Early Palliative Care for Improving Quality of Life and Survival in Patients with Advanced Cancer: A Systematic Review and Meta-analysis
Received: 19-Sep-2018 / Accepted Date: 28-Sep-2018 / Published Date: 05-Oct-2018 DOI: 10.4172/2165-7386.1000343
Keywords: Palliative care; Quality of life; Survival; Neoplasms
Introduction
Despite the advances in the treatment of cancer, the disease at an advanced stage carries a poor prognosis. Typically, in people with advanced cancer, social functions decline in parallel with the physical decline over time, whereas psychological and spiritual wellbeing often fall together into four main moments: around diagnosis, on return home after the initial treatment, the recurrence of the disease and in the terminal phase [1]. These issues can result in a negative impact on the quality of life and survival of these patients [2].
The World Health Organization (WHO) has defined palliative care as "an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” [3].
This does not mean that palliative care should only be considered when all therapeutic options have failed or when a person is considered to be at the end of their life. According to clinical guidelines, palliative care can be offered after diagnosis and combined with standard oncology care [4,5]. Therefore, the objective this systematic review was to determine the effectiveness of Early Palliative Care (EPC) in terms of improving Quality of Life (QoL) and survival in patients with advanced cancer, as compared to standard care.
Methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance (S1 Table) [6]. The protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42017065309).
Eligibility criteria
We included all Randomized Controlled Trials (RCTs) that met the following inclusion criteria:
• Population composed of adult patients diagnosed with advanced cancer
• Patients in the intervention arm received EPC
• Patients in the comparator arm received standard care
• Outcomes evaluated included measurements of QoL and survival.
Search
A literature search was undertaken using multiple electronic databases: MEDLINE (via PubMed), Centre for Reviews and Dissemination (CRD), The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, and Google Scholar. Additionally, International Clinical Trial Registry Platform and ClinicalTrials.com website were also searched for ongoing studies.
The search strategy combined search terms for palliative care, neoplasm, quality of life, and survival. Further, specific search filters for RCTs were also applied. All searches were performed from inception to June 2017. The strategies used in the searched electronic databases are presented in Appendix 1.
Study selection
Search results from the different databases were imported and merged into Cochrane’s tool – Covidence and duplicates were removed automatically or deleted manually. Two reviewers (FZ and IZ) independently assessed the titles and abstracts retrieved to identify potential papers for inclusion. Following which, both reviewers reviewed the remaining full papers. All disagreement was resolved through discussions until consensus was reached. If consensus cannot be met between the two reviewers, the opinion of a third reviewer (CASA) was sought.
Data extraction
Finally with the articles deemed relevant selected, the information on the characteristics of these studies (design, methods of randomization, population, interventions, and results) was extracted using the standardized data collection form. When such information could not be retrieved, an email was sent to authors requesting nonreported data.
Risk of bias
Risk of bias of individual studies was assessed using the Cochrane Risk of Bias (RoB 2.0) tool. This tool considers five domains and each domain was classified as having "low risk" (+), "high risk" (-) or unclear risk (?) of bias.
Risk of bias across studies was assessed using the Grading of Recommendations Assessment, Developing, and Evaluation (GRADE) approach [7,8]. It was intended that potential publication and small study bias across the studies would also be investigated through funnel plots. However, due to the small number of studies identified for the review, this was not carried out [9].
Statistical analysis
The outcomes estimates were pooled in a meta-analysis, using random-effects models based on the DerSimonian-Laird method. The random effects model accounts for sampling error and between study variations. This model is preferable when there is heterogeneity in outcomes and provide estimates that are more conservators [10]. In addition, fixed effect models were also used as the sensitivity analysis. The predictive interval that incorporates the extent of heterogeneity and inconsistency in the analysis, was used to assess the extent of uncertainty in the size of the estimated effect in each measured outcome [11].
Considering that the selected studies used different instruments to measure the outcomes, it is not meaningful to combine the effect size by raw mean differences. In this case usually, the estimated effect size is measured employing the Hedges'g or Cohen's d statistic [12,13].
The two statistics are very similar, however, the Cohen’s tending to overestimate the absolute value in small samples. Meta-analyses performed employed a conservative approach using the Hedges'g statistic. We divided the mean difference between the two groups by pooled and weighted Standard Deviation (SD) of the groups to create a Standardized Mean Difference (SMD) that would be comparable across studies [12]. When not reported, missing SD was imputed using the sample size weighted SD from all of the studies with complete information.
The outcomes measured as SMD have the objective of comparing the results across different studies through of different measures. For this reason, it cannot be interpreted as a difference in rating scale points or percent improvement. One approach for interpreting the magnitude of the SMD is to use the widely accepted guidelines of Cohen [13].
For SMDs, he defined 0.2 as a small effect, 0.5 as a medium effect, and 0.8 as large. Odds Ratio (OR) for survival was calculated using the randomized effect model.
