Research Article Open Access
Effect of Exogenous Phosphocreatine on Ito, Ina And Ica,L in IschemicVentricular Myocytes of Rat
Shi Xiang-Min*, Li Tian-De, Wang Yu-Tang, Chen Qi, Yang Ting-Shu and Shan Zhao-Liang
Department of Cardiology, General Hospital of PLA, Beijing 100853, China
*Corresponding Author:
- Shan Zhao-Liang
Department of Cardiology
General Hospital of PLA
Beijing 100853, China
Tel: +86 10 55499210
Fax: +86 10 55499209
E-mail: shanzl301@sina.com
Received February 10, 2016; Accepted April 20, 2016; Published April 25, 2016
Citation: Xiang-Min S, Tian-De L, Yu-Tang W, Ting-Shu Y, Zhao-Liang S (2016) Effect of Exogenous Phosphocreatine on Ito, Ina And Ica,L in Ischemic Ventricular Myocytes of Rat. J Pharmacokinet Exp Ther 1:104. doi:10.4172/jpet.1000104
Copyright: © 2016 Xiang-Min S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pharmacokinetics & Experimental Therapeutics
Abstract
Aim: The aim of this study is to determine the effect of exogenous phosphocreatine (PCr) with different concentration on transient outward potassium (Ito), natrium (INa) and L-type calcium (ICa,L) current in ischemic ventricular cells of rat and to explore its role in the treatment of ischemic heart disease. Methods: Ventricular cells were isolated enzymatically from left ventricular of rat. Peak Ito, INa and ICa,L current were recorded using patch clamp techniques in the whole-cell configuration in the setting of cells super fused with normal Tyrode solution, simple simulated ischemic solution (SI), ischemic solution containing PCr with concentration of 5, 10, 20, 30 mmol/L for 10 min respectively. Results: Compared with simple simulated ischemic solution, peak Ito, INa and ICa,L current and current density of ischemic solution containing PCr of 5, 10, 20, 30 mmol/L significantly improved (p<0.05). There were statistical significance among PCr of 10 and 0, 5 mmol/L for Ito, INa and ICa,L, no significant difference were found among10, 20 and 30 mmol/L for Ito and ICa,L (p>0.05). Compared with PCr of 10 mmol/L, peak INa current and density decreased in that of 20 and 30 mmol/L (p<0.05). Conclusion: PCr could partly reverse the inhibition of Ito, INa and ICa,L current of rat ventricular cells under ischemic condition, which could be the ionic basis of therapeutic role in the treatment of ischemic heart disease. 0 ~ 10 mmol/L PCr exerted significant dose-effect relationship.
Keywords
Phosphocreatine; Ventricular cells; Patch clamp; Ito, INa and ICa,L current; Ischemia; Rat
Introduction
Coronary Heart Disease (CHD) is one of the most common cardiovascular diseases in clinical practice, which could proceed to Acute Coronary Syndrome (ACS) and result in malignant arrhythmias, sudden death, as well as heart failure [1]. Over the past few decades, percutaneous coronary intervention and Coronary Artery Bypass Graft (CABG) [2] have become established approach in the treatment of CHD, meanwhile, intensive drug therapy, including statin [3], antiplatelet [4], β-blocker and vasodilator, have been proved to effectively prevent progression to ACS and improve prognosis and quality of life for patients with CHD, with the advent of above-mentioned therapy, mortality rate of ACS was significantly reduced, however, the number of patients with ischemic cardiomyopathy and heart failure increased [5], even some novel drugs [6] have been introduced into the treatment of heart failure, the long term effect was still unsatisfactory. Metabolism optimization therapy is another promising alternative, trimetazidine [7], levocarnitine [8] and phosphocreatine (PCr) [9] supplement were believed to exert definite effects in the treatment of ischemia related heart disease, however, which was often ignored by physicians and not listed as the first line medication. PCr has been applied in clinical practice over two decades; early animal experimental and clinical study demonstrated its safety and effectiveness in the presence of ischemia [10], in recent years, PCr has been increasingly administrated by athletes and proved to improve performance [11]. PCr added to arrest solution could facilitate restoration of heart function for patients undergoing cardiac surgery [12], simultaneously, it play a role in the treatment of acute myocarditis, Of which, CHD and ischemia related heart failure are the most common target diseases for PCr administration.
