Effect of Oral Supplement “Lycopene” On Reducing the Signs of Skin Ageing
Received: 13-May-2020 / Accepted Date: 10-Jun-2020 / Published Date: 17-Jun-2020 DOI: 10.4172/2167-065X.1000195
Abstract
In today’s world physical appearance is important not only as a symbol of identity, but also as an indicator of health and youth and there is a growing awareness that beauty starts from within. This study was designed to evaluate the effect of a tomato based oral supplement formulation (Lycoderm) containing different phytonutrients including lycopene the red tomato carotenoid on skin appearance after 16 weeks of use. A panel of 60 subjects was divided in two groups of 28 and 32 subjects provided with the placebo and Lycoderm capsules, respectively. Skin carotenoid level, image analysis of photography, questionnaire for assessment of skin condition and expert visual grading of facial wrinkles were obtained before treatment and every month for the course of the study. Results indicated a statistically significant increase in skin carotenoid levels in subjects who ingested Lycoderm, but no remarkable change in the placebo group. There was also a significant reduction in the appearance of lines and wrinkles in subjects using Lycoderm. The subjects noticed multiple skin benefits such as skin brightness and tonality, skin hydration and overall skin condition after using Lycoderm. Based on the confines and conditions of this study oral supplementation with Lycoderm resulted in significant increase in skin carotenoid level as well as tangible benefits to skin.
Keywords: Lycopene; Wrinkles; Skin; Ageing; Firmness; Elasticity; Hydration; Color
Introduction
The face holds an integral role in human social communication and perception by self and others. In addition to structural facial features, studies have shown that skin appearance in terms of coloration and surface topography plays a serious role in perception of beauty [1]. It is well known that the aging process is affected by two types of processes; the intrinsic and extrinsic that trigger the typical signs expressed on skin. The sun is the primary cause of extrinsic aging and has also been shown to alter the normal course of intrinsic or chronological aging [2]. On the molecular level there are various hypotheses to explain the aging process among which the free radial theory is most prominent. The free radical theory reports that free radicals and other reactive oxygen species (ROS) damage biomolecules, and this damage results in aging of the skin as well as other systems [3]. The aim of this study was to examine the effects of an oral anti-oxidant supplement on reducing the visible signs of skin aging.
Anti-oxidants and ageing
The interaction of free radicals with antioxidants is a topic of increasing interest in the development of strategies to diminish the signs of skin ageing. Free radicals are continuously produced in the human organism as a result of cellular metabolism [4,5]. Intrinsic ageing and illness as well as extrinsic solar irradiation pollution and smoke induce the generation of free radicals [6] which induce cellular signaling that may impair cell cycle, cell growth, and regeneration and/or repair processes [6-9]. As part of its protective system the human body possesses an antioxidative network to quench free radicals [6,10-13]. Typical antioxidants include vitamins (A, C, E and D) [9,14-17], as well as carotenoids (β carotene, lycopene and lutein) [9,17,18], various enzymes [19,20] and hormones [21,22]. Most of these antioxidants cannot be synthesized by the human organism entirely or in sufficient amounts and must be administered via nutrition. It is well known that fruits and vegetables, green tea, cacao and plant extracts are rich sources of antioxidants [23-26]. Interaction of antioxidants with free radicals gives rise to the destruction of antioxidants and neutralization of free radicals [27-31]. Therefore, there is a need to replenish them, especially in order to slow down the ageing process. Carotenoids and retinoids have been reported to act as peroxyl radical scavenging antioxidants in addition to their recognized singlet oxygen-quenching ability [32]. Studies indicate that these compounds can serve important functions in addition to direct antioxidant chemistry, by modulating enzyme activity and gene expression. Changing levels of protein kinase C or of DNA repair enzymes, for example, could impact resilience to photooxidative stress [32].
Antioxidants such as carotenoids, tocopherols and flavonoids, as well as vitamins (A, C, D and E), have been referred as agents capable of promoting skin health and beauty [33-36]. It has been reported that that premature skin ageing is less observable in people with a high level of antioxidants in their tissue [31]. Consequently, the visible signs of ageing like lines and wrinkles have been reported to be not as deep and dense as in the skin of individuals with a low antioxidant level [31]. Amongst the carotenoids, lycopene has been shown to exhibit the highest antioxidative activity [37].
Lycopene
Lycopene is the red pigment naturally present in tomatoes and is a known antioxidant, and free radical absorber. Among the carotenoids, it is a major component found in the serum as well as other tissues. It is known to be the most powerful singlet oxygen quencher and peroxyl radical scavenger among the major carotenoids and may represent an important defense mechanism in the human body [38]. It has received attention for its potential role in preventing cardiovascular disease in humans [39] and its deficiency can result in various health challenges [40-42]. Dietary supplementation studies involving either lycopenecontaining foods or lycopene supplementation have shown prospective short-term improvement in blood and heart conditions [43-49].
Lycopene and β-carotene are usually the dominating carotenoids in human blood and tissues and are known to control skin properties when ingested as supplements or as dietary products. While they cannot be compared with sunscreen, there is indication that they protect skin against sunburn (solar erythema) by increasing the basal defense against UV light-mediated damage [50-52]. It has been reported that amounts of lycopene in plasma and skin are comparable to or even greater than those of β-carotene. When skin is exposed to UV light stress, more skin lycopene is destroyed as compared to β- carotene, suggesting a serious role of lycopene in alleviating oxidative damage in tissues [7].
Mode of action
There are several biochemical mechanisms potentially underlying the protective effects of dietary lycopene. These include antioxidant activity such as the quenching of singlet oxygen and the scavenging of peroxyl radicals and induction of cell-cell communication. Dietary lycopene is absorbed and distributed throughout the body, but its bioavailability depends on various factors such as food processing or co-ingestion of fat [53]. The carotenoids of fruit, vegetables and animal products are usually fat-soluble and are associated with lipid portions of human tissues, cells and membranes. They may also be esterified or complexed with protein [54]. Carotenoids are thus absorbed in the intestinal mucosa with the aid of dietary fat and incorporated into chylomicrons for transport in the serum [54,55]. Adipose tissue is the largest body pool for carotenoid [55] whereas the serum concentrations are somewhat constant and slow to change during periods of low intake. The estimated half-life has been reported to be 11-14 days for lycopene, α-carotene and β-carotene [56,57]. There is, of-course individual variation in the ability to absorb carotenoids such as β-carotene and this variation in the body’s response to β-carotene is gene-based. The distribution of β-carotene and lycopene in human skin strongly depends upon the skin region studied and drastically change inter-individually. Conversely, skin beta-carotene and lycopene concentrations are lower in smokers than in non-smokers and higher in vegetarians [58].