Statistical heterogeneity was assessed and quantified using the Cochran’s Q and I2. Due to the small number of studies included in the meta-analysis, we used p<0.10 as an indication of statistical significance and evidence of heterogeneity [14,15]. All analyses were carried out using Stata 14 [16].
Results
Study selection
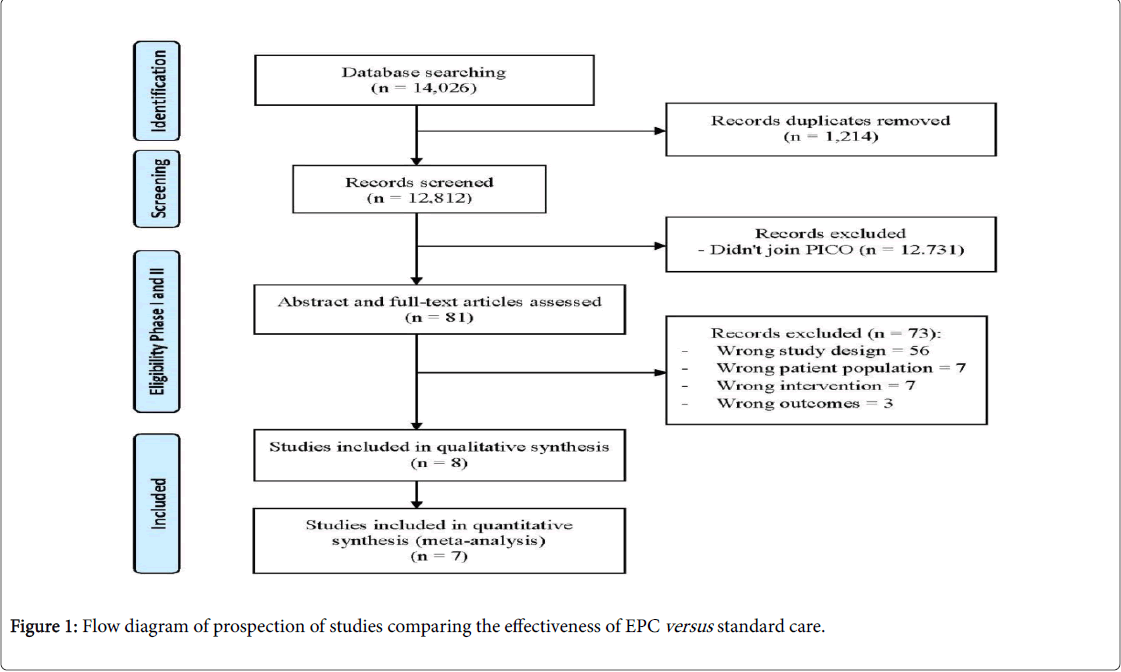
Figure 1 describes a flow diagram of the process of identifying relevant studies to be included in the review. Overall, the databases search retrieved 14,026 articles, of which 81 were read in full. Further exclusions yielded eight studies which met all the inclusion criteria (Table 1). Reasons for exclusion of studies are listed in the S2 Table.
| Characteristics | Bakitas et al. 2009 [18] | Temel el al. 2010 [19] | Tattersall et al. 2014 [20] | Zimmermann et al. 2014 [21] | Bakitas et al. 2015 [22] | McCorkle et al. 2015 [23] | Maltoni et al. 2016 [24] | Temel et al. 2016 [25] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPC | Std. care | EPC | Std. care | EPC | Std. care | EPC | Std. care | EPC | Std. care | EPC | Std. care | EPC | Std. care | EPC | Std. care | |
| N | 145 | 134 | 77 | 74 | 53 | 54 | 228 | 233 | 104 | 103 | 66 | 80 | 97 | 89 | 175 | 175 |
| Mean age (SD) | 65.4 (10) | 65.2 (11) | 64.9 (9) | 64.8 (9) | 63 (11) | 64 (11) | 61.2 (12) | 60.2 (11) | 64.0 (10) | 64.6 (9) | 60 | 60 | 67 | 66 | 65.6 (11) | 64.0 (10) |
| Male (%) | 90 (62) | 78 (58) | 35 (45) | 38 (51) | 32 (53) | 26 (43) | 92 (40) | 108 (46) | 56 (55) | 53 (52) | 19 (29) | 45 (56) | 59 (62) | 47 (53) | 91 (52) | 98 (56) |
| Female (%) | 55 (38) | 56 (42) | 42 (55) | 36 (49) | 28 (47) | 34 (57) | 136 (60) | 125 (54) | 48 (46) | 50 (48) | 47 (71) | 35 (44) | 37 (38) | 42 (47) | 84 (48) | 77 (44) |
| White (%) | 143 (99) | 132 (99) | 77 (100) | 70 (95) | - | - | - | - | 102 (98) | 98 (95) | 58 (88) | 66 (83) | - | - | 156 (89) | 167 (95) |
| American (%) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 (2) | 0 (0) |
| Asian (%) | - | - | 0 | 1 (1) | - | - | - | - | - | - | - | - | - | - | 5 (3) | 3 (2) |
| Black (%) | - | - | 0 | 3 (4) | - | - | - | - | 0 | 1 (1) | - | - | - | - | 6 (3) | 4 (2) |
| Hispanic (%) | - | - | - | - | - | - | - | - | - | - | - | - | 7 (4.0) | 2 (1.1) | ||
| Other (%) | 1 (0.7) | 1 (0.7) | - | - | - | - | - | - | 2 (2) | 3 (3) | 8 (12) | 14 (17) | - | - | 4 (2) | 1 (0.6) |
| Missing (%) | 1 (0.7) | 1 (0.7) | - | - | - | - | - | - | 0 | 1 (1) | - | - | - | - | - | - |
| Lung (%) | 50 (34) | 43 (32) | 77 (51) | 74 (49) | 12 (20) | 11 (18) | 55 (24) | 46 (20) | 46 (44) | 42 (41) | 37 (56) | - | - | - | 95 (54) | 96 (55) |