PCr is a pivotal substrate for ATP synthesis via the shuttle mechanism [13], many studies delineated that patients with CHD could benefit from administration of PCr [14], but the underlying ionic mechanism remain unclear. Intracellular ATP shortage could give rise to inhibition of transient outward potassium (Ito), natrium (INa) and L-type calcium (ICa,L) current, leading to arrhythmia and reduced ventricular contractility [15]. The aim of this study is to investigate the effect of PCr on Ito, INa and ICa,L current of ischemic rat ventricular cells and to explore its role in the treatment of ischemic heart diseases and related heart failure.
Materials and Methods
Materials
The following solutions were prepared: (1) Tyrode solution (in mmol/L): CaCL2 1.8, NaCL 116, KCL 5.4, NaHCO3 15, NaH2PO4 1.4, MgSO4 1, Glucose 15, Taurine 30, pH was adjusted to 7.4 with HCL; (2) Ca++-free Tyrode solution (in mmol/L): NaCL 116.0, KCL 5.4, NaHCO3 15, NaH2PO4 1.4, MgSO4 1, glucose 15, Taurine 30, gassed with 95% O2 plus 5% CO2, pH was adjusted to 7.4 with HCL; (3) KB medium (in mmol/L): KOH 90, L-glutamic acid 70, Taurine 20, KCL 30, KH2PO4 10, HEPES 10, D-glucose 10, EGTA 0.5, pH was adjusted to 7.3 with KOH; (4) Internal pipette solution for Ito current recording (in mmol/L): KCL 140, MgCL2 0.53, EGTA 10, HEPES 10, pH was adjusted to 7.3 with KOH; (5) External pipette solution for Ito current recording (in mmol/L): NaCL 116, KCL 5.4, NaHCO3 15, NaH2PO4 1.4, MgSO4 1, Glucose 15, Taurine 30, BaCl2 1, CoCl2 2, pH was adjusted to 7.4 with HCL; (6) Internal pipette solution for INa current recording (in mmol/L): CsCL 120, EGTA 11, HEPES 10, Na2-ATP 5, MgCL2 5, CaCL2 1.0, Glucose 11, urrent recording (in mmol/L): NaCL 40, Choline-CL 100, KCL 5.4, MgCL2 1.0, CaCL2 0.1, HEPES 10, Glucose 10, NaHPO4 0.33, pH was adjusted to 7.3 with NaOH; (8) Internal pipette solution for ICa,L current recording (in mmol/L): CsCl 120, MgATP 5, MgCL2 0.5, EGTA 14, HEPES 10, Glucose 5.5, pH was adjusted to 7.4 with CsOH; (9) External pipette solution for ICa,L current recording (in mmol/L): Choline-CL 120, CaCL2 2, MgCL2 2, CsCl 4, Glucose 10, pH was adjusted to 7.35 with CsOH; (10) Simulated ischemic solution (SI) (in mmol/L): NaCL 123, KCL 10, NaH2PO4 0.9, MgSO4 0.5, CaCL2 2.7, pH was adjusted to 6.8 with NaOH, 0.5 mmol/L glibenclamide was added to all solution with the aim of blocking IKATP current. All the chemicals including protease E, EGTA, BSA, HEPES, Taurine were obtained from Sigma Chemical (St Louis, MO). Exogenous phosphocreatine (PCr) was provided from Aassermann company (Italy) ├ú┬?┬?30 wista rats (weight 150 ± 15 g) were provided by animal experiment center of general hospital of PLA. The instance age of rats ranged from 80-90 days, male and female rats accounted for 50% respectively.
Ventricular cells isolation
Single left ventricular cells were isolated enzymatically from rat, using previously described methods [16]. Briefly, the heart was rapidly excised and perfused via the coronary artery with Ca++-free Tyrode solution for 5 min, followed by Ca++-free Tyrode solution containing protease E, 0.5 mg/mL BSA and 150 μmol/mL CaCl2 for 5 min until the heart became soft, all solutions were gassed with 95% O2 plus 5% CO2 and warmed to 37°C, the perfusion pressure maintained at 70 cm H2O. Left ventricular was separated and cut into small pieces; isolated ventricular cells were stored in KB medium after filtration. The obtaining rate of calcium-tolerant ventricular M cells was 70-80%. Only rod shaped non-contracting cells with clear cross striations were used for the whole-cell patch clamp studies.
