Review Article Open Access
Effect of Time-of-Day on Muscle Fatigue: A Review
| Hamdi Chtourou1,2*, Omar Hammouda1,3, Asma Aloui1and Nizar Souissi1,3 | |
| 1Research Laboratory “Sport Performance Optimization” National Center of Medicine and Sciences in Sport (CNMSS), Tunis, Tunisia | |
| 2Research Unit (EM2S), High Institute of Sport and Physical Education, Sfax University, Sfax, Tunisia | |
| 3High Institute of Sport and Physical Education, Ksar-Saïd, Manouba University, Manouba, Tunisia | |
| Corresponding Author : | Hamdi Chtourou Research Laboratory Sport Performance Optimization National Center of Medicine and Sciences in Sport (CNMSS), Tunis Tel: + 216-22-87-20-95 E-mail: h_chtourou@yahoo.f |
| Received May 06, 2013; Accepted June 10, 2013; Published June 12, 2013 | |
| Citation: Chtourou H, Hammouda O, Aloui A, Souissi N (2013) Effect of Time-of- Day on Muscle Fatigue: A Review . J Nov Physiother 3:160. doi:10.4172/2165-7025.1000160 | |
| Copyright: © 2013 Chtourou H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
To date, although the effect of time-of-day on short-term maximal performance has been well established with early morning nadirs and peak values in the late afternoon, data concerning the diurnal variations in muscle fatigue appear to be equivocal. Most studies reported higher muscle fatigue (represented by a decrease in muscle power or strength during short-term maximal exercises) in the evening compared with the morning. Moreover, the underlying mechanisms of the diurnal variation in muscle fatigue are not well known. In this context, some biomarkers of cellular damage were higher in the evening compared with the morning, while markers of antioxidant status were higher in the morning. This article focuses on the diurnal variations in short-term maximal performance and some biochemical parameters. Moreover, we aimed to review the effect of time-of-day on muscle fatigue.
| Keywords |
| Circadian rhythm; Sport; Fatigue; Chronobiology; Biochemistry |
| Abbreviations |
| TAS: Total Antioxidant Status; UA: Uric Acid TBIL: Total Bilirubine; Hcy: Homocysteine; SCN: Suprachiasmatic Nuclei; MVC: Maximal Voluntary Contraction; PP: Peak Power; MP: Mean Power; Mg2+: Magnesium concentrations; ATP: Adenosine Triphosphate; IMP: Ionosine Monophosphate; AMP: Adenosine Monophosphate; ADP: Adenosine Diphosphate; Pi: Inorganic Phosphate; K+: Potassium; Ca2+: Calcium; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; NAC: Thiol on or N-acetyl-cysteine; Na+-K+: Sodium-Potassium; CK: Creatine Kinase; LDH: Lactate Deshydrogenase; ALAT: Alanine; ASAT: Aspartate Aminotransferase; WBC: White Blood Cells; Lac: Blood Lactate; RMS: Root Mean Square; NME: Neuromuscular Efficiency; MPF: Mean Power Frequency; HG: Hand Grip; RPE: Rating of Perceived Exertion |
| Introduction |
| The existence of circadian rhythms is recognized by the scheduling of athletic training sessions at different times of the day. However, competition schedules are often set to suit the typical activities of spectators and television audiences rather than those of the athletes [1]. |
| It is generally established that peak short-term maximal performance is achieved in the afternoon/evening at about the time when body temperature is at its circadian peak, with amplitudes ranging between 3% and 22%. For example, during a 30 second Wingate test, Souissi et al. [2] showed that peak (PP) and mean (MP) power fluctuate with time-of-day, with morning nadirs, afternoon/ early evening peak values and a peak to trough amplitude of 7.6% and 11.3% respectively. However, the literature regarding circadian or time-of-day effects on muscle fatigue yields inconclusive results. |
| Muscle fatigue is generally characterized by an impaired performance [3] with origins generally linked to peripheral and/or central mechanisms [4]. Peripheral fatigue refers to biochemical and ionic changes at the muscle level that could alter contractile process and/or excitation-contraction coupling, while central fatigue is attributed to a decline in motoneuronal output and/or central motor command [5]. |