| Gastrointestinal (%) | 61 (42) | 58 (43) | - | - | 20 (33) | 24 (40) | 74 (32) | 65 (28) | 26 (25) | 24 (23) | - | 53 (66) | 97 (52) | 89 (48) | 80 (46) | 79 (45) |
| Genitourinary (%) | 19 (13) | 18 (13) | - | - | - | - | 27 (12) | 51 (22) | 7 (7) | 9 (9) | - | - | - | - | - | - |
| Breast (%) | 15 (10) | 15 (11) | - | - | 5 (8) | 12 (20) | 41 (18) | 31 (13) | 10 (10) | 13 (13) | - | - | - | - | - | - |
| Gynaecological (%) | - | - | - | - | 11 (18) | 8 (13) | 31 (14) | 40 (17) | - | - | 29 (44) | - | - | - | - | - |
| Other solid tumour (%) | - | - | - | - | 12 (20) | 3 (5) | - | - | 10 (10) | 10 (10) | - | - | - | - | - | - |
| Hematologic malignancy (%) | - | - | - | - | - | - | - | - | 5 (5) | 5 (6) | - | - | - | - | - | - |
| Prostate (%) | - | - | - | - | 0 (0) | 2 (3) | - | - | - | - | - | - | - | - | - | - |
| Head and neck (%) | - | - | - | - | - | - | - | - | - | - | - | 27 (34) | - | - | - | - |
| Never smoker or < 10 pack-years (%) | - | - | 18 (24) | 16 (22) | - | - | - | - | 72 (70) | 73 (71) | - | - | - | - | 75 (43) | 67 (38) |
| Current or former smoker (%) | - | - | - | - | - | - | - | - | 17 (24) | 14 (20) | - | - | - | - | 93 (53) | 95 (54) |
| Unknown (%) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 (4) | 13 (7) |
| High School or Less (%) | 17 (12) | 20 (15) | - | - | - | - | 18 (8) | 24 (10) | 8 (8) | 3 (3) | 18 (27) | 24 (30) | - | - | 58 (33) | 73 (42) |
| Some or Complete college (%) | 83 (57) | 74 (55) | - | - | 23 (38) | 32 (53) | 56 (25) | 57 (24) | 61 (59) | 50 (49) | 48 (73) | 56 (70) | - | - | 76 (43) | 69 (40) |
| Graduate School (%) | 43 (30) | 38 (28) | - | - | - | - | 152 (65) | 151 (65) | 35 (34) | 50 (49) | - | - | - | - | 41 (23) | 33 (19) |
| Chemotherapy (%) | 107 (74) | 96 (72) | 50 (65) | 46 (65) | - | - | 174 (76) | 182 (78) | 76 (73) | 80 (78) | 28 (42) | 36 (45) | - | - | - | - |
| Radiation therapy (%) | 30 (21) | 30 (22) | 27 (35) | 26 (35) | - | - | 16 (7) | 13 (6) | 20 (19) | 20 (19) | - | - | - | - | - | - |
Table 1: Characteristics of included studies.
Study characteristics
Of the eight studies included, three were undertaken in the USA, two in the Lebanon, and one each in Canada, Australia, and Italy [17-24]. Across the studies selected, the number of patients assessed in each study ranged from 107-461, with the mean age of the patients ranging from 60 to 67 years old. The most evaluated cancer types were lung, gastrointestinal, genitourinary, breast and gynecological cancer.