Whole-cell patch clamp recording
Based on the conventional Hamil method, ventricular cells were placed in a chamber on the stage of an inverted microscope (Nikon Japan). After 5 min of deposition, cells adhered to the bottom of chamber. Ito, INa and ICa,L current were recorded with whole-cell patch-clamp techniques (patch clamp amplifier, Axon patch 700 A, Axon Instrument Co, USA). Amplifier was connected with personal computer. The experimental protocol and data acquisition were performed with Pclamp 8.2 software (Clampex, Axon Instrument Co, USA). Micropipettes were pulled by a continuous two-step puller (P-97, Sutter Instrument Co, USA), which had a tip resistance of 2-4 MΩ with average diameter of 2-3 um when filled with internal pipette solution. Liquid junction potentials were corrected after the pipette immersed in the solution. After a tight pipette–membrane seal obtained (seal resistance>1 GΩ), fast capacitance was compensated, then the membrane was ruptured with gentle suction to obtain the whole-cell voltage-clamp configuration and slow capacitance as well as series resistance were compensated.
Method of recording Ito, INa and ICa,L
Isolated cells were assigned to the following 6 groups: Tyrode solution; simple simulated ischemic solution; ischemic solution containing PCr with concentration of 5, 10, 20, and 30 mmol/L. In each group 8 cells were tested. The chamber was continuously superfused with test solution at a speed of 2 ml/min at 37°C after suction of cells. Tyrode solution was gassed with 95% O2+5% CO2; other groups gassed with 95% N2+5% CO2. 10 min later, peak Ito, INa and ICa,L current were recorded in every group and expressed by current density. Currents amplitudes were calculated as difference between peak inward and steady-state currents. To get rid of the effect of capacitance on the peak amplitude, current density was compared between different groups. The Ito current was evoked by 250 ms step depolarization between –30 mV and +70 mV with a 10 mv increment from a holding potential of -40 mV, stimulation frequency 0.5 Hz. The INa current was evoked by 60 ms step depolarization between –90 mV and +60 mV with a 10 mv increment from a holding potential of -90 mV. The ICa,L current was evoked by 250 ms step depolarization between –40 mV and +50 mV with a 10 mv increment from a holding potential of -40 mV. Currentvoltage relationship (I-V) curves was generated by applying a series depolarizing pulse from a holding potential to different membrane potential with a 10 mV increment.
During the process of experiment, PO2 and SO2 of perfusion solution were measured by blood gas analyzer (Nova biomedical USA), which indicated PO2>60 mm Hg and SO2>90% in Tyrode solution, as well as PO2<60 mm Hg and SO2<90% in simulated ischemic solution. The osmolality of perfusion solution was monitored (JC216-8P, Beijing) and varied between 290-320 mOsm.
Data Analysis
Statistical analysis was performed by SPSS 12.0 (Beijing Stats Data Mining Co. Ltd, Beijing, China). One-Way Analysis Of Variance (ANOVA) was adopted to test the difference of all groups, and Dunnett (double side) test was used in the comparisons among groups. All results were expressed as mean ± SE, P<0.05 was considered statistical significant.
Results