| Concerning the effect of time-of-day on muscle fatigue, in active males, Lericollais et al. [6] showed that muscle power decrement during a 60 second Wingate test defined two phases: (i) during the first 20 seconds, power decreased rapidly and values were higher at 18:00 h compared with 06:00 h; and (ii) during the last 40 seconds, power decreased slightly and values were not significantly different between 18:00 h and 06:00 h. However, in healthy male road competitive cyclists, Lericollais et al. [7] observed that power output values recorded during a 60 second Wingate test were higher at 18:00 h than 06:00 h indicating that fatigue is not affected by time-of-day. During a repeated sprint exercise, Racinais et al. [8] reported that power output decrement throughout the sprints was greater at 17:00 h than 08:00 h. However, this time-of-day effect was not present when participants produced the same initial power output in the afternoon and in the morning. The authors concluded that the higher power decrement observed in the afternoon could be due to a greater initial power output developed at this time-of-day as compared to the morning rather than higher muscle fatigability. |
| The underlying mechanisms of the diurnal variation in muscle fatigue are not well known. In this context, it has been reported that some resting biochemical markers of muscle fatigue (i.e., lactate (Lac), muscle damage, oxidative stress) are time-of-day dependent [9]. Also, biomarkers of cellular damage and leukocytes measured after a 30 second Wingate test [10] and a repeated sprint test [11] were higher in the evening compared with the morning. However, markers of antioxidant status (i.e., Total Antioxidant Status (TAS), Uric Acid (UA), and Total Bilirubine (TBIL)) were higher in the morning compared with the evening only before the repeated sprint test [11]. Thus, the higher muscle fatigue in the evening hours during shortterm maximal exercise could be, in part, explained by the higher levels of homocysteine (Hcy) and biological markers of muscle injury and lower resting antioxidant status at this time-of-day. |
| In view of the above considerations, this paper aimed to review the effect of time-of-day on muscle fatigue and to appraise the diurnal variations in short-term maximal performance and some biochemical parameters. |
| Effect of Time-of-Day on Sport Performance |
| Chronobiology is the science that investigates the time-dependent changes in many variables (e.g., physiological, biochemical). A biological rhythm has been defined as a sequence of events which, in a steady state, repeat themselves in time in the same order and same interval [12]. Although biological rhythms are present in a wide range of periods, circadian rhythms have been the most widely investigated and have the farthest reaching implications for humans. Circadian rhythms refer to variations that recur periodically every ~24 hours. Circadian-based variations have been described extensively in most of physiological, biochemical, behavioral, and cognitive parameters in humans [13-17]. The expression of these circadian rhythms is representative of 24 hour fluctuations in both endogenous (i.e., the “body clock”) and exogenous (i.e., called ‘zeitgebers’; the most important one is the external light-dark cycle) factors [18]. Therefore, circadian rhythms can be attributed to a mixture of influences from the external environment, the individual’s sleep-wake cycle, and an internal “body clock” (i.e., suprachiasmatic nuclei (SCN)) [19]. However, precise information on the relative importance of endogenous or exogenous factors is lacking [20]. |
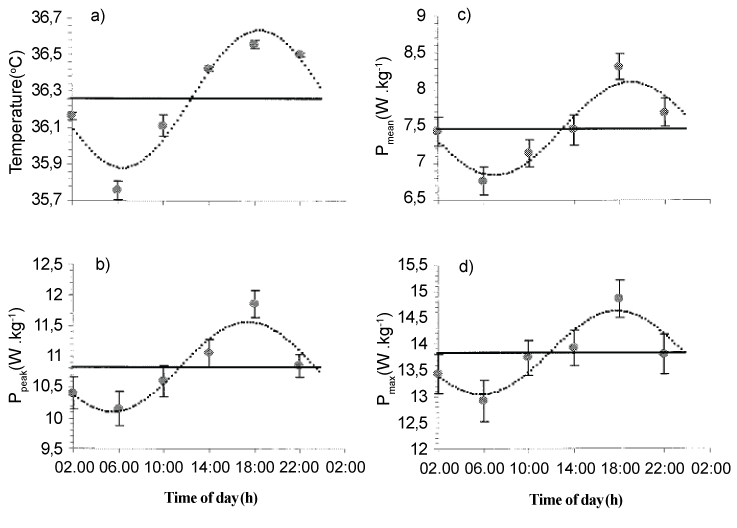
| The circadian rhythm of core temperature is often considered as a marker of the body clock due to its strong endogenous component. Body temperature falls to a minimum during the night (i.e., between 04:00 h and 06:00 h) and begins to rise before wakefulness until the peak value (i.e., acrophase) of the rhythm is typically observed in the late afternoon (i.e., between 17:00 h and 19:00 h [12]. The amplitude of these 24 hour variations is 0.4 to 0.5°C in young adults [12]. In this context, Souissi et al. [2] reported that body temperature fluctuates with time-of-day with an acrophase observed at 18:22 ± 00:34 h and an amplitude of 0.39 ± 0.03°C (Figure 1). Also, various components of physical, mental, and psychomotor performance display circadian rhythms closely in phase with the 24 hour fluctuation in body temperature [20]. However, circadian rhythms of complex mental tasks and mood states are not in phase with that of body-temperature [21]. |
