reached significance. Although Sennaroglu and Belgin [20] reported low-frequency hearing sensitivity (125 to 500 Hz) that became poorer throughout the pregnancy but returned to normal levels post-partum, and their ABR recordings revealed no differences similar to the Tandon et al. [18] study. The combined results of these studies suggest that the hormonal changes in pregnancy are probably not likely to cause clinically-significant deviations in the ABR for at least three reasons. First, click-evoked ABRs are biased towards higher audiometric frequencies. Second, the hormonal changes associated with pregnancy occur over a long period of time (as compared to the 28-day menstrual cycle) and adaptation may occur. Finally, group studies, especially crosssectional groups, may contain one or more outliers that skew the data. As with the studies involving menstruation and menopause, Yadav et al. [15] recorded the MLR and LLR in 20 pregnant women and nonpregnant controls. They found no statistically-significant changes in the MLR waves; however, LLR waves P1, N1, P2, and N2 were all significantly delayed in the pregnant participants. Taken together, these studies suggest that later cortical potentials, beginning with P1, are most susceptible to the effects of pregnancy. |
| Rationale for case study approach |
| The impetus for the study of hormonal effects associated with pregnancy (as well as the menstrual cycle and menopause) is to minimize or avoid potentially misleading electrophysiological results that could further alter or mask a true, underlying, existing neural disorder. Though rare, misleading electrophysiological results have been reported in the literature for other auditory disorders [21,22]. Furthermore, although studies involving groups are generally powerful on a statistical basis and in terms of evidence-based practice, the effects within the individual are often blurred or lost by such studies. Case studies, such as the one by Souaid and Rappaport [14], have the potential to provide meaningful information appropriate for a particular individual towards intervention [23]. Thus, the purpose of this investigation was to examine, in two individuals with two different audiologic histories, both audiometric and electrophysiologic effects of healthy pregnancy in a longitudinal manner. These two case studies were conducted for at least three important reasons: First, there is a paucity of information about how hearing sensitivity is impacted by pregnancy in both normal hearing listeners and in patients with sensorineural hearing loss. Second, the literature does not appear to show examination of various auditory evoked potential measures with pregnant mothers as their own control. Finally, there does not appear to be sufficient evidence in the literature to preclude pregnant mothers from participating from all auditory research experiments, if they so choose to volunteer and meet the audiologic criteria. Although this is a longitudinal study of only two individuals throughout their pregnancy and one-month post-partum, we hypothesized that there would be no measureable changes for any of the transient auditory evoked potentials recorded. |
| Materials and Methods |
| Participants |
| Due to the long-term commitment of this study and the potential inconveniences associated with pregnancy, only two medically-healthy (non-otosclerotic and non-preeclamptic) and neurologically-intact volunteers experiencing normal, healthy pregnancies were recruited. The first participant was 29-yrs. of age during her pregnancy, and this pregnancy was her first. She had bilateral mild sloping to moderatelysevere sensorineural hearing loss from early childhood due to ototoxicity, and she compensated for her hearing loss with hearing aids. For this participant, we were able to collect data for 9 sessions beginning with the second trimester, including 1 session 1-month part-partum (i.e., after birth). The second participant was 24-yrs. of age undergoing her second pregnancy. She had normal hearing bilaterally, and we were able to collect data for 5 sessions also beginning with the second trimester, including 1 session 1-month post-partum. Each participant carried their child to term with no reported obstetric or medical concerns. All testing sessions were identified by the number of weeks gestation relative to their last known menstrual period. The protocol of the study was approved by the Institutional Review Board (IRB) of the University of Arkansas at Little Rock (# 08-093M1), and each participant gave her informed consent. |
