Effect of Carvacrol Tested on Different in vivo and in vitro Experimental Studies: Systematic Review
Received: 12-Feb-2018 / Accepted Date: 21-Feb-2018 / Published Date: 23-Feb-2018 DOI: 10.4172/2167-065X.1000181
Abstract
The objective of the study was to show carvacrol isolad in in vitro and in vivo assays to verify the effects of their dosages on different application areas. The searches were realized in the following databases, pubmed, and academic google using the descriptors carvacrol; carvacrol and in vitro; carvacrol and in vivo. Thus that in academic google we still use in the advanced search, exclusion of patents and references, as well the inclusion the articles that had the descriptors present in the title. For eligibility, there were 13 articles for analysis. In which, the carvacrol proved to be effective with antimicrobial action, anti-carcinogenic, immune system cell protector, reducer of gastrointestinal lesions, optimizer anti-carcinogenic drug in nanoparticles, as well as mitotic index reducer in bone marrow cells used in high dosages. In conclusion, it is understood that the dosage applied in the right amount can be efficient in the cancer control and elimination of bacteria, in which the carvacrol acts as promising herbal remedy for clinical use.
Keywords: Carvacrol; Dosage; Systematic review
Introduction
Some phytochemicals derived from plants have been very commonly used for the prevention and treatment of different types of diseases due to the belief in their safety from the use in ancient cultures, traditions and societies [1].
The Carvacrol is a monoterpenoid (C10H14O) phenolic component extracted of thyme, oregano and marjoram that belongs to a class of phenols that have a ten-carbon unit and are present in the essential oils of many plants (Origanum vulgare, Pepperwort, Wild Bergamot, Satureja, Origanum marjorana, Nigella sativa and Tequila), being recognized as a safe substance by the Food and Drug Administration [2-4].
Carvacrol molecules have been incorporated as useful ingredients in various food products, in the agricultural, pharmaceutical, perfumery, cosmetic, and flavor industries. Besides the use in these types of industries, its biological and pharmaceutical properties have been used in anti-inflammatory, antimicrobial, analgesic, anticancer, and antioxidant processes [2,5].
Thus, in recent decades, the pharmaceutical industry has shown great interest in the research of plants as a source for new structures and for the development of standardized herbal agents with proven effectiveness, safety and quality. This interest is increased for several reasons such as: consumer preferences for natural therapies, high cost and side effects of synthetic drugs and the belief that natural products are innocuous [6].
There are many cases in which natural products exert multiple pharmacological effects, being a practical prerequisite to identify highly effective multi-acting drugs for simultaneous treatment of multifactorial symptoms of chronic diseases [7,8].
Since carvacrol is a substance found in several plants as mentioned above, have as goal to show the effect of carvacrol alone in in vitro and in vivo tests and their dosages for different application areas.
Methods
The searches were conducted in the following databases, pubmed and academic google using the descriptors “carvacrol; carvacrol and in vitro; carvacrol and in vivo “. So in academic google we still use in the advanced search to exclude patents and references, as well as include the articles that had the descriptors present in the title and publication period from 1990 to 2017.
We selected the original studies that used animals or animal tissue. Being excluded articles where the carvacrol was in the extract or combined with other substances, dietary intake, vegetable action or microcapsule and review articles.
Results And Discussion
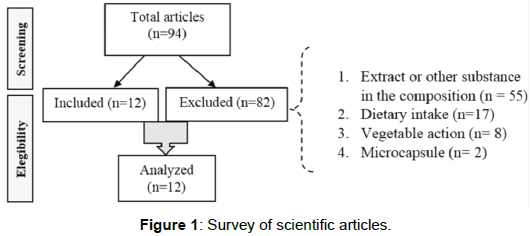
The Figure 1 shows the screening of scientific articles, demonstrate a total of 94 articles found in the search, where 12 articles were included for analysis. A total of 82 articles were excluded because they presented the carvacrol still in the form of extract or still to be used in the protocol associated with other substances, vegetal action, dietary intake and microcapsule.
In the Table 1 we have the approach of two articles with in vitro and in vivo analysis, showing important aspects such as reduction in colony formation in Campylobacter sp infected chickens and reduction of Echinococcus granulosus cyst in mice [9,10].
| Carvacrol (Dosage) | Study methods | Effects | Reference |
|---|---|---|---|
| 120, 200 and 300 mg/kg | Infection of chickens by Campylobacter sp. | Delay in formation of Campylobacter sp. colonies inducing changes in the intestinal microflora. | [9] |
| 40 mg/kg | Against Echinococcus granulosus cyst in mice developed over 4 months. | Reduction of treated cyst weight for 20 days. | [10] |
Table 1: Different approaches to carvacrol in vitro and in vivo.