| It has been well documented that short-term maximal performances vary with time-of-day, with morning nadirs, afternoon acrophases, and amplitudes ranging from 3 to 22% [22-28]. The amplitude of the diurnal or circadian rhythm of short-term maximal performance depends on the population tested, the muscle groups and/or the experimental design [29]. For example, concerning muscle strength, Martin et al. [30] examined the effect of time-of-day (07:00 h vs. 18:00 h) on maximal voluntary contraction (MVC) and reported that the force produced during a MVC is higher at 18:00 h than 07:00 h (+8.9%). Likewise, Gauthier et al. [31] examined the effect of time-ofday (i.e., circadian rhythm: 01:00 h, 05:00 h, 09:00 h, 13:00 h, 17:00 h, 21:00 h) on isometric and isokinetic (i.e., 1.05 rad•s-1, 2.09 rad•s-1, 3.14 rad•s-1, 4.19 rad•s-1, and 5.24 rad•s-1) torque of the elbow flexor muscles. They showed a significant circadian rhythm in muscle isometric and isokinetic torque with higher values observed between 17:50 h and 18:30 h and amplitudes of ~9-12%. Additionally, Nicolas et al. [22] reported a significant diurnal variation in muscle strength during 50 MVC of the knee extensors at 2.09 rad•s-1 (i.e., significant time-of-day effect only for the first 26 repetitions) with an acrophase observed at 18:00 h. In the same way, performance during explosive exercise (i.e., squat-jump, counter-movement-jump (CMJ) [32-34]) or intermittent [10,35,36] and continuous [10,37] high-intensity cycling exercise is time-of-day dependent. For example, Souissi et al. [2] studied the circadian rhythm of PP and MP during a 30 second Wingate test and reported a significant time-of-day effect, with morning nadirs (i.e., ~06:00 h), afternoon/early evening acrophases (i.e., 18:24 h and 18:00 h for PP and MP respectively), and an amplitude of ~7.6% and 11.3% for PP and MP, respectively (Figure 1). Likewise, during intermittent exercise, Chtourou et al. [36] showed that muscle power was significantly higher in the evening (17:00 h) compared with the morning (07:00 h) only for the first two sprints during a 5×6 second sprint exercise. Recently, Pallarés et al. [38] investigated the effect of time-of-day (i.e., 10:00 h and 18:00 h) on short-term maximal performance (i.e., bench press maximum strength and muscle power, CMJ, crank-arm peak power (i.e., a 10 second Wingate test), and time to complete 25 m freestyle). The authors reported that performance was significantly enhanced in the afternoon compared with the morning in 25 m swimming time (1.7%), bench press maximum strength (3.6%), bench press muscle power (5.1%), and CMJ height (5.8%), but not in crank arm power. |
| However, the literature regarding circadian or time-of-day effects on long duration exercise or muscle fatigue yields inconclusive results. This updated review aims to present the current state of knowledge of the effect of time-of-day on muscle fatigue. |
| Muscle Fatigue |
| Muscle fatigue is generally characterized by an impaired performance [3]. The origins of this phenomenon are generally linked to peripheral and/or central fatigue [4,39]. Peripheral fatigue refers to biochemical and ionic changes at the muscle level that alter contractile process and/or excitation-contraction coupling, while central fatigue is described as the decrease in muscle force attributable to a decline in motoneuronal output and/or central motor command [5]. |