Intervention class
We include all types of professional EPC services provided to patients in advanced stages of cancer. In addition, patients needed to be enrolled in palliative care soon after diagnosis of advanced disease. We did not include palliative therapies to prolong life (e.g. palliative chemotherapy) or relieve symptoms (e.g. palliative radiotherapy). We did not restrict the type of delivery of EPC (inpatient, outpatient) or place of consultation (clinic, patient’s home).
Beyond standard cancer treatment, the EPC included monitoring patients’ status, symptom management, problem-solving, communication and social support, and advance care planning. Patients met with nurse and palliative care physicians to routine assessment and discussion of goals of care, or patient and family support needs. The clinical practices were guided by specific guidelines for people and families living with cancer. Two main ways of delivering the EPC were identified across of selected RCTs:
• intervention carried out mainly by telephone and face to face (called and face to face) [17,19-22] and
• patients have met with a member of the palliative care team (faceto- face) [18,23,24].
Patients assigned to standard care group were allowed to use all standard cancer treatments.
Outcome assessment
Quality of life was measured using the Functional Assessment of Chronic Illness Therapy (FACIT) tool [25–27]. This tool have a score range of 0 to 184 and measures the physical, emotional, social, and functional well-being of people with life-threatening illnesses. Higher scores indicate better QoL. Symptoms intensity was measured by Edmonton Symptom Assessment Scale (ESAS) and Symptom Distress Scale (SDS) [28,29]. Scoring ranges from 0 to 900 and 13 to 65, respectively. Both tools assess the intensity of the most common symptoms in cancer, such as pain, activity, nausea, depression, anxiety, drowsiness, appetite, feeling of well-being, and shortness of breath. Higher scores indicate a greater intensity of symptoms. The mood was measured by the instruments, Patient Health Questionnaire-9 (PHQ-9), Center for Epidemiological Studies Depression Scale (CESD), and Hospital Anxiety and Depression Scale (HADS) [30-32]. Their scores range from 0 to 27, 0 to 60, and 0 to 21, respectively. In both instruments, higher scores indicate a greater level of depressed mood. The survival rate was assessed through a log-rank test to compare Kaplan-Meier survival curves between the two groups. Mean and SD values were requested when provided only the mean observed change from baseline [20]. The studies varied in relation to follow-up and assessment time: 3, 6, and 12 months.
Risk of bias
According to Cochrane risk of bias tool, the methods used for allocation concealment and measurement of the outcomes were not clearly stated and show some concerns. Conversely, the methods for deviations from intended interventions, missing outcomes data, and selection of the reported results were appropriately described in all studies. Overall, the risk of bias in the studies showed some concerns (S3 Table).
Considering that the study by Tattersall et al. showed a failure in randomization and high risk of bias due the patients assigned to the EPC group had an initial diagnosis of cancer of more than 3 months and with a worse survival estimate, this study was not considered in the meta-analysis [19].
Synthesis of results
We identified heterogeneity in relation to the type of intervention provided to patients in the EPC group, we observed also differences in follow-up time, time of enrollment in the study after the initial diagnosis, and in estimates of survival (prognosis) (S4 Table). In this case, a sensitivity analysis was performed, separating the studies according to the follow-up time to evaluate the effect of the intervention over time.
Quality of Life – QoL
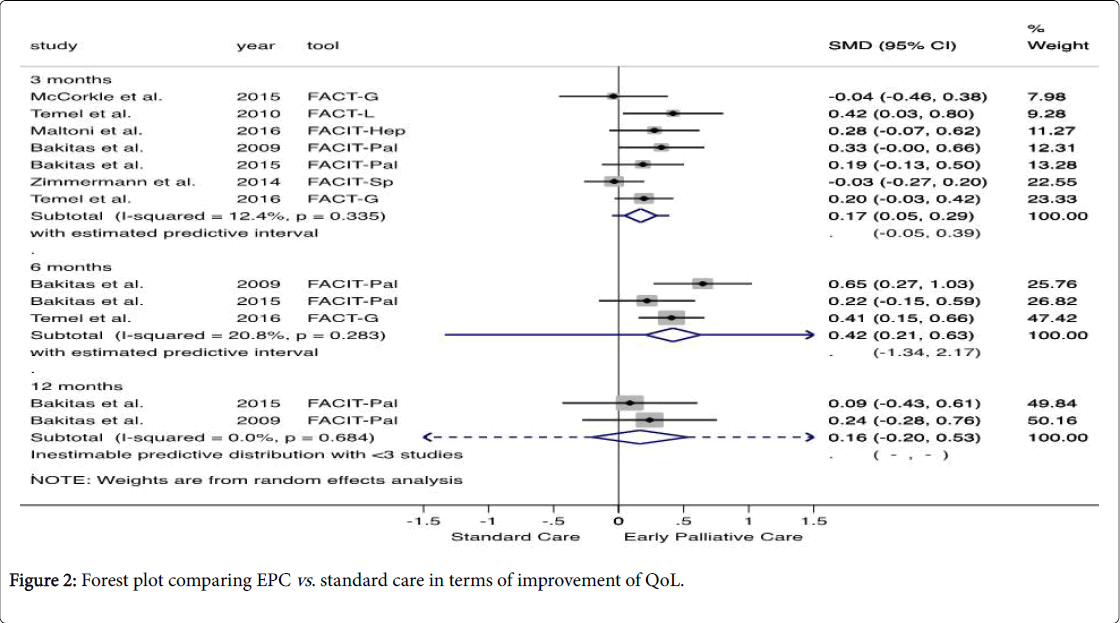
Pooled data showed that patients enrolled in EPC presented a significantly higher QoL than those attributed to standard care (SMD 0.17, 95% CI 0.05, 0.29 at 3 months and, SMD 0.42, 95% CI 0.21, 0.63 after 6 months). According to Cohen's criteria, the magnitude of the outcome assessed was small [13]. A qualitatively similar result was obtained by the fixed effect model. Considering the level of heterogeneity and statistical uncertainty observed, the estimated effect of a new study would be between (-0.05, 0.39) and (-1.34, 2.17) after 3 and 6 months, respectively.