Although, the antioxidant properties of lycopene are thought to be primarily responsible for its beneficial properties, evidence is accumulating to suggest involvement of other mechanisms such as intercellular gap junction communication, hormonal and immune system modulation and metabolic pathways [49].
Cholesterol hydroperoxides formed on exposure to UVA contribute to the expression of metalloproteinases like MMP-9, resulting in photoaging. Dietary β-carotene prevents the expression of MMP-9, at least partly, by inhibiting photodynamic action implicated in the formation of cholesterol hydroperoxides [59] as well as by attenuating the expression of MMP family proteins [60]. Topical retinoid treatments have also been reported to inhibit the UV-induced, MMPmediated breakdown of collagen and also protect against UV-induced decreases in procollagen expression [61,62].
Effect on skin
It is well known that chronological and photoaging of the skin results in increased wrinkling and loss of elasticity. Antioxidant supplementation with combination of carotenoids, vitamin E and selenium has been reported to increase serum levels of selected carotenoids and increased skin density and thickness as well as skin smoothness [63]. Dietary antioxidants provide protection against skin damage from sunlight [23,32,51,64,65] including the erythematous UV-B as well as the longer wavelength UV-A [60]. Intake of lycopene‐rich supplements has been shown to be associated with a significant reduction of UVR‐induced mRNA expression of MMP‐ 1 and ICAM‐1 proteins implicated in inflammatory responses. There is substantial evidence that carotenoids, vitamin C and E prevent sunburn-associated erythema following UV exposure. UV radiation generates reactive oxygen species in the skin, leading to damaging reactions which have been associated with photo carcinogenesis, photosensitivity or premature skin ageing [66]. Dietary supplementation of humans with β carotene has been shown to provide protection against sunburn in a time‐dependent manner [67]. The precise mechanism by which these supplements inhibit UVA radiation‐induced gene expression is currently not known but most likely involves antioxidative mechanisms [60].
Thus, scavenging reactive oxygen species could be one mechanism of action underlying skin protective effects of antioxidants [68,69]. However, it has been shown that several dietary antioxidants exhibit biological properties not directly related to antioxidant activity; they influence cellular signaling pathways and may trigger cell cycle progression, cell growth and repair systems [70,71].
Objective
The current study was designed to evaluate the effect of a novel dietary supplement formulation containing a specific combination of phytonutrients from the tomato and rosemary. This specific formula was found effective in reducing UV damage [52] and hence, leading the authors to investigate whether supplementation with these dietary micronutrients would improve skin condition and signs of skin ageing.
Materials and Methods
Study design
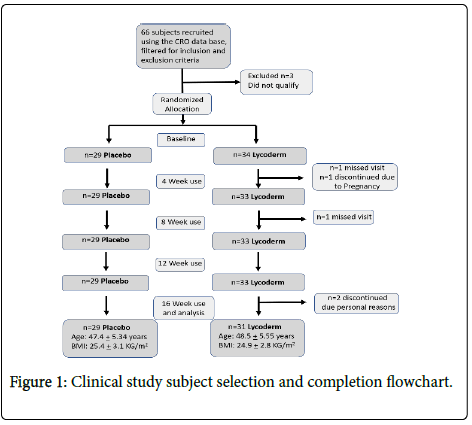
This study was approved by the local Ethics Committees of Allendale Institutional Review Board according to the ethical rules stated in the principles of the Declaration of Helsinki and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. This was a 16-Week Double- Blind, Randomized, Placebo-Controlled Study Evaluating the Nutritional Supplement “Lycoderm” on its Impact on Skin Parameters in Healthy Female Subjects.
Subject inclusion and exclusion criteria
Sixty six subjects were recruited for the study out of which sixty completed the study. Six subjects discontinued due to product unrelated reasons (Figure 1).
• The subjects were all healthy females between ages of 35 and 55 years old.
• Caucasians and Hispanics mainly with Fitzpatrick Skin Type II-III.
• The BMI of the subjects did not exceed 30Kg/m2.
• The subjects were healthy with no acute or chronic disease or medical condition, including dermatological problems, which could put them at risk in the opinion of the Principal Investigator or compromise study outcomes.
• Subjects who used nutritional supplementation regularly (within the last month) especially carotenoids were excluded.
• The subjects were not participating in any other clinical studies.
• Also excluded were subjects with a history of allergic reactions, skin sensitization and/or known allergies to cosmetic ingredients, toiletries, sunscreens, etc. as well as those who were immunocompromised, on hormone replacement therapy or on oral contraception for less than three months before the screening visit.
• Also excluded as were pregnant and lactating women. Employees of testing lab or other testing firms/ laboratories, cosmetic or raw goods manufacturers or suppliers were also excluded from the panel.
• The subjects were self-reported non-smokers who agreed to maintain specified dietary instructions and cosmetic regimen defined in the study protocol.
• Expert skin grading determined that the subjects possessed facial lines and wrinkles.
• They exhibited the ability to read, understand and sign an informed consent form (including HIPAA and State requirements). They were willing and able to co-operate and follow all study directions, attend study visits as scheduled and willing to accept the restrictions of the study.
• Exclusion criteria for the subjects included known allergies to soybeans, coconut, tomatoes, or rosemary.
• Also subjects with extreme diet habits including those who consume fruit, vegetables and lycopene rich food above the dietary instructions.
• Subjects with a history of malabsorption diseases, liver diseases, or diseases of lipid metabolism as well as those with a drastic change in body weight (greater than 10%) one month prior to screening or during the study were excluded.
• The subjects did not have a history of immunological as well as photosensitizing disorders and did not use medications which could interact with the study procedures according to the principal investigator (during or prior to the study).
General instructions
The subjects agreed to avoid extensive sun exposure during the study and a month prior. They were instructed to refrain from using any facial treatments and procedures such as Botox® and fillers during the study period and two months prior.