| Many muscle properties, including action potentials, extracellular and intracellular ions, and intracellular metabolites, change during a fatiguing task [40]. Muscle fatigue could be induced by alteration of ionic movements. Indeed, the increase in magnesium (Mg2+) concentrations decreased the adenosine triphosphate (ATP) concentrations. Adenosine diphosphate (ADP), adenosine monophosphate (AMP), and ionosine monophosphate (IMP) have much lower affinity for Mg2+ than does ATP [41]. Moreover, inorganic phosphate (Pi) and potassium (K+) accumulation may decrease the myofibrillar force production and calcium (Ca2+) sensitivity and release from the sarcoplasmic reticulum [42,43]. Accordingly, increased Pi is considered to be a major cause of fatigue [43]. Moreover, anaerobic glycolysis is of central importance in muscle fatigue. The breakdown of glucose to Lac ions and H+ protons may induce early acidosis associated with rapid onset of muscle fatigue [42]. The accumulation of lactic acid in the muscle has been suggested to be among major causes of muscle fatigue (for review see Fitts [42]). Furthermore, the gradual accumulation of extracellular lactate around muscle fibers during exercise probably reduces any tendency for intracellular lactate to trigger vacuolation of the T system [44]. Although correlations do not necessarily imply cause and effect relationships, the explanation that lactate formation causes acidosis and muscular fatigue is present in the scientific literature and in biochemistry, physiology, and exercise physiology textbooks [42,43]. |
| On the other hand, there is a large and rapidly growing literature establishing that reactive oxygen species (ROS) are produced in active muscles and have some roles in fatigue (Allen et al. [40]). The most convincing evidence that ROS contribute to fatigue comes from experiments with exogenously added ROS scavengers which can reduce the rate of fatigue in isolated muscles and intact muscles of animals. A range of ROS scavengers have subsequently been shown to slow fatigue including superoxide dismutase (SOD), catalase [45,46], Tiron, and Tempol [47]. Furthermore, it is generally agreed that ROS production is accelerated by muscle activity and by increased muscle temperature with exercise. Moopanar and Allen [47] found that fatigue was unaffected by ROS scavengers at 22°C but was substantially slowed by ROS scavengers at 37°C. The increased sensitivity of muscle fatigue to ROS scavengers at 37°C may result from an increased rate of ROS production as temperature increases. Although attenuation of fatigue in response to ROS scavengers has been frequently reported in isolated muscles or intact muscles of animals, improvements in human performance in response to dietary antioxidants (e.g., vitamins C and E) have not usually been observed. |
| As already described by Reid et al. [45,46] and McKenna et al. [48], an exception is the thiol donor N-acetyl-cysteine (NAC) which seems to have beneficial effects on muscle fatigue. McKenna et al. [48] showed that NAC may minimize the reduction in sodium-potassium (Na+-K+) pump activity that usually occurs in exercise, and attenuate the rise in plasma K+ [49]. Given that the Na+-K+ pump is redox sensitive [50], the authors proposed that ionic changes associated with Na+-K+ pump contribute to fatigue and can be attenuated by the ROS scavenger ‘NAC’. Bruton et al. [51] showed that fatigue-induced prolonged low-frequency force depression can be caused by two ROSdependent mechanisms: (i) decreased sarcoplasmic reticulum Ca2+ release associated with low SOD activity and (ii) reduced myofibrillar Ca2+ sensitivity associated with high SOD activity. The reduced sarcoplasmic reticulum Ca2+ release may be related to increased superoxide levels. Additionally, previous research demonstrated that markers of muscle damage, e.g., creatine kinase (CK), lactate deshydrogenase (LDH), alanine (ALAT), aspartate aminotransferase (ASAT), as well as white blood cells (WBC) and blood Lac, increased significantly during high-intensity maximal tasks [52]. These parameters are indicative of muscle fatigue and overtraining [53-55]. Also, during long duration sports (e.g., triathlon), leg muscle fatigue was correlated with blood markers of muscle damage [56]. Indeed, Del Coso et al. [56] indicated that 35% of jump height loss experienced by the triathletes could be explained by blood markers of muscle damage (i.e., myoglobin and CK). The authors concluded that muscle damage is a good predictor of leg muscle fatigue after running. |
| Effect of Time-of-Day on Muscle Fatigue |
| Muscle fatigue can be defined as an exercise-induced decrease in maximal force-generating capacity of muscles [57]. |