After 12 months of follow-up, the evidence shows that there is no statistically significant difference between the two groups assessed (SMD 0.16, 95% CI -0.20, 0.53). Based on Cochran’s Q and Higgin’s I2, there is no statistic evidence of heterogeneity between the studies (Figure 2).
Using approach GRADE, we downgrade the quality of the evidence to moderate because the studies presented some concerns regarding the bias arising from the randomization process and the outcome assessor was aware of the intervention received [18,20–22] (S5 Table). Additionally, McCorkle assessed in the intervention group, older patients with more chronic conditions, and diagnosed with later-stage cancers [22].
Symptom intensity
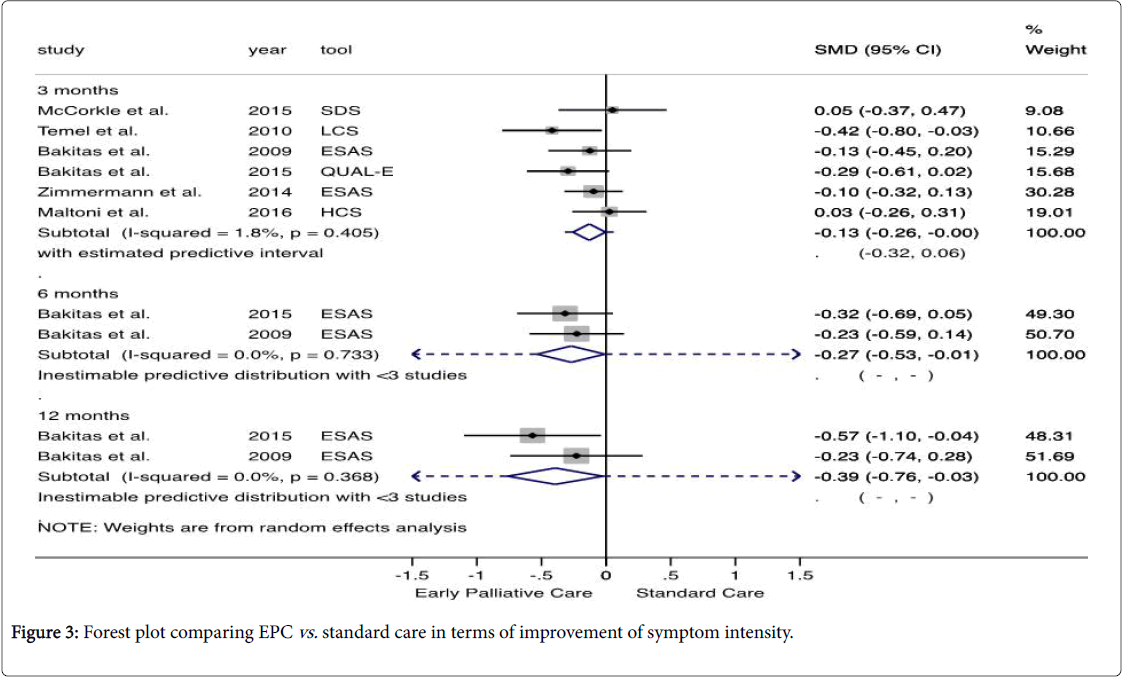
Data pooled showed that the symptom intensity was significantly lower in EPC group after 3, 6, and 12 months follow-up (SMD -0.13, 95% CI -0.26, -0.00; SMD -0.27, 95% CI -0.53, -0.01; and SMD -0.39, 95% CI -0.76, -0.03) (Figure 3). The effect size was small and no heterogeneity across studies was found. A qualitatively similar result was obtained through of fixed effect analysis.