Dietary instructions
The subjects were instructed to limit their food consumption to no more than 3 servings/day of vegetables and fruit and no more than 3 serving/week of lycopene-rich foods (e.g. tomatoes and tomato products; including all sauces containing tomatoes, ketchup, etc. as well as mango, cantaloupes, zucchini and squash). A list of suggested foods was provided to the subjects to aid their dietary restrictions.
Cosmetic regimen
Use of facial products and color-cosmetics which subjects were regularly using for at least two weeks prior to study enrollment was allowed during study period, however, the subjects were instructed not to change products during the course of the study. Use of certain other systemic and topical medications and treatments products was prohibited during the study. The subjects were provided with a cleanser (Cetaphil Cleanser) and a sunscreen (Neutrogena SPF Oil Free SPF 30) to be used for the course of the study.
Test material and instruction of use
Placebo: Gelatin soft gel capsule containing Medium Chain Triglycerides (MCT) (Cremer Care, Cincinnati, OH). Lycoderm: Gelatin soft gel capsule containing tomato extract standardized to 7.5 mg Lycopene and additional tomato phytonutrients as well as Rosemary extract standardized for 2mg carnosic acid (Lycored Inc, Beer Sheva, Israel).
The subjects ingested a soft gel capsule twice a day (i.e. 15mg lycopene per day) preferably with a meal.
Measurements
This was a 16 week, in use study. A total of 60 female subjects participated in the study. The panel was randomly divided in two groups of 28 and 32 subjects provided with the placebo and Lycoderm capsules, respectively. Measurements were obtained before treatment and every month for the course of the study.
• Expert Visual grading using a 1-10cm scale where 1 was none and 10 was extreme for the appearance of lines/wrinkles (crow’ s feet), texture/smoothness (visual).
• Subject self-assessment via questionnaire with approval rating scale of: 1-strongly agree, 2- agree, 3 neither agree nor disagree, 4 disagree and 5 strongly disagree. The percent of population showing approval was calculated for each time point.
• Photographs were obtained using a 2D Clarity pro (BrighTex Bio- Photonics, San Jose California, USA) followed by analysis of wrinkles in the crows feet area at baseline and 16 weeks after use. Image analysis involves digitizing the photograph and subsequent count of pixels depicting depth and length.
• Skin carotenoid level: The Pharmanex S3 Biophotonic Scanner (NuSkin, Provo, UT, USA) is a non-invasive tool that measures carotenoid levels in living tissue thus providing an indication of overall antioxidant levels. The scanner provides a Skin Carotenoid Score (SCS), using an optical measurement known as Resonant Raman Spectroscopy. Carotenoid measurements were obtained from the palm of the hands at baseline and weeks 4, 8, 12 and 16.
Statistics
All statistics were computed using SPSS (Statistical Package for the Social Sciences). Simple frequency/percentage and other summary statistics were provided for each variable within each product group. A student’s t-test was used to compare product groups across all continuous data for: Raw scores, Differences and Percent changes. Crosstabs with Chi-square were employed to analyze category data. For subject self assessment questionnaires both student’s t-test and Wilcoxon signed rank test were used along with chi-square (for positive/negative rankings). In addition, simple frequency/percentage distributions were calculated within each product group for each question.
Results
Carotenoid
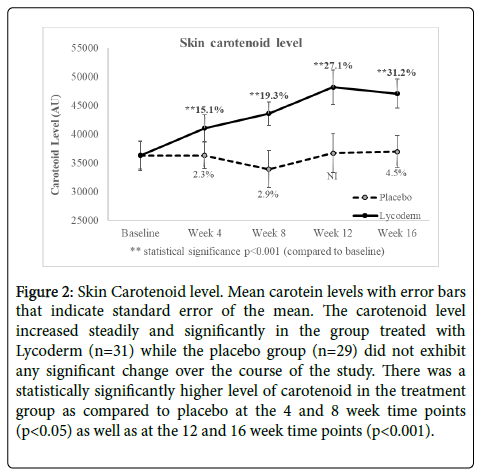
Figure 2 shows average of carotenoid level with error bars showing standard error of the mean. As observed in this figure the skin carotenoid level increased steadily and significantly in the group treated with Lycoderm while the placebo group did not exhibit any significant change over the course of the study. After 4, 8, 12 and 16 weeks of treatment the Lycoderm treated panel exhibited 15.1% (p<0.001), 19.3% (p<0.001), 27.1% (p<0.001) and 31.2% (p<0.001) respectively, compared to baseline. The placebo group did not exhibit a notable change over the course of the study. There was a statistically significantly higher level of carotenoid in the treatment group as compared to placebo at the 4 and 8 week time points (p<0.05) as well as at the 12 and 16 week time points (p<0.001) (Table 1).
Figure 2: Skin Carotenoid level. Mean carotein levels with error bars that indicate standard error of the mean. The carotenoid level increased steadily and significantly in the group treated with Lycoderm (n=31) while the placebo group (n=29) did not exhibit any significant change over the course of the study. There was a statistically significantly higher level of carotenoid in the treatment group as compared to placebo at the 4 and 8 week time points (p<0.05) as well as at the 12 and 16 week time points (p<0.001).
| Age and Body Mass Index distribution | ||||||
|---|---|---|---|---|---|---|
| Average | Minimum | Maximum | ||||
| Placebo | Lycoderm | Placebo | Lycoderm | Placebo | Lycoderm | |
| Age (Years) | 47.4 ± 5.34 | 48.5 ± 5.55 | 36 | 35 | 55 | 55 |
| BMI (Kg/M2) | 25.4 ± 3.1 | 24.9 ± 2.8 | 20.1 | 20.5 | 30.7 | 30.7 |
Table 1: Describes t he even distribution of age and body mass index between the two groups.