| As mentioned previously, short-term maximal performances are time-of-day dependent. However, data concerning the effect of time-of-day on muscle fatigue appear to be equivocal (Table 1). In the literature, the time-of-day effect on fatigue has been investigated either during discontinuous (i.e., repeated brief maximal efforts) or continuous (i.e., single sustained maximal efforts) short-term maximal exercises. |
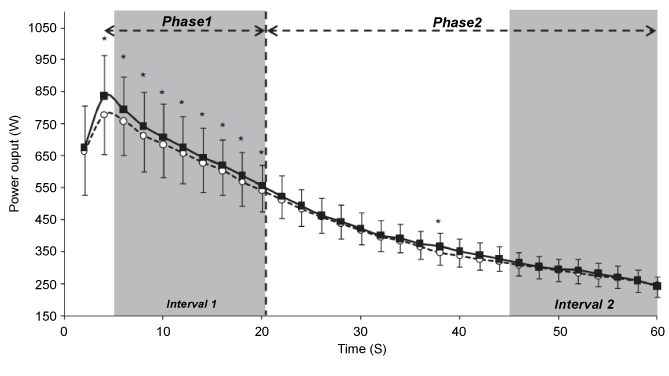
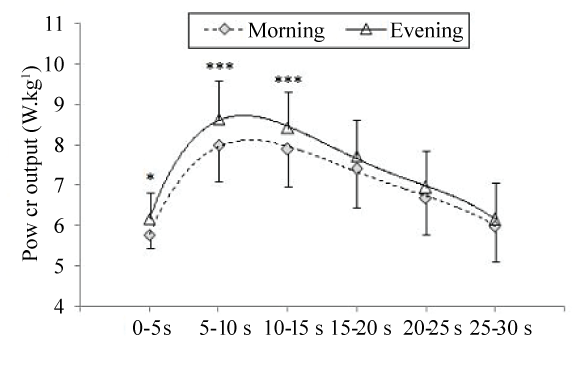
| Deschenes et al. [58] investigated the effect of time-of-day on torque decrease during 50 MVC. The authors did not observe any significant time-of-day effect on muscle fatigue during this exercise. In contrast, Nicolas et al. [22] showed that the diurnal variation in torque decrease defined two phases: (i) from the 1st to the 26th repetition (i.e., the first phase), torque decreased quickly and values were higher at 18:00 h than 06:00 h; and (ii) from the 27th to the 50th repetition (i.e., the second phase), torque values were not significantly different between the two times-of-day. During this latter phase, the torque decreased slightly and reached a floor value that appeared constant with time-of-day (Figure 2). Moreover, the authors investigated the evolution of the EMG parameters (Root Mean Square; RMS) and the neuromuscular efficiency (NME) for the vastus medialis and the vastus lateralis muscles during the 50 MVC at the two times-ofday. They showed that the NME decrease was higher in the evening than the morning only during the first phase of fatigue (i.e., the 1st to the 26th repetition). However, the RMS values were not different between 18:00 h and 06:00 h. Therefore, Nicolas et al. [22] concluded that the diurnal variation in muscle fatigue during 50 MVC seems to reflect on the muscle, with a greater contractile capacity but a higher fatigability at 18:00 h compared with 06:00 h. Likewise, Lericollais et al. [6] investigated the evolution of muscle power during a 60 second Wingate test at 06:00 h and 18:00 h in active males. In agreement with Nicolas et al. [22], Lericollais et al. [6] showed that muscle power decrement during a 60 second Wingate test defined two phases: (i) during the first phase (i.e., first 20 seconds of the exercise), power decreased rapidly and values were higher in the evening compared with the morning; and (ii) during the second phase (i.e., from 21 seconds to 60 seconds), power decreased slightly and values were not significantly different between 18:00 h and 06:00 h (Figure 3). Moreover, the authors found that fatigue index calculated during a 60 second Wingate test was significantly higher at 18:00 h (71.4%) than 06:00 h (69.2%). The same findings were observed by Chtourou et al. [37] in adults and by Souissi et al. [59] in boys. In these studies, fatigue index calculated during a 30 second Wingate test was significantly higher in the evening compared with the morning (i.e., morning vs. evening: 49% vs. 51% [37] and 30% vs. 34% [59]). Moreover, in agreement with Lericollais et al. [6], Chtourou et al. [37] and Souissi et al. [59] showed that power decrement during a 30 second Wingate test defined two phases. In the study of Chtourou et al. [37], the first phase was observed during the first 20 seconds during which muscle power was significantly higher in the evening compared with the morning. However, the second phase was observed during the last 10 seconds during which the evolution of power output was not significantly different between 07:00 h and 17:00 h. In the study of Souissi et al. [59], the first phase was observed during the first 15 seconds and the second phase was observed during the last 15 seconds of the test (Figure 4). The slight discrepancies between data of Chtourou et al. [37] and those of Souissi et al. [59] could be explained by the difference in fatigability during the Wingate test between adults and boys. In this context, Hebestreit et al. [60] showed that during the Wingate test, children are more fatigue resistant than adults. Moreover, Chtourou et al. [37] and Souissi et al. [59] reported that power output decrement during the 30 seconds was concomitant to an increase in