Mood
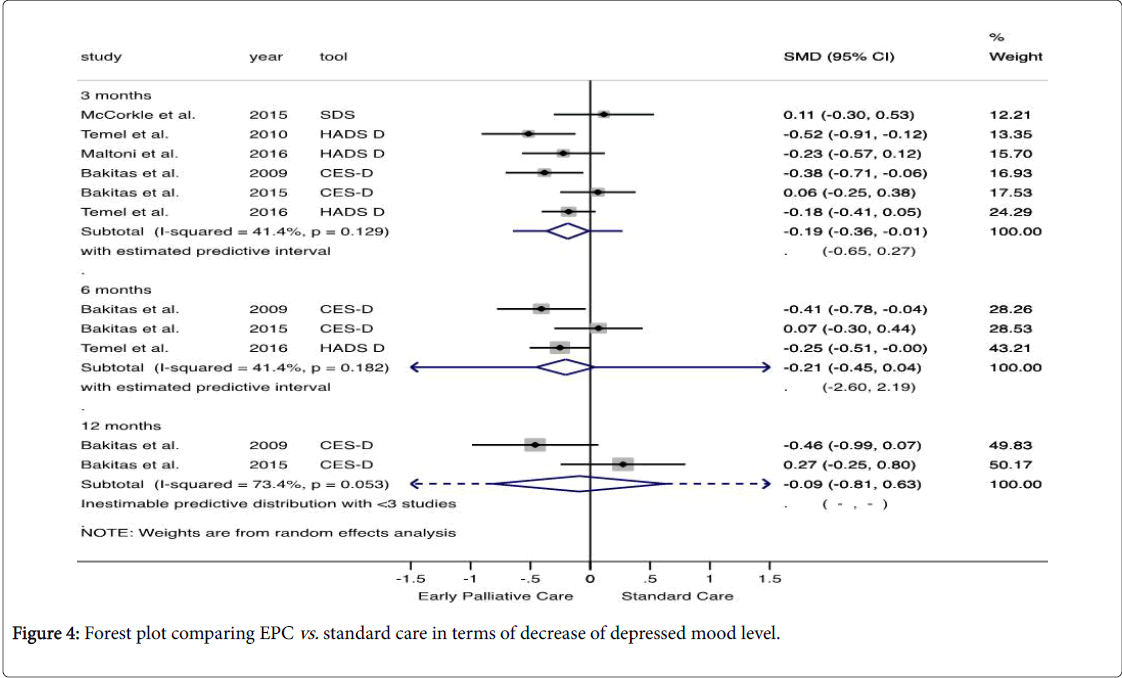
The analysis showed that the depressed mood level did not differ between the evaluated groups. Given the level of heterogeneity and statistical uncertainty observed, the effect of a new study would be between (-0.65, 0.27) and (-2.60, 2.19) after 3 and 6 months, respectively (Figure 4).
Based on Cochran’s Q and Higgin’s I2, the outcome is characterized by qualitative heterogeneity between the studies. Some factors that influenced the inconsistency and imprecision are shown in S3 and S4 Table. Using approach GRADE, we downgrade the overall quality of evidence in the following criteria: risk of bias [19,21-23], inconsistency [20–22], and imprecision [17,20-24] (S5 Table).
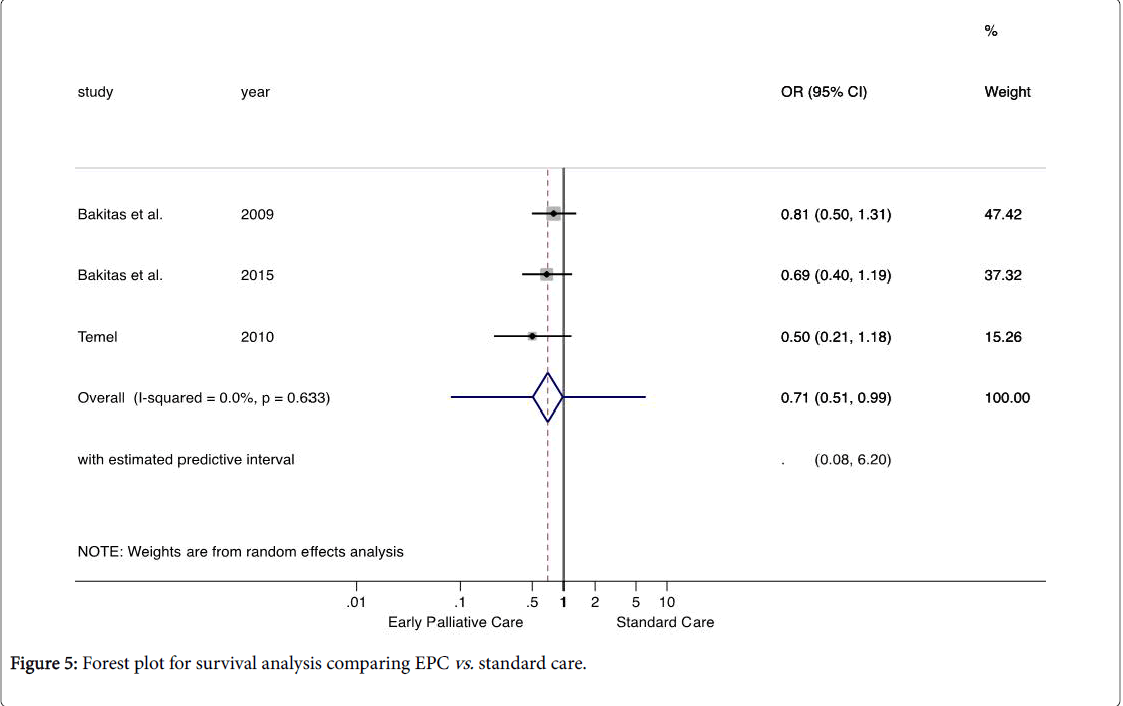
Survival
The outcome showed that the OR below 1.0, represent a lower odds of mortality in the EPC group. The median survival time ranged from 11.6 to 18 months for the EPC group and 8.5 and 11.8 months for the standard care group. Available evidence indicates that patients undergoing the EPC have 29% lower chance of mortality than patients undergoing standard care, with the true effect in the population between 49% and 1% lower: OR=0.71, 95% CI 0.51, 0.99, P=0.04). This outcome was based on a set of studies homogeneous from the statistic viewpoint: PQ=0.63 with I2=0% (Figure 5). Using approach GRADE, the confidence of evidence was rated as moderate. Making a practical interpretation of the effect size, the EPC reduces 85 deaths per 1,000 patients (95% CI 2 to 166).
Discussion
Main results
Considering a decrease in health over time, we combined the evidence on the effectiveness of EPC in patients with advanced cancer in subgroups according to the time of follow-up. On average, the Quality of Life in the early palliative care group was higher after 3 and 6 months of follow-up. Additionally, patients enrolled in EPC group presented lower symptoms intensity and longer survival. A similar benefit was observed between EPC services provided through telehealth and face-to-face.
According to Murray et al. , a fall in the trajectory of psychological and spiritual wellbeing can occur at four moments during the evolution of disease: around diagnosis, on return home, at recurrence, and in the terminal phase [1]. The limited effect on the improvement of QoL and depressed mood observed after 12 months of follow-up can be explained by the variation in the trajectory of wellbeing and disease progression.
Overall, the quality of evidence and the strength of the recommendation showed some concerns and had been classified between very low and moderate. Second Zimmermann, the evidence on the effectiveness of palliative care is scarce and with methodological limitations, such as contamination of the control group, substantial failure in recruitment, attrition, and adherence [33].