Fine lines and wrinkles
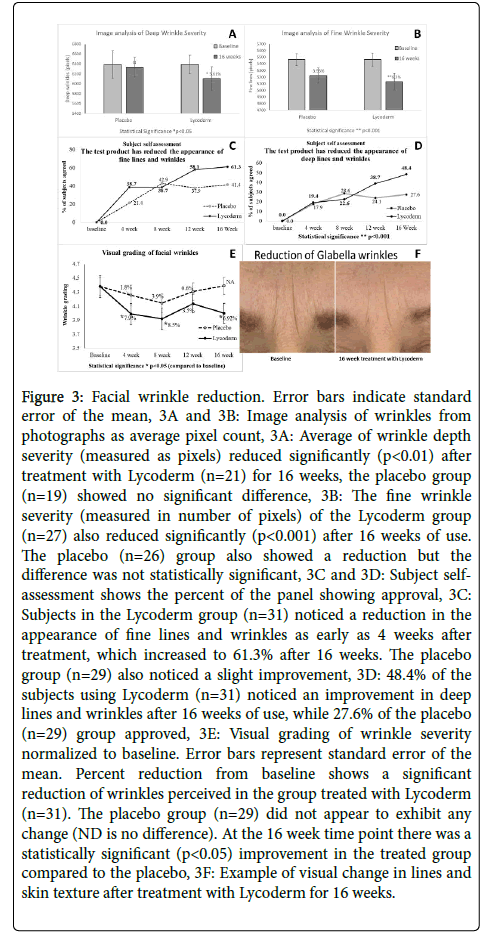
Visual change in facial wrinkles is reported in Figure 3. Figure 3F shows a photographic example of improvement in lines and wrinkles after using Lycoderm for 16 weeks. Image analysis of photographs is displayed in Figure 3A and 3B as average of number of pixels. Average of wrinkle depth severity reduced 5.61% (p<0.05) after treatment with Lycoderm for 16 weeks while the placebo group showed no significant difference. 78.9% of the population on Lycoderm exhibited an improvement. The fine wrinkle severity of the Lycoderm group also significantly reduced by 6.2% (p<0.001) with 84.6% positive responders. The placebo group also showed a reduction of 4.41% in the severity of fine lines, possibly due to hydration effect of topical moisturizer, however the difference was not statistically significant.
Figure 3: Facial wrinkle reduction. Error bars indicate standard error of the mean, 3A and 3B: Image analysis of wrinkles from photographs as average pixel count, 3A: Average of wrinkle depth severity (measured as pixels) reduced significantly (p<0.01) after treatment with Lycoderm (n=21) for 16 weeks, the placebo group (n=19) showed no significant difference, 3B: The fine wrinkle severity (measured in number of pixels) of the Lycoderm group (n=27) also reduced significantly (p<0.001) after 16 weeks of use. The placebo (n=26) group also showed a reduction but the difference was not statistically significant, 3C and 3D: Subject selfassessment shows the percent of the panel showing approval, 3C: Subjects in the Lycoderm group (n=31) noticed a reduction in the appearance of fine lines and wrinkles as early as 4 weeks after treatment, which increased to 61.3% after 16 weeks. The placebo group (n=29) also noticed a slight improvement, 3D: 48.4% of the subjects using Lycoderm (n=31) noticed an improvement in deep lines and wrinkles after 16 weeks of use, while 27.6% of the placebo (n=29) group approved, 3E: Visual grading of wrinkle severity normalized to baseline. Error bars represent standard error of the mean. Percent reduction from baseline shows a significant reduction of wrinkles perceived in the group treated with Lycoderm (n=31). The placebo group (n=29) did not appear to exhibit any change (ND is no difference). At the 16 week time point there was a statistically significant (p<0.05) improvement in the treated group compared to the placebo, 3F: Example of visual change in lines and skin texture after treatment with Lycoderm for 16 weeks.
Figure 3C and 3D shows subject self-assessment of product effect. Figure 3C reports the percent of the panel showing agreement to the question “Has the product reduced the appearance of fine lines and wrinkles”? At the 12 and 16 week time points 58.1% and 61.3% of the Lycoderm users, respectively, remarked that they noticed a reduction in the appearance of facial fine lines and wrinkles. Some (less than 42%) subjects in placebo group also noticed improvement. Figure 3D shows percent of the panel in agreement to the question “Has the product reduced the appearance of deep lines and wrinkles”? Very few subjects were in agreement initially, but after 12 weeks of use 38.7% of the panel on Lycoderm and 24.1% of the subjects on placebo were in agreement of improvement. At the 16 week time point 48.4% of the panel on Lycoderm and 27.6% of the subjects on placebo expressed approval.
Figure 3E shows average of visual grading of facial wrinkles by a trained technician. There was no statistically significant difference between the wrinkles grades of the Lycoderm vs the placebo group, nevertheless, it appears that the subjects treated with Lycoderm exhibited some reduction in visually perceivable wrinkles. The group treated with Lycoderm showed a 7.9% (p<0.05) and 8.5% (p<0.05) reduction in wrinkle intensity after 4 and 8 weeks of use, respectively while the placebo group exhibited only a 1.8% and 3.9% reduction after 4 and 8 week use, respectively. At 4 and 8 week time points 67.7% and 64.5% of the population on Lycoderm exhibited an improvement and 50% and 57% of the population on placebo showed an improvement. It is possible that the topical use of moisturizers slightly improved the skin texture of the placebo group. At the 12 week time point both groups exhibited an elevation, nevertheless, the average intensity of facial wrinkles of the placebo was still higher than the Lycoderm treated. At the 16 week time point the Lycoderm group exhibited 6.92% (p<0.05) reduction in wrinkles from baseline while the placebo group appeared to look worse. Count of positive responders shows that 48.3% of the subjects on placebo and 61.3% on Lycoderm exhibited an improvement and the difference between the placebo and Lycoderm group was statistically significant at the 16 week time point (p<0.05).
Radiance
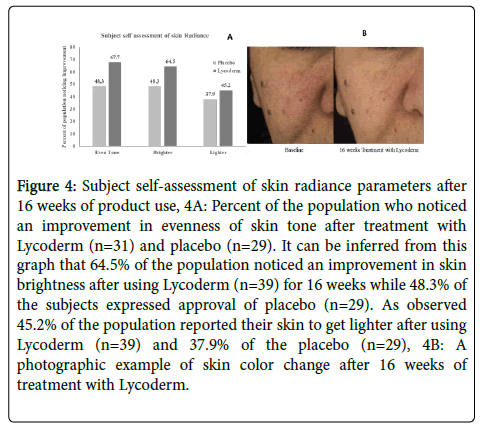
Figure 4 exhibits subject self-assessment of skin radiance parameters. The graphs represent percent of subjects showing approval. Figure 4A indicates that 64.5% of the population noticed an improvement in skin brightness after using Lycoderm for 16 weeks while 48.3% of the subjects expressed approval of placebo at the same time point. After using Lycoderm for 16 weeks, 45.2% subjects reported their skin to be lighter while in the placebo group, 37.9% noticed a skin lightening. As observed in this figure, 67.7% of the Lycoderm users and 48.3% of the placebo users reported of improvement in their skin tone after 16 weeks of use.