mean power frequency (MPF) of the EMG and NME. Indeed, during the first phase, NME and MPF were higher in the evening than the morning. However, during the second phase, NME and MPF were independent of time-of-day of measurement. In addition, the authors observed no significant difference between 07:00 h and 17:00 h for RMS during the whole 30 seconds of the exercise, and concluded that changes in muscle power and fatigue during a 30 second Wingate test are likely linked to modifications prevailing at the peripheral rather than central level. However, in healthy male road competitive cyclists, Lericollais et al. [7] observed that power output values recorded during a 60 second Wingate test were higher at 18:00 h than 06:00 h indicating that fatigue induced by this exercise was not affected by time-of-day (Figure 5). These results indicate a similar power output decrement at 06:00 h and 18:00 h. The total decrease in power output was ~59% at the two times-of-testing. The authors hypothesized that the freedom and complexity of cycling exercises could allow adaptations in movement patterns, as a function of time-of-day, in order to maintain higher performance in the evening. Indeed, in the study of Lericollais et al. [7], subjects were highly trained cyclists and familiar with cycling exercises. Moreover, the self-selected exercise intensity used to distribute work and energy (i.e., pacing-strategy) throughout a 60 second Wingate test could explain the no-significant difference between the morning and the evening in the fatigue index. In contrast, Souissi et al. [61] showed that fatigue index during a 30 second Wingate test was higher in the morning (06:00 h) than the evening (18:00 h). Moreover, the authors observed better aerobic participation in energy production during the 30 second Wingate test in the afternoon than the morning. They concluded that the higher fatigue index observed in the morning compared with the afternoon could be due to better contribution of aerobic pathway in the afternoon. The discrepancies between studies concerning the fatigue index calculated during the Wingate test could be explained by its intrinsic variability [7]. For example, in the study of Souissi et al. [61], the fatigue index was calculated as the percentage of the difference between peak and minimal power recorded during the test. Nevertheless, Lericollais et al. [6,7], Chtourou et al. [37], and Souissi et al. [59] determined the fatigue during the Wingate test by taking into account all power output values recorded during the exercise. Recently, Chtourou et al. [62] investigated the effect of time-of-day (i.e., 09:00 h, 12:30 h, and 16:00 h) on short-term maximal performance (i.e., hand grip (HG) and a 30 second Wingate test) before and after a judo match in young judokas. The results indicated a significant decrease in HG and muscle power during the Wingate test at the three times of measurement after the combat compared with before the combat. However, the decrease in performance was higher at 16:00 h than 09:00 h and 12:30 h (e.g., 3.7% vs. 3.7% vs. 6.4% for HG at 09:00 h, 12:30 h, and 16:00 h respectively). The authors concluded that the diurnal variations in muscle strength and power disappeared after a judo match by a higher fatigue (i.e., decrease in performance) in the afternoon than the morning. However, Chtourou et al. [62] reported that the subjective estimation of fatigue (i.e., ratings of perceived exertion (RPE)) was not significantly different between 09:00 h, 12:30 h, and 16:00 h. In this context, the literature presents equivocal findings concerning the effect of time-of-day on the subjective estimation of fatigue (Table 2). Indeed, although Hammouda et al. [10] reported higher RPE scores at 17:00 h than 07:00 h, Reilly et al. [21] showed that fatigue estimated by the mood-adjective check-list of McNair et al. [63] was higher in the morning than the evening. However, other studies did not show significant difference between the morning and the evening for subjective estimation of fatigue [27,64]. |
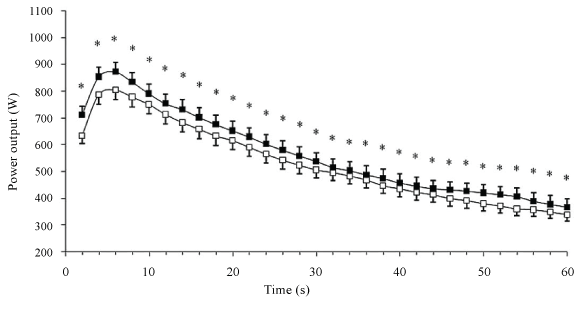
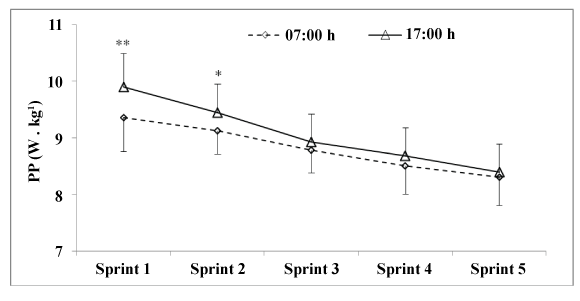