| Measures and procedures |
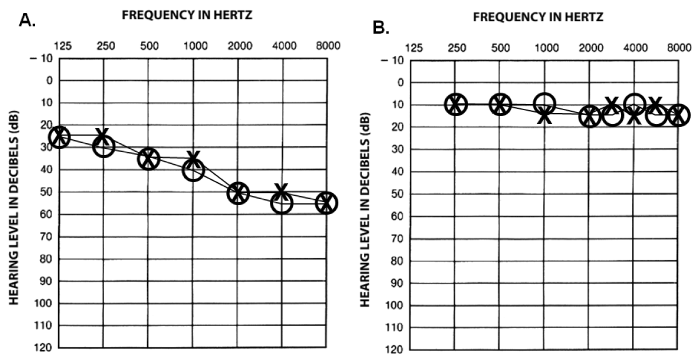
| Air-conduction pure tone audiometry was conducted at the onset of the study, when time permitted during the pregnancy, and one-month post-partum. Thresholds were collected at audiometric frequencies in octave intervals from 250 to 8000 Hz for both participants. For the participant with hearing loss, audiometric threshold at 125 Hz was also included. Pure-tone audiometry confirmed a bilateral mild sloping to moderately-severe sensorineural hearing loss for the first participant and hearing within normal limits for the second participant. More importantly, there were no clinically-significant changes in auditory sensitivity when compared to pre-pregnancy records for either case. Tympanometry was always conducted prior to each auditory evoked potential recording to ensure that any middle ear pathology would not negatively influence the results in any session. Figure 2 shows the airconduction audiometric results which, at no time, exceeded acceptable test-retest variability of ±5 dB HL throughout the pregnancies and 1-month post-partum. |
| A one-channel recording was implemented using disposable snapelectrodes with non-inverting electrode at Fz (i.e., high forehead), reference electrode on the stimulus ear lobe, and ground on the nonstimulus ear lobe. Electrode impedances 2 kΩ or less was obtained prior to each recording. To minimize ocular artifact, participants were instructed to rest with eyes closed during ABR, and were asked to focus on a stationary target for MLR and LLR. ABRs, MLRs, and LLRs were collected and repeated for each ear in every session. All electrophysiologic recordings were obtained in a quiet, non-anechoic clinical test room using a Bio-logic Navigator Pro evoked potential system (Mundelein, IL, USA). Specific stimulus and recording parameters are presented in Table 1. |
| Analysis |
| Absolute peak latency and amplitude values were obtained for the following peaks: I, III, and V of the ABR; Na and Pa of the MLR; and P1, N1, and P2 of the LLR. All recordings were marked independently by two individuals with any marking disagreements resolved prior to data analysis. For data reduction purposes, the latency and amplitude values for repeated runs were mathematically averaged. |
| Results |
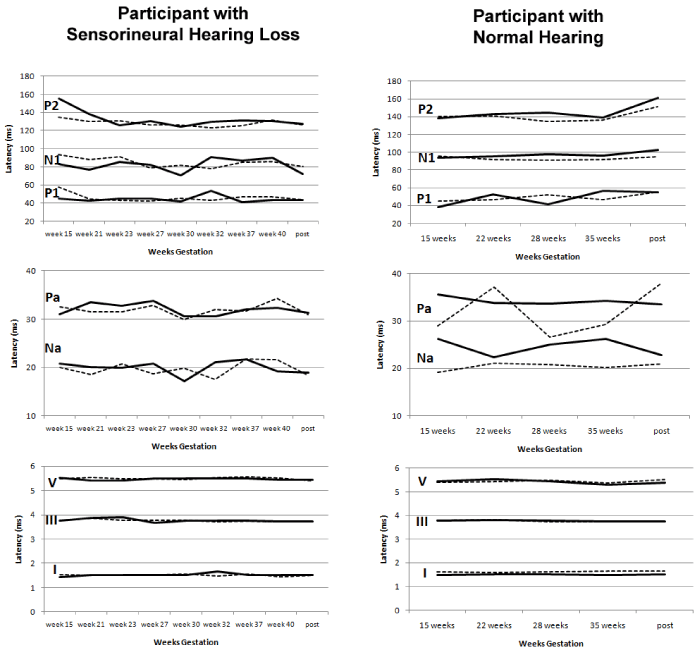
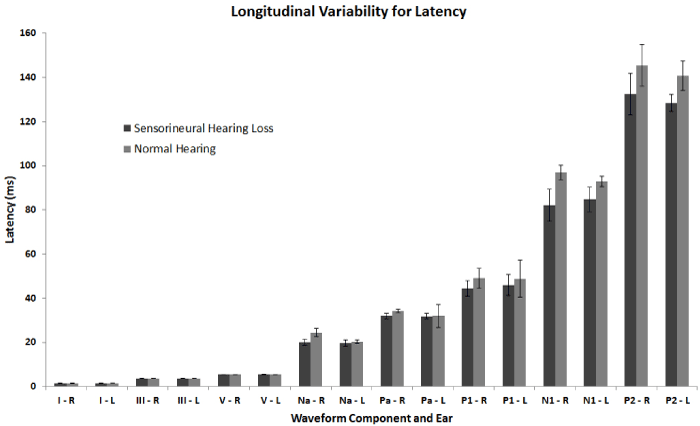
| All recordings yielded typical waveform morphologies. The absolute peak latencies and amplitudes for ABR (I, III, and V), MLR (Na and Pa), and LLR (P1, N1, and P2) were obtained over the course of two pregnancies and 1-month post-partum. Figure 3 shows latency values plotted longitudinally across session and separately for each ear of stimulation. When viewed longitudinally, the participant with normal hearing had greater latency variability across sessions and between ear of stimulation than for the participant with sensorineural hearing loss. The relative lack of MLR latency ear-related variability seen in the participant with sensorineural hearing loss may reflect the existing pathology of the cochlea that might otherwise be sensitive to hormonal changes. When latency is collapsed across sessions and reported descriptively as means and standard deviations, Figure 4 shows noticeably greater variability for the participant with normal hearing for left Pa, left P1, and left P2 compared to the participant with sensorineural hearing loss. In contrast, left and right N1 was noticeably more variable for the participant with sensorineural hearing loss. Figure 4 should be interpreted with caution as it masks the trend over time and does not take into account expected signal averaging variability. Neither Figures 3 nor 4 show much variability for ABR latencies (maximum standard deviation was no more than 0.07). ABR interpeak latencies (i.e., I-III, III-V, and I-V) were reportedly prolonged in pregnant women as suggested by Tandon et al. [18]; however, after further examination, this finding was not validated in our recordings during pregnancy when compared to recordings 1-month post-partum. |