Figure 4: Subject self-assessment of skin radiance parameters after 16 weeks of product use, 4A: Percent of the population who noticed an improvement in evenness of skin tone after treatment with Lycoderm (n=31) and placebo (n=29). It can be inferred from this graph that 64.5% of the population noticed an improvement in skin brightness after using Lycoderm (n=39) for 16 weeks while 48.3% of the subjects expressed approval of placebo (n=29). As observed 45.2% of the population reported their skin to get lighter after using Lycoderm (n=39) and 37.9% of the placebo (n=29), 4B: A photographic example of skin color change after 16 weeks of treatment with Lycoderm.
As observed in photographic example (Figure 4B) skin was lot clearer after using Lycoderm for 16 weeks. The skin of this subjects appears lighter and a lot more even toned than baseline.
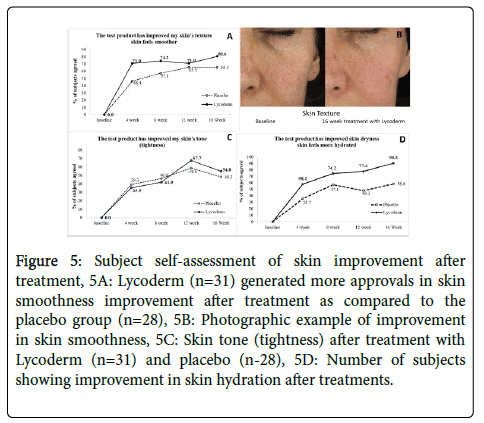
Skin smoothness
Figure 5 displays subject self-assessment of other parameters after treatment. Figure 5A shows that the subjects on Lycoderm noticed a lot more improvement in skin smoothness after treatment as compared to the placebo group. Over 71% of the subjects exhibited approval after using Lycoderm after 4, 8 and 12 weeks of use which increased to 80.6% after 16 weeks. A small percentage of the placebo group also appeared to notice improvement but not as much as the Lycoderm group. Figure 5B shows visual example of skin smoothness.
Figure 5: Subject self-assessment of skin improvement after treatment, 5A: Lycoderm (n=31) generated more approvals in skin smoothness improvement after treatment as compared to the placebo group (n=28), 5B: Photographic example of improvement in skin smoothness, 5C: Skin tone (tightness) after treatment with Lycoderm (n=31) and placebo (n-28), 5D: Number of subjects showing improvement in skin hydration after treatments.
Skin tightness and hydration
Figure 5C shows skin tone (tightness) after treatment. Both groups appeared to show some improvement after treatment, however there was not much difference between the groups until 12 week use when 67.7% of the Lycoderm group professed that the treatment improved their skin tightness. 58.6% of the subjects in the placebo group also noticed an improvement. The effect dropped slightly after 16 week use, nevertheless the Lycoderm treated group still appeared to be somewhat better than the placebo.
Figure 5D illustrates the percent of subjects showing improvement in skin hydration after treatments. It is evidant from this graph that the group on Lycoderm noticed a lot more improvement than the placebo. After 4, 8, 12 and 16 weeks of use, 58.1%, 74.2%, 77.4% and 90.3% of the subjects on Lycoderm noticed improvement in skin hydration, respectively. A small percentage of subjects in the placebo group also noticed improvement (35.7%, 57.1%, 48.3% and 58.6% after 4, 8, 12 and 16 weeks of use, respectively).
Subjects on Lycoderm (n=31) noticed a lot more improvement than the placebo (n=28).
Placebo effect
Subject self-assessment of most parameters show a change in the placebo group indicating a “placebo effect”. The subjects were provided with a topical moisturizer and SPF lotion to apply on the face through the course of the study. It is possible that consistent use of these topical agents resulted in some change that was perceived by them as improvement. In spite of the topical effect, the the treatment group perceived greater change indicating that the supplement did induce biological changes which were greater than the placebo effect.
Discussion and Conclusion
Public interest in diverse and sophisticated ways of enhancing appearance has expanded the beauty field into the realm of improved nutrition, psychological well-being as well as physical fitness. Healthy and nutritious diet has a big impact on the way one looks and feels, so while good quality cosmetic products are important, good dietary habits as well as nutrition supplementation are gaining impetus as important factors to radiate beauty from the inside out.
Skin has been reported to reflect chronological and photoaging as well as general inner-health status. Nutrition and its reflection on skin is a topic of increasing popularity worldwide. In the last 4 decades various natural and synthetic retinoids have been explored for the treatment of aging and many of them have shown histological and clinical improvement, Although retinoids are considered a gold standard in the treatment of skin aging, irritant reactions such as burning, scaling or dermatitis associated with retinoid therapy limit their acceptance by consumers. Beta Carotene has an important nutritional role as the principal precursor of vitamin A which is less irritating. It is of primary importance in vision, cell differentiation, synthesis of glycoprotein, mucus secretions from epithelial tissues, reproduction, overall growth and development of bones [72]. Carotenoids are the main dietary source of vitamin A. β-Carotene is cleaved at its central double to yield vitamin A (retinal or β-apo-15′- carotenal) [73]. Other carotenoids in the Lycoderm formula such as lycopene may also be subject to central or asymmetrical cleavage [74] and their retinoid-like derivatives may take part in the overall effect of the formula [75,76].
In spite of these conversions it is evidant from this study that there is accumulation of carotenoids in skin from dietary treatment. Studies have shown that there is a variation in distribution of beta carotene in skin with highest basal values in skin of the forehead, palm of the hand and dorsal skin and these skin sites exhibited high accumulations of beta carotene after ingestion [77]. Clearly there are large disparities between skin areas in terms of embedded micronutrient, as shown for instance in the high levels of β-carotene in the palm of the hand as compared to other skin areas [77] nevertheless, the main strength of this study shows that ingestion of a carotene rich supplement leads to accumulation in skin. This increase is beneficial in many ways including its effect on balancing inflammation [52] epithelial differentiation and proliferation [78,79]. It is possible that the reduction in lines and wrinkles observed in this study was partially due to improvement in epidermal differentiation as well as the potent antioxidant properties of carotenoids [80,81].