| During discontinuous cycling test, Falgairette et al. [65] did not find any diurnal variation in fatigue. Likewise, Giacomoni et al. [66] found that patterns of fatigue during 10×6 second repeated all-out maximal sprints interspaced by 30 second rest periods were similar between the morning (08:00 h) and the evening (17:00 h). However, Racinais et al. [64] observed a higher decrease in power output between the first and the second sprint during 5×6 second maximal sprints interspersed by 24 seconds of passive recovery in the evening (17:00 h) compared with the morning (07:00 h). The higher muscle power observed in the evening compared with the morning disappeared during the last 4 sprints leading to a higher power decrement at 17:00 h (11% vs. 7% for the evening and the morning, respectively). The same results were observed by Chtourou et al. [36] (Figure 6) and Zarrouk et al. [67] (Figure 7) who reported a higher power decrement during a repeated sprint test in the evening compared with the morning. Moreover, Zarrouk et al. [67] found that the EMG RMS values were not significantly different between the morning and the evening. This provides evidence that the diurnal increase in muscle power and fatigue is not due to a modification in neural drive but rather to an improvement in muscle contractile properties at the time of peak performance. |
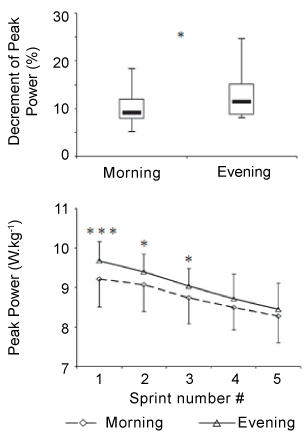
| To explain the higher muscle fatigue observed in the evening, it can be argued that reaching a higher muscle power or strength at this time-of-day predisposes the subject to a subsequent greater decrease in performance. In this context, Racinais et al. [8] investigated if the higher fatigue observed in the afternoon compared with the morning during a repeated sprint exercise represents a time-of-day effect on fatigability or a consequence of a higher initial power output. In this study, subjects performed 3 different 10×6 second maximal cycling sprint exercises with a recovery period of 30 seconds between repetitions at 08:00 h, 17:00 h, and 17:00 h controlled (i.e., the initial power was controlled to be the same as the first two sprints recorded at 08:00 h). The authors showed that power output was higher in the afternoon than the morning during the first 5 sprints, but not significantly different between 08:00 h and 17:00 h during the last 5 sprints of the exercise. However, this greater decrement in power output observed at 17:00 h was no longer present when participants were producing the same initial performance in the afternoon as in the morning (i.e., during 17:00 h controlled) (Figure 8). The authors concluded that the higher torque and power decrements observed in the afternoon could be a consequence of a greater initial torque or power output in the afternoon as compared to the morning rather than higher muscle fatigability. Similarly, previous reports showed a positive relationship between initial performance and power decrement during a repeated sprint exercise [68]. Therefore, the greater power decrement observed at 17:00 h is simply a mathematical/statistical consequence of a greater initial power output [8]. The aforementioned findings of Racinais et al. [8] could explain why both Falgairette et al. [65] and Giacomoni et al. [66] did not observe any time-of-day effect on muscle fatigue during the repeated sprint exercises when initial sprints were not statistically different between the morning and the afternoon. |
| Diurnal Variations in Biochemical Measures |
| Diurnal fluctuations in resting biochemical markers of muscle fatigue |
| The exact underlying mechanisms of the diurnal variation in muscle fatigue are not well known [69,70]. Although Nicolas et al. [22] suggested that the diurnal variation in muscle fatigue is linked to changes in muscle contractile properties, e.g., NME; Lericollais et al. [6] explained muscle fatigue during a 60 second Wingate test by a reduction in the range of motion of the ankle angle in the evening. Otherwise, many biochemical markers of muscle fatigue (e.g., Lac, muscle damage, oxidative stress) are time-of-day dependent. Indeed, resting biochemical markers of cellular damage [71,72], as well as WBC and their subpopulations [73], are time-of-day dependent with acrophases generally observed in the evening. In addition, plasma Hcy levels, an indirect marker of oxidative stress, were also shown to vary in a daily manner in humans with an evening peak and a morning nadir [74]. Also, respiratory and motor activities, which directly determine the production of free radicals, are diurnally variable [75]. |