| Peak amplitude values were difficult to interpret due to changes about the zero baseline. In order to compensate for this issue, we calculated the peak-to-peak amplitude (as well as the ranges) of Na- Pa of the MLR and N1-P2 of the LLR for each participant (Tables 2 and 3). Again, no clear longitudinal trend is observable. However, one interesting finding for both participants is that left ear stimulation peak-to-peak amplitudes more variable than for right ear stimulation for both MLR and LLR with greater variability for the participant with sensorineural hearing loss. LLR latencies for P1, N1, and P2 were largely stable across session and between ear of stimulation for both participants. |
| Discussion |
| The results presented here support the conclusions of Sennaroglu and Belgin [20] that there are no changes in ABR latencies over time, but they are in some contrast to the results reported by Tandon et al. [18] and Egeli and Gürel [19]. While Yadav et al. [15] indicated no changes in MLR during pregnancy for their participants, our participant with normal hearing showed the greatest changes in MLR latencies, not only over the course of her pregnancy, but also between ears of stimulation. Unfortunately, we are unable to attribute this variability to any single reason, but may be due to changes in arousal levels [24] and due to poor signal-to-noise ratio during the recordings. For both participants, LLRs showed little differences between ears and were largely stable over time, which is in contrast to Yadav et al. [15] indicating that LLRs are prolonged in pregnant women. We observed no such result here in our two participants when viewed in a longitudinal fashion. The important distinction between our data and those reported by Yadav et al. [15] is the use of different stimulus types. Specifically, we used short duration tonebursts, whereas Yadav and colleagues used click stimuli. Although click stimuli are often used for suprathreshold neurologic applications, McPherson, Ballachanda, and Kaf [25] suggest that toneburst stimuli are more reliable than clicks for LLRs, which guided our decision to use tone bursts in this study. |
| The primary strength of this two-case study is taking into account individual variability over the course of pregnancy. Limitations, however, include lack of information in the first trimester, lack of information about trends within and between groups, and lack of ability to examine hemispheric amplitudes for the MLR. Future studies should consider examining participants on the individual level starting with the first trimester as well as be compared to the results of the whole group through the pregnancy and post-partum. A comparison of auditory evoked potentials beyond the ABR in pregnant women with sensorineural hearing loss, otosclerosis, and preeclampsia could also be worthwhile. Lastly, future studies could consider adding endrocrinologic measures, incorporating a rigorous timeline, conducting regular audiologic examinations (including otoacoustic measures with and without contralateral suppression), incorporating the study of the P300 component, and exploring a variety of different acoustic stimuli to come to more solid conclusions about the effect of pregnancy on auditory evoked potentials. |
| Acknowledgements |
| Portions of this study were presented at the 2009 American Speech- Language-Hearing Association Convention in New Orleans, LA. The authors thank Drs. Rebecca S. Atcherson and Michelle Richardson for their assistance with data collection. |
References
- Baker MA, Weiler EM (1977) Sex of listener and hormonal correlates of auditory thresholds. Br J Audiol 11: 65-68.