The result of our meta-analysis contributes to the increasing evidence that early palliative care may improve quality of life and survival of patients with advanced or metastatic cancer, which are two of the main target of care [3,34].
Completeness, applicability, and overall quality of evidence
Although we have made a highly sensitive electronic search, the result of our systematic review identified few studies published in the literature. We believe that the best evidence available was found, however, the pooled data from seven studies maintains the uncertainties surrounding the effect of EPC. According to search made in the International Clinical Trial Registry Platform and ClinicalTrials.gov website, new RCTs are ongoing and can contribute to raising the strength of evidence.
Some studies showed differences related to the patients assigned to the EPC group and this may have affected the quality and heterogeneity of the evidence [20,22,24]. Others concerns that deserve to be addressed are: comparing the effect of providing early versus delayed palliative care and the trade-off of recruit highly distressed patients due to newly diagnosed advanced cancer may limit the ability to identify the differences and affect outcomes [21,22].
Potential biases in the review process
A potential bias that we can highlight is the non-recovery of studies carried out but not published. At this stage, it is difficult to say such studies actually exist. We consider this number to be too small or nonexistent. Moreover, it is reasonable to assume that such studies would probably not qualitatively change the overall outcome obtained. Although traditional meta-analysis methods are robust to an inadequate assumption of normality, the confidence intervals of the summarized estimates should be seen as reasonable approximations of the true population parameter [35].
Implications for clinical practice and health services
The provision of palliative care aims to alleviate suffering and achieve the quality of life of patients and their family caregivers. Results found shown that EPC is appropriate and potentially beneficial when introduced after diagnosis and combined with standard oncology care. Some questions still need to be clarified. For example, the reason for greater survival in the EPC group is unknown. This benefit may be due to improved QoL or improved symptom severity. However, the studies assessed were not designed to assess this question.
Implication for research
New studies with robust, stratified samples by cancer type are needed to assess the effectiveness and to identify the best ways to provide EPC. Additionally, studies that assess the effect of EPC on overall health care costs need to be performed.