Characteristics of Ito, INa and ICa,L current and steady-state current-voltage relation
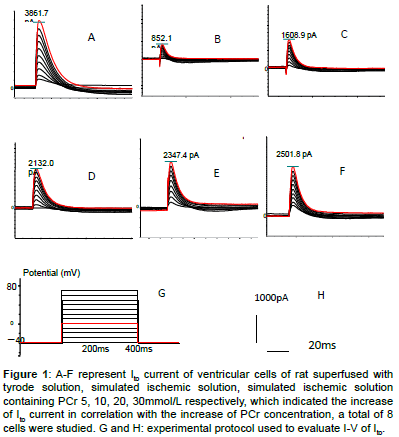
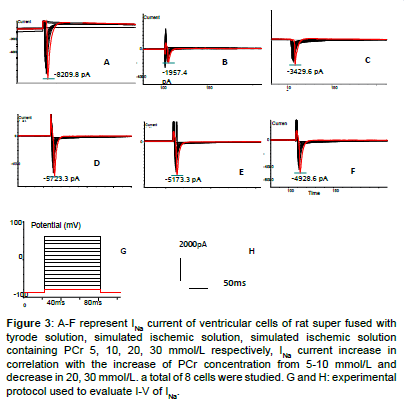
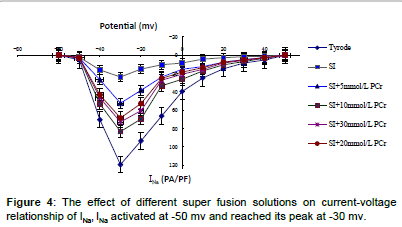
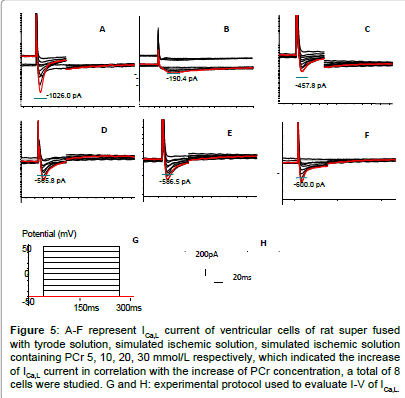
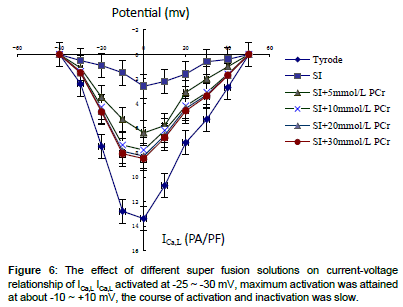
High amplitude outward (Ito) and inward (INa) (ICa,L)current was recorded in Tyrode solution group which presented quick activation and inactivation process, as well as voltage-dependent character (Figures 1-5). Ito activated at -30 mv and reached its peak at 70 mv. INa activated at -50 mv and reached its peak at -30 mv. Threshold for ICa,L activation was around -25 ~ -30 mV, maximum activation was attained at about -10 ~ +10 mV, the course of activation and inactivation was slow (Figure 6). Compared with Tyrode solution, peak Ito, INa and ICa,L current in cells superfused with simulated ischemic solution remarkably inhibited (p<0.05) (Table 1).
Figure 1: A-F represent Ito current of ventricular cells of rat superfused with tyrode solution, simulated ischemic solution, simulated ischemic solution containing PCr 5, 10, 20, 30mmol/L respectively, which indicated the increase of Ito current in correlation with the increase of PCr concentration, a total of 8 cells were studied. G and H: experimental protocol used to evaluate I-V of Ito.
Figure 3: A-F represent INa current of ventricular cells of rat super fused with tyrode solution, simulated ischemic solution, simulated ischemic solution containing PCr 5, 10, 20, 30 mmol/L respectively, INa current increase in correlation with the increase of PCr concentration from 5-10 mmol/L and decrease in 20, 30 mmol/L. a total of 8 cells were studied. G and H: experimental protocol used to evaluate I-V of INa.
Figure 5: A-F represent ICa,L current of ventricular cells of rat super fused with tyrode solution, simulated ischemic solution, simulated ischemic solution containing PCr 5, 10, 20, 30 mmol/L respectively, which indicated the increase of ICa,L current in correlation with the increase of PCr concentration, a total of 8 cells were studied. G and H: experimental protocol used to evaluate I-V of ICa,L.
| Solution | Peak current├»┬╝┬?PA├»┬╝┬? | Peak current density├»┬╝┬?PA/PF├»┬╝┬? | |
|---|---|---|---|
| Tyrode | Ito | 3668.4 ± 444.3 | 53.9 ± 6.5 |
| INa | 8360.8 ± 628.4 | 119.4 ± 8.9 | |
| ICa,L | 955.4 ± 113.2 | 13.4 ± 1.4 | |
| SI | Ito | 877.1 ± 232.3 | 12.9 ± 3.4 |
| INa | 1734.3 ± 211.8 | 23.7 ± 3.0 | |
| ICa,L | 184.6 ± 47.8 | 2.6 ± 0.7 | |
| SI+5mmol/LPCr | Ito | 1574.6 ± 354.2Δθ | 23.2 ± 5.2Δθ |
| INa | 3523.5 ± 384.2Δθ | 52.4 ± 5.6Δθ | |
| ICa,L | 439.1 ± 63.5Δθ | 6.2 ± 0.9Δθ | |
| SI+10mmol/LPCr | Ito | 2187.5 ± 379.3Δ | 32.2 ± 5.6Δ |
| INa | 5803.7 ± 433.5Δ | 83.2 ± 6.4Δ | |
| ICa,L | 560.7 ± 73.5Δ | 7.8 ± 1.1Δ | |
| SI+20mmol/LPCr | Ito | 2407.8 ± 355.8Δ* | 35.4 ± 5.2Δ* |
| INa | 5112.4 ± 461.7Δθ | 72.8 ± 6.6Δθ | |
| ICa,L | 588.4 ± 70.5Δ* | 8.3 ± 1.0Δ* | |
| SI+30mmol/LPCr | Ito | 2486.3 ± 386.3Δ* | 36.6 ± 5.7Δ* |
| INa | 4835.4 ± 396.4Δθ | 68.5 ± 5.7Δθ | |
| ICa,L | 605.8 ± 81.5Δ* | 8.5 ± 1.3Δ* | |
Table 1: Peak Ito, INa and ICa,L current and density in rat ventricular myocytes, super fused with different solution ( x ± s ; n=8). Δ p<0.05 vs SI; * p>0.05 vs SI +10m mol/L PCr; θp<0.05 vs SI+10m mol/L PCr.