In the Table 2 we have three large areas related to the approach in the studies, firstly effects with anticancer activity being evidenced in four studies, demonstrating cellular apoptosis and reduction of Bcl-2, reduction of gastric cancer with use of nanoparticles, reduction of intestinal lesions, and genotoxicity in lymphomas. In addition, antimicrobial activity was also found. Finally, effects such as inhibition of dendritic cells as well as protective effect of lymphocytes.
| Carvacrol (Dosage) | Study methods | Effects | Reference |
|---|---|---|---|
| 31.21 and 62.5 µg mL-1 | Aplication of Coleus aromaticus | A375 cells treated with carvacrol for 24 h induced apoptosis by cleavage of PARP and reduction of Bcl-2 gene expression. It can induce apoptosis by direct activation of the mitochondrial pathway, which plays a vital role in the anticancer effect. | [11] |
| 30, 1070 and 120 μg/ml | Activity of human serum albumin (HSA) nanoparticles against gastric cancer. | Carvacrol in combination with chemotherapy agents in HSA nanoparticles can treat cancer cells better than single drug loaded nanoparticles. | [12] |
| 0,20 μg/g | Controled of Clostridium perfringens in broilers. | Relief of intestinal lesions in broiler chickens, which may contribute to the control of C. perfringens infection in broiler chickens. | [13] |
| 0-2500 mM 700 Μm |
Carvacrol in vitro genotoxicity test performed in micronucleus (MN) and rat lymphomas assay. | Carvacrol presented small genotoxic effects, but only in the MN test at the highest concentration tested (700 μM) and in the absence of metabolic activation. | [14] |
| 50 and 100 μg/ml | Inhibitory effects of carvacrol on dendritic cells (DCs). | Suppressive effects of carvacrol on DCs on maturation and function, as well as T-cell responses. | [14] |
| MBC90=600 μg/ml | Antimicrobial effects of five natural substances against 50 clinical isolates of Mycoplasma hominis. | The Carvacrol had strong antimicrobial activity and the antimicrobial agent in the treatment of mycoplasmatic infections. | [15] |
| 0,257 mg/ml | Tratamento contra Staphylococcus aureus, Bacillus cereus Escherichia coli. |
Antimicrobial activity against Staphylococcus aureus, Bacillus cereus and Escherichia coli. | [16] |
| 5 – 100 mM | Antibiotic growth promoters in the pig nutrition. | Protective effect on lymphocytes, significantly increasing the IC50 to 516 ± 87 lM. | [17] |
Table 2: Different approaches to carvacrol in vitro.
Carvacrol at the non-cytotoxic concentrations for DCs used in the compounds did not decrease the viability of DCs at concentrations up to 10 mg/ml. Therefore, concentrations of 0.1, 1 and 10 mg/ml for and subsequent experiments in DC, at where doses were harmful to both DC and T cells, a decrease in mouse lymphocytes was also obtained in the present study (reducing the proliferation index at doses greater than 10 mg/ml), indicating its immunosuppression [4].
However, the dosage of cravacrol being used and adjusted and balanced can be very efficient as anticancer as presented in some studies.
The antiproliferative activity and cytotoxicity on human melanoma cancer (A375) cells; its effect on cell cycle control, DNA fragmentation and apoptosis by the cleavage of poly-(ADPribose)-polymerase (PARP) and Bcl-2 gene expression was examined, the same can induce apoptosis by direct activation of the mitochondrial via, which plays a vital role in the anticarcinogen effect [11].
This aspect is also demonstrated in the use of carvacrol by optimizing other drugs to combat gastric cancer by improving the action of nanoparticles [12], as well as in stomach lesions decreasing infections that can progress to cancer [13]. As well as decreased metabolic activity in rat lymphoma with genotoxic action at high doses [14].
In this way the dosages applied in the correct proportions can be very effective in the fight against cancer.
In addition, the studies point to another important antimicrobial action demonstrated in the combat against various types of bacteria such as Mycoplasma hominis [15], Staphylococcus aureus, Bacillus cereus and Escherichia coli [16] and Campylobacter sp, where this immunoprotective aspect may assist in strengthening the organism against pathological agents, improving, for example, the action of lymphocytes [17].