- Wharton JA, Church GT (1990) Influence of menopause on the auditory brainstem response. Audiology 29: 196-201.
- McFadden D (2000) Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear Res 142: 23-33.
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, et al. (2003) Auditory brainstem response in premenopausal women taking oral contraceptives. Hum Reprod 18: 85-89.
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J (1994) Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res 80: 209-215.
- BRUCE J, RUSSELL GF (1962) Premenstrual tension. A study of weight changes and balances of water, sodium, and postassium. Lancet 2: 267-271.
- McEwen BS (1991) Non-genomic and genomic effects of steroids on neural activity. Trends PharmacolSci 12: 141-147.
- Klinke R, Galley N (1974) Efferent innervation of vestibular and auditory receptors. Physiol Rev 54: 316-357.
- Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF (1992) Estrogen influences auditory brainstem responses during the normal menstrual cycle. Hear Res 60: 143-148.
- Cox JR (1980) Hormonal influence on auditory function. Ear Hear 1: 219-222.
- Fagan PL, Church GT (1986) Effect of the menstrual cycle on the auditory brainstem response. Audiology 25: 321-328.
- Yadav A, Tandon OP, Vaney N (2002) Auditory evoked responses during different phases of menstrual cycle. Indian J PhysiolPharmacol 46: 449-456.
- Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM (2010) Alteration in auditory function during the ovarian cycle. Hear Res 268: 114-122.
- Souaid JP, Rappaport JM (2001) Fluctuating sensorineural hearing loss associated with the menstrual cycle. J Otolaryngol 30: 246-250.
- Yadav A, Tandon OP, Vaney N (2003) Mid latency and slow vertex responses during pregnancy. Indian J PhysiolPharmacol 47: 423-428.
- Tandon OP, Khaliq F, Goel N (2001) Auditory evoked potential responses in menopausal women: a normative study. Indian J PhysiolPharmacol 45: 361-366.
- Schmidt PM, Flores Fda T, Rossi AG, Silveira AF (2010) Hearing and vestibular complaints during pregnancy. Braz J Otorhinolaryngol 76: 29-33.
- Tandon OP, Misra R, Tandon I (1990) Brainstem auditory evoked potentials (BAEPs) in pregnant women. Indian J PhysiolPharmacol 34: 42-44.
- Egeli E, Gürel, S (1997) The aspect of the ABR in pregnancy. Turk Arch Oto Rhino Larynogol 37: 11-14.
- Sennaroglu G, Belgin E (2001) Audiological findings in pregnancy. J LaryngolOtol 115: 617-621.
- Leggett JM, Reid A (1987) Misleading auditory brainstem responses in a case of acoustic neuroma. J LaryngolOtol 101: 179-183.
- Warren FM, Wiggins RH, Pitt C, Harnsberger HR, Shelton C (2010) Apparent cochlear nerve aplasia: to implant or not to implant? OtolNeurotol 31: 1088-1094.
- Barlow D, Hersen M (1984) Single Case Experimental Designs: Strategies for Studying Behavior Change. Pergamon Press, New York.
- Mendel MI, Hosick EC, Windman TR, Davis H, Hirsh SK, et al. (1975) Audiometric comparison of the middle and late components of the adult auditory evoked potentials awake and asleep. ElectroencephalogrClinNeurophysiol 38: 27-33.
- McPherson D, Ballachanda B, Kaf W (2007) Middle and long latency auditory evoked potentials. In: Roeser R, Valente M, Hosford-Dunn H, editors. Audiology: Diagnosis. Thieme Medical Publishers, New York.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16432
- [From(publication date):
December-2014 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 11779
- PDF downloads : 4653