Effect of PCr with different concentration on Ito INa and ICa,L in the presence of ischemia
In relation to elevated PCr concentration in simulated ischemic solution, peak Ito, INa and ICa,L current and density gradually improved, Compared with cells superfused with simple simulated ischemic solution, for ischemic solution with PCr of 5, 10, 20, 30 mmol/L, peak Ito current density increased by 79.8%, 149.6%, 174.4%, 183.7% respectively; peak INa current density improved by 121.0%, 251.0%, 207.1%, 189.0% respectively, peak ICa,L current density improved by 138.4%, 200%, 219.2%, 226.9% respectively. There were statistical significance among PCr of 10 and 0, 5 mmol/L for Ito, INa and ICa,L, no significant difference were found among10, 20 and 30 mmol/L for Ito and ICa,L (p>0.05). Compared with PCr of 10 mmol/L, peak INa current and density decreased in PCr of 20 and 30 mmol/L (p<0.05).Within the concentration of 0-10 mmol/L, PCr exerted a prominent dosedependent manner on current density improvement, however, if the concentration was greater than 10 mmol/L, this effect no longer existed (Table 1 and Figures 1-6).
The I-V curve position of cells superfused with Tyrode solution was higher than that of simulated ischemic solution or mixed with different concentration of PCr, but the maximum Ito INa and ICa,L current amplitude still occurred at 70 mv, -30 mv and 0 mv, meanwhile, the activation potential and the morphology of I-V curve remained unchanged (Figures 2, 4 and 6).
Discussion
Coronary Heart Disease (CHD) and ischemic cardiomyopathy are prevalent in the world, subsequent heart failure and potential malignant ventricular arrhythmia are life threatening, although Implantable Cardioverter/Defibrillator (ICD) [17] or Cardiac Resynchronization Therapy (CRT) [18] can decrease mortality, the high cost and devicerelated complication prevent them from being available to majority of patients, meanwhile, some Antiarrhythmia Drugs (AAD) have potential proarrhythmic effects and been restricted. Pharmacological interventions are still key approach in the treatment of heart failure.
The result of this study demonstrated that exogenous PCr could partly restore the inhibited ion currents of ventricular cells in the setting of ischemia, even the concentration of PCr was not lineally correlated with the increase of Ito, INa and ICa,L current, the significant improvement still could be observed in lower PCr concentration group compared with that of simple ischemia solution. It is well known that ion channel disturbance exerts great effect in impaired cardiac contractility and arrhythmia formation, so we deduced that ion current increase contributed to the clinical improvement for CHD patients receiving PCr treatment.