In this study the Lycoderm formulation appeared effective in improving some signs of ageing like wrinkles, overall skin appearance, skin hydration and smoothing of skin tonality. It is possible that some of these effects were partially due to the effect of carotenoids on improvement of cell-cell communication. Gap junctional channels link the cytoplasm of two cells, and provide a means for the exchange of ions (K and Ca), second messengers (cAMP,cGMP, and inositol), and small metabolites (glucose), allowing electrical and biochemical coupling between cells [82,83]. Gap junctional communication is essential for many physiological events, including cell synchronization, differentiation, cell growth, and metabolic coordination of avascular organs including epidermis and lens [84,85]. Major dietary carotenoids, including β-carotene, lycopene, and several xanthophylls can increase connexin expression and, consequently, cell-to-cell communication growth, differentiation and metabolism [84,86-90] as well as sebaceous secretion [85]. There is evidence that metabolites or oxidation products of carotenoids such as retinoids or apo carotenals are the ultimate active agents [51,64,89]. Various connexins are found in skin and are of importance for normal development and differentiation of human epidermis [91]. Improvement in keratinocyte differentiation results in a healthier looking skin as is implicated in this study.
Skin inflammation has long been associated with signs of ageing on sun exposed as well as unexposed sites. Chronic intrinsic inflammation as well as other external stressors such as UV exposure causes skin aging. Skin photoaging may be the result of singlet oxygen mediated generation of matrix metalloproteases (MMPs). UVA irradiation massively reduces the cellular carotenoids content (eg β-carotene) while supplementation allows suppression of UVA-induction of MMP proteins involved in photoaging. β-Carotene is effective in quenching O2-mediated induction of MMP-1 and MMP-10 [92] thus reducing inflammation which in turn, can improve the general health of skin as well as reduce the signs of ageing as observed in this study. Lycopene has been shown to reduce inflammation by inhibiting the release of TNF-α and stimulating IL-10 production [93] indicating an additional pathway of reducing inflammation from this Lycoderm supplementation.
As observed in this study that use of Lycoderm formulation for 16 weeks resulted in skin brightening and evening as well as smoothing of tonality after long term ingestion of Lycopene. Although effects could be due to improvement in differentiation there is a possibility that Lycoderm reduced melanogenesis. One mechanism linked to this is via stimulation of cGMP synthesis by Nitros oxide from inflammation, which in turn stimulates melanogenesis [94]. Additionally, in a study of carotenoids, fucoxanthin was found to inhibit tyrosinase activity as well as melanogenesis in melanoma and UVB-induced skin pigmentation [95]. It is possible that lycopene could also exert a similar effect on tyrosinase, however, further studies need to be conducted to investigate the effect of this active on melanogenesis.
Limitations
As discussed, there could be several pathways leading to the effect of Lycoderm formulation on skin. The reported results do not shed light on the cellular mechanism of action of the actives. Although consumer perception is important, additional instrumental data would have strengthened the message of this manuscript. These weaknesses will be addressed in further investigational studies.
Author Contributions
Conceptualization, K.H, E.T, S.S.; methodology, K.H, E.T, S.S.; software, S.S.; validation, S.S.; formal analysis, S.S.; investigation, S.S.; resources, K.H, E.T, S.S..; data curation, S.S.; writing-third party; writing-review and editing, K.H, E.T; visualization, K.H, E.T; supervision, K.H, E.T.; project administration, E.T.; funding acquisition, K.H, E.T.
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The study was fully funded by the sponsor- Lycored.
Acknowledgments
Product development - Tanay Sedlov.
Technical support - Oleg Braverman.
Conflicts of Interest
K.H and ET are employed by Lycored that is the supplier of the dietary supplement tested in this study and its funder. All other authors declare no conflict of interest.
KL and ET contributed to the study design. SS played a role in designing the study, collecting and analyzing the data and interpreting it. Data analysis and preparation of manuscript by third party.
References
- Samson N, Fink B, Matts PJ (2010) Visible skin condition and perception of human facial appearance. Int J Cosmet Sc 32:167-184.
- Baumann L (2007) Skin aging and its treatment. J Pathology 211:241-251.
- Balcerczyk A, Gajewska A, Macierzyńska-Piotrowska E, Pawelczyk T, Bartosz G, et al. (2014) Enhanced antioxidant capacity and anti-ageing biomarkers after diet micronutrient supplementation. Molecules 19:14794-14808.
- Droge W (2002) Free radicals in physiological control of cell function. Physiol Rev 82:47-95.
- Fujimori T, Yasui H, Hiromura M, Sakurai H (2007) Suppressive effect of orally administered copper (II)-aspirinate (Cu2(asp)4) complex on the generation of reactive oxygen species in the skin of animals subjected to UVA exposure. Exp Dermatol 16:746-752.
- Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Molecular aspects of medicine 24:345-351.
- Ribaya-Mercado JD, Garmyn M, Gilchrest BA, Russell RM (1995) Skin lycopene is destroyed preferentially over beta-carotene during ultraviolet irradiation in humans. J Nutr 125:1854-1959.
- Biesalski HK, Hemmes C, Hopfenmuller W, Schmid C, Gollnick HP (1996) Effects of controlled exposure of sunlight on plasma and skin levels of beta-carotene. Free Radic Res 24:215-224.
- Heinrich U, Neukam K, Tronnier H, Sies H, Stahl W (2006) Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J Nutr 136:1565-1569.
- Fuchs J (1998) Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, L-ascorbic acid and beta-carotene in cutaneous photoprotection. Free Radic Biol Med 25:848-873.
- Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ (2004) Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol 43:326-335.
- Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L (2001) The antioxidant network of the stratum corneum. Curr Probl Dermatol 29:26-42.
- Packer L, Valacchi G (2002) Antioxidants and the response of skin to oxidative stress: vitamin E as a key indicator. Skin Pharmacol Appl Skin Physiol 15:282-290.
- Lin JY, Selim MA, Shea CR, Grichnik JM, Omar MM, et al. (2003) UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol 48:866-874.
- Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, et al. (2005) Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol 124:304-307.
- Popadic S, Ramic Z, Medenica L, Mostarica Stojkovic M, Trajković V, et al. Antiproliferative effect of vitamin A and D analogues on adult human keratinocytes in vitro. Skin Pharmacol Physiol 21:227-234.