In Table 3, the in vivo approach demonstrated fundamental aspects such as half-life, half-life and depletion of carvacrol, as well as the effect of mitotic index reduction on bone marrow cells.
| Carvacrol (Dosage) | Study methods | Effects | Reference |
|---|---|---|---|
| 0.001 and 0.0005 μg ml-1, respectively, in plasma and all tissues except for fat both LOQ of 0.01 μg ml-1 and a LOD of 0.005 μg ml-1 | Topical and intra-mammary product. The levels were measured in plasma, liver, kidney and fat up to 72 h after the last dose. | Semi-life plasma levels were reduced to carvacrol approximately 1.5 h, while wich the estimated half-lives in tissues ranging from 16.9 at 25 h for carvacrol. The predicted amount of time of the molecules found in the body based on the slower depletion time in liver tissue was 10 days for carvacrol. |
[18] |
| 30, 50 and 70 mg/kg b.w. | Bone marrow cells from rats. | Decreased of mitotic index. | [19] |
Table 3: Different approaches to carvacrol in vivo.
Important aspects are still fundamental in preclinical research, since carvacrol presents great clinical potential to combat pathologies related of bacteria or disorders of excess cell proliferation, thus understanding the half-life and the half-life in the carvacrol [18] is elementary to use effectively in the clinic, as well as the moment of intervention with this phytotherapic, since the same in certain dosages can decrease the cellular division [19], being thus a strong combatant of cells carcinogenic in the early stages.
As study limitations we include the use of only two databases, although they are the most visited and used in the research and no further investigation of the selected studies, such as the statistical analysis study.
Conclusion
Therefore, it is understood that the dosage applied in the right amount can be efficient in the cancer control and elimination of bacteria, using the carvacrol as a promising herbal remedy for clinical use.
References
- Bacanl M, Aydın S, Başaran AA, Başaran N (2017) Are all phytochemicals useful in the preventing of DNA damage? Food Chem Toxicol 109: 210-217.
- Rajput JD, Bagul SD, Pete UD, Zade CM, Padhye SB, et al. (2017) Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol Divers 2017: 1-21.
- Thompson JD, Chalchat JC, Michet A, Linhart YB, Ehlers B (2003) Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J Chem Ecol 29: 859-880
- Amirghofran Z, Ahmadi H, Karimi MH, Kalantar F, Gholijani N (2016) In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm Biol 54: 1125-1132.
- Arunasree K (2010) Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 17: 581-588.
- Calixto J (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 33: 179-189.
- Kimura I (2006) Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi 126: 133-143.
- Pothier J, Galand N, El Ouali M, Viel C (2001) Comparison of planar chromatographic methods (TLC, OPLC, AMD) applied to essential oils of wild thyme and seven chemotypes of thyme. Farmaco 56: 505-511.
- Kelly C, Gundogdu O, Pircalabioru G, Cean A, Scates P, et al. (2017) The in vitro and in vivo effect of carvacrol in preventing campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog Dis 14: 341-349.
- Fabbri J, Maggiore MA, Pensel PE, Denegri GM, Gende LB, et al. (2016) In vitro and in vivo efficacy of carvacrol against Echinococcus granulosus. Acta Tropica 164: 272-279.
- Govindaraju S, Arulselvi PI (2017) Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. J Herbs Spices Medicinal Plants 2017: 1-15.
- Maryam K, Shakeri S, Kiani K (2015) Preparation and in vitro investigation of antigastric cancer activities of carvacrol-loaded human serum albumin nanoparticles. IET Nanobiotechnology 9: 294-299.
- Du E, Gan L, Li Z, Wang W, Liu D (2015) In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol 6: 58.
- Maisanaba S, Prieto AI, Puerto M, Gutiérrez-Praena D, Demir E, et al. (2015) In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays. Mutat Res Genet Toxicol Environ Mutagen 784: 37-44.
- Sleha R, Mosio P, Vydrzalova M, Jantovska A, Bostikova V, et al. (2014) In vitro antimicrobial activities of cinnamon bark oil, anethole, carvacrol, eugenol and guaiazulene against Mycoplasma hominis clinical isolates. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 158: 208-211.
- Keawchaoon L, Yoksan R (2011) Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B Biointerfaces 84: 163-171.
- Bimczok D, Rau H, Sewekow E, Janczyk P, Souffrant WB, et al. (2008) Influence of carvacrol on proliferation and survival of porcine lymphocytes and intestinal epithelial cells in vitro. Toxicol In Vitro 22: 652-658.
- Mason SE, Mullen KAE, Anderson KL, Washburn SP, Yeatts JL, et al. (2017) Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2017: 1-10.
- Azirak S, Rencuzogullari E (2008) The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol 23: 728-735.
Citation: Ferreira AC, Mostarda CT, Dias CJ, Paixao LC, Pires FDO, et al. (2018) Effect of Carvacrol Tested on Different in vivo and in vitro Experimental Studies: Systematic Review. Clin Pharmacol Biopharm 7: 181. DOI: 10.4172/2167-065X.1000181
Copyright: ©2018 Ferreira AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8702
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 7662
- PDF downloads: 1040