Ito is the key outward potassium current in early stage of action potential with character of rapid voltage-dependent activation and inactivation [19], it has been proved that inhibition of Ito in ischemia would more significantly prolong the Effective Refractory Period (ERP) of Mid-Cardium Cells (M Cells) than that of endocardial and epicardial cells, consequently amplifying Transmural Repolarization Dispersion (TRD) [20], which could be one of the underlying mechanism of arrhythmia. INa is responsible for 0 phase of action potential; therefore, influencing conduction velocity between ventricular cells, which exhibits rapid activation and inactivation pattern similar to that of Ito, many studies demonstrated that INa could be remarkably inhibited in the presence of ischemia, leading to impaired intercellular conduction and facilitating reentry formation, which contribute to development of malignant arrhythmia [21]. ICa,L plays an important role in initiating myocardial contraction, ischemia related ICa,L inhibition closely correlated with extent of heart failure [22]. It was clear that intracellular ATP shortage gave rise to Ito, INa and ICa,L inhibition due to ischemia, therefore increasing intracellular ATP synthesis could probably reverse impaired current. It has been reported that IKATP could augment largely and was responsible for the increased outward potassium during ischemia [23]. With the purpose of eliminating influence of IKATP, IKATP blocker, glibenclamide was added to all perfusion solution. Some studies confirmed that intercellular ATP level rapidly dropped with the accumulation of ADP during ischemia, as a normal physical response with the purpose of maintaining ATP level, ATP synthesis was initiated by means of Lomman reaction employing substrate of ADP and PCr (ADP+PCr→ATP+Cr), which was closely correlated with decomposition of ATP, finally the net intercellular metabolic result was expressed as PCr→Cr+Pi, the level of PCr dramatically decreased [24]. In our experiment, supplement of different concentration of exogenous PCr exerted its remarkable effect on increasing Ito, INa and ICa,L peak current and current density, PCr is known as the fundamental substrate for ATP synthesis, therefore supplement of exogenous PCr could be a effective and feasible way to improve intercellular ATP under the condition of ischemia, Conway [25] demonstrated that PCr marked with 14C, 97P could readily penetrate membrane of myocytes in ischemia, meanwhile, Perepech [9] suggested that PCr or PCr combined with thrombolysis therapy could significantly reduce fatal arrhythmia within 6 hours in acute myocardial infarction.
Our study showed that the concentration of exogenous PCr did not lineally correlate with current improvement, PCr with concentration ranging from 0-10 mmol/L significantly improved peak current and current density, as concentration greater than 10 mmol/L, PCr displayed minor improvement on Ito and ICa,L current, there were no statistical difference among simulated ischemic solution containing PCr of 10, 20, 30 mmol/L. This result suggested exogenous PCr could promote ATP synthesis via another pathway rather than simple substrate of ATP. If PCr merely serve as substrate of ATP, the dosage in our experiment could not meet the abundant ATP need in whole body, another key mechanism responsible for this sign is that PCr could protect the pool of Σ(ATP+ADP+AMP) via inhibiting 5’-nucleotidase and maintain the substrate of ATP [24-26], this function may reach a plateau when the concentration exceed 10 mmol/L [27], which could explain the dose-effect curve between PCr and current, this result reconfirmed that 10 mmol/L of PCr was the optimal concentration for the treatment of ischemia heart disease in many clinical practice [28].
In terms of INa current, PCr at concentration of 0-10 mmol/L could significantly improve its current and current density similar to Ito and ICa,L, however, as concentration greater than 10 mmol/L, there was a trend of current decrease, and statistical significance could be found among10, 20 and 30 mmol/L. The underlying mechanism of this phenomenon could be attributable to inhibitive effect of higher concentration of PCr on INa, previous study revealed that PCr could exert its effect similar to class I antiarrhythmia drugs [29]. This character supported that 10 mmol/L could be the best therapeutic concentration for ischemia heart disease.
References
- Hung J, Teng TH, Finn J, Knuiman M, Briffa T, et al. (2013) Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population-based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc 2: e000172.
- Silvain J, Cayla G, Collet JP, Fargeot C, Montalescot G (2014) [Coronary stents: 30 years of medical progress]. Med Sci (Paris) 30: 303-310.
- Pursnani A, Mayrhofer T, Ferencik M, Hoffmann U (2014) The 2013 ACC/AHA cardiovascular prevention guidelines improve alignment of statin therapy with coronary atherosclerosis as detected by coronary computed tomography angiography. Atherosclerosis 237: 314-318.
- Messori A, Fadda V, Gatto R, Maratea D, Trippoli S (2014) New oral anticoagulants in acute coronary syndrome: is there any advantage over existing treatments? IntCardiovasc Res J 8: 124-126.
- Faxon DP (2005) Coronary interventions and their impact on post myocardial infarction survival. ClinCardiol 28: I38-44.
- George M, Rajaram M, Shanmugam E, VijayaKumar TM (2014) Novel drug targets in clinical development for heart failure. Eur J ClinPharmacol 70: 765-774.
- Chrusciel P, Rysz J, Banach M (2014) Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs 74: 971-980.
- DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH (2013) L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo ClinProc 88: 544-551.
- Perepech NB, Nedoshivin AO, Nesterova IV (2001) [Neoton and thrombolytic therapy of myocardial infarction]. TerArkh 73: 50-55.