- Fernández-GarcÃa E (2014) Skin protection against UV light by dietary antioxidants. Food Funct 5:1994-2003.
- Stahl W, Sies H (2005) Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 1740:101-107.
- Shindo Y, Witt E, Han D, Epstein W, Packer L (1994) Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol 102:122-124.
- Lopez-Torres M, Thiele JJ, Shindo Y, Han D, Packer L (1998) Topical application of alpha-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br J Dermatol 138:207-215.
- Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, et al. (2008) Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol 17:713-730.
- Moosmann B, Behl C (2002) Secretory peptide hormones are biochemical antioxidants: structure-activity relationship. Mol Pharmacol 61:260-268.
- Heinrich U, Gärtner C, Wiebusch M, Eichler O, Sies H et al. (2003) Supplementation with beta-carotene or a similar amount of mixed carotenoids protect humans from UV induced erythema. J Nutr 133:98-101.
- Verstraeten SV, Hammerstone JF, Keen CL, Fraga CG, Oteiza PI (2005) Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J Agric Food Chem 53:5041-5048.
- Katiyar SK (2003) Skin photoprotection by green tea: antioxidant and immunomodulatory effects. Curr Drug Targets Immune Endocr Metabol Disord 3:234-242.
- Mulero M, RodrÃguez-Yanes E, Nogués MR, Giralt M, Romeu M, et al. (2008) Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp Dermatol 17:653-658.
- Bonnefoy M, Drai J, Kostka T (2002) Antioxidants to slow aging, facts and perspectives. Presse Med 31:1174-1184.
- Varadraj VP, Shukla P, Kikkeri NN (2014) Antioxidants in dermatology. Indian Dermatol Online J 5:210-214.
- Hadad, N, Levy R (2012) The synergistic anti-inflammatory effects of lycopene, lutein, b-carotene, and carnosic acid combinations via redox-based inhibition of NF-kB signaling. Free Radical Biology and Medicine 53:1381-1391.
- Lademann J, Meinke MC, Sterry W, Darvin ME (2011) Carotenoids in human skin. Experimental Dermatol 20:377-382.
- Sies H, Stahl W (1998) Lycopene: antioxidant and biological effects and its bioavailability in the human. Proc Soc Exp Biol Med 218:121-124.
- Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51:327-336.
- Ristow M, Zarse K, Oberbach A, Klöting, N, Birringer M, et al. (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106:8665-8670.
- Boelsma E, Hendriks HF, Roza L (2001) Nutritional skin care: health effects of micronutrients and fatty acids. The American journal of clinical nutrition 73:853-864.
- De Spirt S, Sies H, Tronnier H, Heinrich U (2012) An encapsulated fruit and vegetable juice concentrate increases skin microcirculation in healthy women. Skin pharmacology and physiology 25:2-8.
- Di Mascio P, Kaiser S, Sies H (1989) Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 274:532-538.
- Shixian Q, Dai Y, Kakuda Y, Shi J, Mittal G, et al (2005) Synergistic Anti-Oxidative Effects of Lycopene with Other Bioactive Compounds. J Food Reviews International 21:295-311.
- Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. Can Med Assoc J 163:739-744.
- Rissanen T, Voutilainen S, Nyyssönen K, Salonen R, Salonen JT (2000) Low plasma lycopene concentration is associated with increased intima-media thickness of the carotid artery wall. Arterioscler Thromb Vasc Biol 20:2677-2681.
- Kim OY, Yoe HY, Kim HJ, Park JY, Kim JY et al. (2010) Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 208:581-586.
- Yeo HY, Kim OY, Lim HH, Kim JY, Lee JH (2010) Association of serum lycopene andbrachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 60:537-543.
- Bub A, Watzl B, Abrahamse L, DelinceÌe H, Adam S (2000) Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr 130:2200-2206.
- Deplanque X, Muscente-Paque D, Chappuis E (2016) Proprietary tomato extract improves metabolic response to high-fat meal in healthy normal weight subjects. Food Nutr Res 60:32537.
- Devaraj S, Mathur S, Basu A, Aung HH, Vasu VT, et al. (2008) dose–response study on the effects of puriï¬ed lycopene supplementation on biomarkers of oxidative stress. J Am Coll Nutr 27:267-273.
- Engelhard YN, Gazer B, Paran E (2006) Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: a double-blind, placebo-controlled pilot study. Am Heart J 151:1-6.
- Wolak T, Sharoni Y, Levy J, Linnewiel-Hermoni K, Stepensky D, et al. (2019) Effect of Tomato Nutrient Complex on Blood Pressure: A Double Blind, Randomized Doseâ»Response Study. Nutrients 11:950-963.
- Kim JY, Paika JK, Kima OY, Park HW, Leec JH, et al. (2011) Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 215:189-195.
- Rao AV, Agarwal DS (1999) Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutrition Research 19:305-323.
- Stahl W, Heinrich U, Aust O, Tronnier H, Sies H (2006) Lycopene-rich products and dietary photoprotection. Photochem Photobiol Sci 5:238-242.
- Aust O, Stahl W, Sies H, Tronnier H, Heinrich U (2005) Supplementation with tomato-based products increase lycopene, phytofluene and phytoene levels in human serum and protects against UV-induce erythema. Int J Vit Nutr Res 75:54-60.
- Groten K, Marini A, Grether-Beck S, Jaenicke T, Ibbotson SH, et al. (2019) Tomato Phytonutrients Balance UV Response: Results from a Double-Blind, Randomized, Placebo-Controlled Study. Skin Pharmacol Physiol 32:101-108.
- Sies H, Stahl W (2004) Nutritional protection against sun damage from sunlight. Annu Rev Nutr 24:173-200.
- Simpson KL, Katayama T, Chichester CO (1981) Carotenoids as colorants and Vitamin A precursors. Bauernfeind JC (ed), Academic Press, New York, USA, p:463.
- Bendich A, Olson JA (1989) Biological actions of carotenoids. FASEB J 3:1927-1932.
- Rock CL, Swendseid ME (1992) Plasma β-carotene response in humans after meals supplemented with dietary pectin. Am J Clin Nutr 55:96-99.
- Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, et al. (1992) Plasma carotenoid response to chronic intake of selected foods and β-carotene supplements in men. Am J Clin Nutr 55:1120-1125.