Conclusion
Our findings suggest that EPC effectively improves QoL, reduces symptoms, and increases the likelihood of survival of patients with advanced cancer. Although the effect size has been small, clinically it may be relevant considering a decrease in health over time. Finally, the results should be interpreted with caution due there are few published studies and the quality of evidence observed.
Acknowledgement
Zanghelini would like to thank the University of Glasgow, the Health Economics and Health Technology Assessment (HEHTA) team, especially my supervisor Olivia Wu, for the opportunity to participate as a visiting PhD student. In addition, I thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for a doctorate scholarship.
References
- Murray SA, Kendall M, Mitchell G, Moine S, Amblà s-Novellas J, et al. (2017) Palliative care from diagnosis to death. BMJ 356: j878
- Davis MP, Bruera E, Morganstern D (2013) Early integration of palliative and supportive care in the cancer continuum: challenges and opportunities. Am Soc Clin Oncol Educ Book 2013: 144-150.
- Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, et al. (2012) American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 30: 880-887
- Improving supportive and palliative care for adults with cancer, NICE cancer service guidance. London Natl Inst Heal Clin Excell 2004.
- Moher D, Altman DG, Liberati A, Tetzlaff J (2009) PRISMA Statement. Epidemiol 22: 128.
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, et al. (2011) GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 64: 1311-1316.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, et al. (2009) GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Chinese J Evidence-Based Med 9: 8-11.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: d4002.
- Pereira TV., Ioannidis JP (2011) Statistically significant meta-analyses of clinical trials have modest credibility and inflated effects. J Clin Epidemiol 64: 1060-1069.
- Riley RD, Higgins JP, Deeks JJ (2011) Interpretation of random effects meta-analyses. BMJ 342: d549.
- Hedges LV (1981) Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J Educ Behav Stat 6: 107-128.
- Lachenbruch PA, Cohen J (1989) Statistical power analysis for the behavioral sciences. J Am Stat Asso 84: 1096
- Pereira T V., Patsopoulos N, Salanti G, Ioannidis JP (2010) Critical interpretation of Cochran’s Q test depends on power and prior assumptions about heterogeneity. Res Synth Methods 1: 149-161.
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539-1558.
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. 2015 2015.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, et al. (2009) Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 302: 741-749.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, et al. (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363: 733-742.
- Tattersall MH, Martin A, Devine R, Ryan J, Jansen J, et al. (2014) Early Contact with Palliative Care Services: A Randomized Trial in Patients with Newly Detected Incurable Metastatic Cancer. J Palliat Care Med 4:170.
- Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, et al. (2014) Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 383: 1721-1730.
- Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, et al. (2015) Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 33: 1438-1445.
- McCorkle R, Jeon S, Ercolano E, Lazenby M, Reid A, et al. (2015) An Advanced Practice Nurse Coordinated Multidisciplinary Intervention for Patients with Late-Stage Cancer: A Cluster Randomized Trial. J Palliat Med 18: 962-969.
- Maltoni M, Scarpi E, Dall’Agata M, Zagonel V, Bertè R, Ferrari D, et al. (2016) Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 65:61-68.
- Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, et al. (2016) Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol 2016;35:834-841.
- Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, et al. (2009) Reliability and Validity of the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-Pal) Scale. J Pain Symptom Manage 37: 23-32.
- Cella D, Hahn EA, Dineen K (2002) Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual Life Res 11: 207-221.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, et al. (1995) Reliability and validity of the functional assessment of cancer therapy-lung (FACT-L) quality of life instrument. Lung Cancer 12: 199-220.
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7: 6-9.
- Cooley ME, Short TH, Moriarty HJ (2003) Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Â Psychooncology 12: 694-708.
- Kroenke K, Spitzer RL, Williams JB (2001) Â The PHQ-9. Validty of a Brief Depression Severity Measure. J Gen Intern Med 16: 606-613.
- Okun A, Stein RE, Bauman LJ, Silver EJ (1996) Content validity of the Psychiatric Symptom Index, CES-depression Scale, and State-Trait Anxiety Inventory from the perspective of DSM-IV. Psychol Rep 79: 1059-1069.
- Zigmond AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67:361-370.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I (2008) Effectiveness of specialized palliative care: a systematic review. Jama 299: 1698-1709.
- Parikh RB, Kirch RA, Smith TJ, Temel JS (2013) Early Specialty Palliative Care — translating data in oncology into practice. N Engl J Med 369: 2347-2351.
- Kontopantelis E, Reeves D (2012) Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: A simulation study. Stat Methods Med Res 21: 409-426.
Citation: Zanghelini F, Zimmermann IR, Andrade CAS, Wu O (2018) Early Palliative Care for Improving Quality of Life and Survival in Patients with Advanced Cancer: A Systematic Review and Meta-analysis. J Palliat Care Med 8: 343. DOI: 10.4172/2165-7386.1000343
Copyright: © 2018 Zanghelini F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7175
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 6134
- PDF downloads: 1041