- Prabhakar G, Vona-Davis L, Murray D, Lakhani P, Murray G (2003) Phosphocreatine restores high-energy phosphates in ischemic myocardium: implication for off-pump cardiac revascularization. J Am CollSurg 197: 786-791.
- Hall M, Trojian TH (2013) Creatine supplementation. Curr Sports Med Rep 12: 240-244.
- Guo-han C, Jian-hua G, Xuan H, Jinyi W, Rong L, et al. (2013) Role of creatine phosphate as a myoprotective agent during coronary artery bypass graft in elderly patients. Coron Artery Dis 24: 48-53.
- Soboll S, Conrad A, Eistert A, Herick K, Krämer R (1997) Uptake of creatine phosphate into heart mitochondria: a leak in the creatine shuttle. BiochimBiophysActa 1320: 27-33.
- Ferraro S, Codella C, Palumbo F, Desiderio A, Trimigliozzi P, et al. (1996) Hemodynamic effects of creatine phosphate in patients with congestive heart failure: a double-blind comparison trial versus placebo. ClinCardiol 19: 699-703.
- Liu XS, Jiang M, Zhang M, Tang D, Clemo HF, et al. (2007) Electrical remodeling in a canine model of ischemic cardiomyopathy. Am J Physiol Heart CircPhysiol 292: H560-571.
- Hamill OP (2006) Twenty odd years of stretch-sensitive channels. Pflugers Arch 453: 333-351.
- Arenja N, Schaer B, Sticherling C, Kühne M (2014) [Current indications for an implantable cardioverter defibrillator (ICD)]. TherUmsch 71: 111-116.
- Leyva F, Nisam S, Auricchio A (2014) 20 years of cardiac resynchronization therapy. J Am CollCardiol 64: 1047-1058.
- Antzelevitch C (2005) Modulation of transmural repolarization. Ann N Y AcadSci 1047: 314-323.
- Calloe K, Soltysinska E, Jespersen T, Lundby A, Antzelevitch C, et al. (2010) Differential effects of the transient outward K(+) current activator NS5806 in the canine left ventricle. J Mol Cell Cardiol 48: 191-200.
- Lipkind GM, Fozzard HA (2008) Voltage-gated Na channel selectivity: the role of the conserved domain III lysine residue. J Gen Physiol 131: 523-529.
- Kubalová Z (2003) Inactivation of L-type calcium channels in cardiomyocytes. Experimental and theoretical approaches. Gen PhysiolBiophys 22: 441-454.
- Day YJ, Gao Z, Tan PC, Linden J (1999) ATP sensitive potassium channel and myocardial preconditioning. ActaAnaesthesiol Sin 37: 121-131.
- Greenhaff PL (2001)Thecreatine-phosphocreatine system: there's more than one song in its repertoire. J Physiol., 537(Pt 3):657.
- Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, et al. (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338: 973-976.
- Saks VA, Dzhaliashvili IV, Konorev EA, Strumia E (1992) [Molecular and cellular aspects of the cardioprotective mechanism of phosphocreatine]. Biokhimiia 57: 1763-1784.
- Menin L, Panchichkina M, Keriel C, Olivares J, Braun U, et al. (2001) Macrocompartmentation of total creatine in cardiomyocytes revisited. Mol Cell Biochem 220: 149-159.
- Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27: 241-261.
- KryzhanovskiÄ SA, Kacharava VG, Marko R, Kelemen K, Kaverina NV, et al. (1991) [Electrophysiologic study of the anti-arrhythmic mechanism of action of phosphocreatine in acute myocardial ischemia and reperfusion]. Kardiologiia 31: 66-69.
--
Relevant Topics
- Adalimumab Pharmacokinetics
- ADME Studies
- Atorvastatin Pharmacokinetics
- Clinical Pharmacokinetics
- Clopidogrel Pharmacokinetics
- DMPK Studies
- Esomeprazole Pharmacokinetics
- Etanercept Pharmacokinetics
- Fluticasone Pharmacokinetics
- Infliximab Pharmacokinetics
- Linear Pharmacokinetics
- Morphine Pharmacokinetics
- Nonlinear Pharmacokinetics
- Olanzapine Pharmacokinetics
- Pharmacokinetics Modelling
- Quetiapine Pharmacokinetics
- Rosuvastatin Pharmacokinetics
Recommended Journals
Article Tools
Article Usage
- Total views: 11313
- [From(publication date):
May-2016 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 10371
- PDF downloads : 942