- Darvin ME, Gersonde I, Meinke M, Sterry W, Lademann J (2005) Non-invasive in vivo determination of the carotenoids beta-carotene and lycopene concentrations in the human skin using the Raman spectroscopic method. J Phy Appl Phys 38:2696-2700.
- Minami Y, Kawabata K, Kubo Y, Arase S, Hirasaka K, et al. (2009) Peroxidized cholesterol-induced matrix metalloproteinase-9 activation and its suppression by dietary beta-carotene in photoaging of hairless mouse skin. J Nutr Biochem 20:389-398.
- Gretherâ€Beck S, Marini A, Jaenicke T, Stahl W, Krutmann J (2017) Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: results from a doubleâ€blinded, placeboâ€controlled, crossover study. Br J Dermatol 176:1231-1240.
- Fisher GJ, Wang ZQ, Datta SC, Varan J, Kang S, et al. (1997) Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 337:1419-1428.
- Fisher GJ, Datta S, Wang Z, Li XY, Quan T, et al. (2000) c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest 106:663-670.
- Heinrich U, Tronnier H, Stahl W, Béjot M, Maurette JM (2006) Antioxidant supplements improve parameters related to skin structure in humans. Skin Pharmacol Physiol 19:224-231.
- Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H (2000) Carotenoids and carotenoids plus vitamin E protect against UV-light induced erythema in humans. Am J Clin Nutr 71:795-798.
- Mukhtar H (2003) Eat plenty of green leafy vegetables for photoprotection: emerging evidence. J Invest Dermatol 121:399-405.
- Krutmann J (2000) Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci 23:22-26.
- Köpcke W, Krutmann J (2008) Protection from Sunburn with βâ€Carotene—A Metaâ€analysis. Photochem Photobiol 84:284-288.
- Césarini JP, Maurette JM (2003) Immediate effects of UV radiation on the skin, modiï¬cation by an antioxidant complex containing carotenoids. Photodermatol Photoimmunol Photomed 19:182-189.
- Césarini JP, Ruche G (1991) Protective effect of oral selenium plus copper associated with vitamin complex on sunburn cell formation in human skin. Photodermatol Photoimmunol Photomed 8:232-235.
- Klotz LO, Holbrook NJ, Sies H (2001) UVA and singlet oxygen as inducers of cutaneous signaling events. Curr Probl Dermatol 29:95-113.
- Sen CK, Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10:709-720.
- Dutta D, Chaudhuri UR, Chakraborty R (2005) Structure, health benefits, antioxidant property and processing and storage of carotenoids. African Journal of Biotechnology 4:1510-1520.
- Eroglu A, Harrison EH (2013) Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lipid Res 54:1719-1730.
- Caris-Veyrat C, Schmid A, Carail M, Böhm V (2003) Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J Agric Food Chem 51:7318-7325.
- Linnewiel-Hermoni K, Motro Y, Miller Y, Levy J, Sharoni Y (2014) Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic Biol Med 75:105-120.
- Sharoni Y, Linnewiel-Hermoni K, Zango G, Khanin M, Salman H, et al. (2012) The role of lycopene and its derivatives in the regulation of transcription systems: implications for cancer prevention. Am J Clin Nutr 96:1173-1178.
- Stahl W, Heinrich U, Jungmann H, von Laar J, Schietzel M, et al. (1998) Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlate with serum levels in women ingesting Betatene. J Nutr 128:903-907.
- Fritsch PO, Pohlin G, Längle U, Elias P (1981) Response of epidermal cell proliferation to orally administered aromatic retinoid. J Invest Dermatol 77:287-291.
- Klein-Szanto AJP, Martin DH, Pine AH (1980) Cutaneous manifestations in rats with advanced vitamin A deficiency. J Cutan Pathol 7:260-270.
- Nagata C, Nakamura K, Wada K, Oba S, Hayashi M, et al. (2010) Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br J Nutr 103:1493-1498.
- Darvin M, Patzelt A, Gehse S, Schanzer S, Benderoth C, et al. (2008) Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur J Pharm Biopharm 69:943-947.
- De Maio A, Vega VL, Contreras JE (2002) Gap junctions, homeostasis, and injury. J Cell Physiol 191:269-282.
- Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, et al. (2003) Lycopene oxidation product enhances gap junctional communication. Food Chem Toxicol 41:1399-1407.
- Hertzberg EL, Lawrence TS, Gilula NB (1981) Gap junctional communication. Annu Rev Physiol 43: 479-491.
- Meda P (2018) Gap junction proteins are key drivers of endocrine function. Biochim Biophys Acta Biomembr 1860:124-140.
- Bertram JS (1999) Carotenoids and gene regulation. Nutr Rev 57:182-191.
- Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, et al. (1997) Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 18:89-92.
- Stahl W, von Laar J, Martin HD, Emmerich T, Sies H (2000) Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch Biochem Biophys 373:271-274.
- Teicher VB, Kucharski N, Martin HD, van der Saag P, Sies H, et al. (1999) Biological activities of apo-canthaxanthinois acids related to gap junctional communication. Arch Biochem Biophys 365:150-155.
- Fuchs E, Green H (1981) Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell 25:617-625.
- Richard G (2000) Connexins: a connection with the skin. Exp Dermatol 9:77-96.
- Wertz K, Seifert N, Hunziker PB, Riss G, Wyss A, et al. (2004) Free Radical Biology and Medicine β-carotene inhibits UVA-induced matrix metalloprotease 1 and 10 expression in keratinocytes by a singlet oxygen-dependent mechanism. Free Radic Biol Med 37:654-670.
- Hazewindus M, Haenen GRMM, Weseler AR, Bast A (2012) The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chemistry 132:954-958.
- Romero-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, et al. (1996) Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem 271:28052-28056.
- Shimoda H, Tanaka J, Shan SJ, Maoka T (2010) Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J Pharm Pharmacol 62:1137-1145.
Citation: Tarshish E, Hermoni K, Schwartz SR (2020) Effect of Oral Supplement “Lycopene” On Reducing the Signs of Skin Ageing. Clin Pharmacol Biopharm 9: 195. DOI: 10.4172/2167-065X.1000195
Copyright: © 2020 Tarshish E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13828
- [From(publication date): 0-2020 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 12686
- PDF downloads: 1142