| Considerable attention has been recently focused on the chronobiological aspects of the antioxidant system. Indeed, total antioxidant capacity [75], activities of some antioxidant enzymes [76], and rate of lipid peroxidation [77,78] were higher in the morning compared with the evening. From these studies, the antioxidant system is more efficient in the early morning than the evening. Although origins of these fluctuations are still unclear, it has been identified that the physiological levels of melatonin contribute to total antioxidant capacity of human serum [79]. This hormone has a powerful antioxidant role [80]. |
| Recently, Hammouda et al. [9] confirmed the diurnal variations in biological markers of muscle injury and antioxidant status in young soccer player. The authors showed that resting levels of Hcy, biomarkers of cellular damage, and leucocytes were higher in the evening compared with the morning [9]. These diurnal variations in leucocytes and muscle damage enzymes could be linked to the circadian rhythm of core temperature [72,73]. In fact, Dalton et al. [81] supposed that the evening elevation in body temperature would increase the activity of some enzymes such as phosphofructokinase and LDH. In agreement, Hammouda et al. [9] showed that core temperature was higher in the evening than the morning. Also, Hammouda et al. [9] observed that markers of antioxidant status (i.e., TAS, UA, and TBIL) were higher in the morning than the evening. Similarly, peak TAS [75,79] and UA [71,82] were observed in the early morning. UA is a powerful antioxidant [83] and has a protective role of vitamins E and C [84]. Hence, inflammation and fatigue could be more important in the evening due to a more efficient antioxidant status in the morning. |
| Diurnal fluctuations in biochemical markers of muscle fatigue during exercise |
| Fluctuations in the biomarkers cited previously at rest could affect performance during short-term maximal exercise. However, research on the diurnal variations in biochemical markers of muscle fatigue during exercise are lacking. Recent studies showed that biomarkers of cellular damage and leukocytes were higher in the evening compared with the morning during a repeated sprint exercise [11] and a 30 second Wingate test [10]. However, markers of antioxidant status, i.e., TAS, UA, and TBIL, were higher in the morning only before the repeated sprint test. Moreover, Hammouda et al. [10] showed that Lac levels measured after the Wingate test were higher in the evening than the morning. Also, Racinais et al. [64] reported that plasma Lac levels during a repeated sprint exercise were higher in the evening than the morning, which could explain the higher muscle fatigue at this timeof- day. The difference between the morning and the evening in Lac responses to exercise might be linked to an increased catecholamine activity since catecholamines follow very similar patterns in response to exercise [58]. Furthermore, Dalton et al. [81] suggested that the circadian variation in core temperature could affect Lac production during exercise. In addition, Hammouda et al. [11] showed that plasma levels of muscle damage enzymes and oral temperature were higher at 17:00 h than 07:00 h. The acrophases of these enzymes [72], as well as immunological process [73] correlate with the peak of oral temperature rhythm, which may explain the diurnal variation in muscle power [61]. The higher increase in these enzymes in the evening compared with the morning in response to exercise could be due to their higher resting values and the greater initial power output during short-term maximal exercises in evening hours. The higher muscle fatigue in the afternoon during short-term maximal exercise could be, also, due to higher resting levels of Hcy and biological markers of muscle injury and lower resting antioxidant status at this time-of-day [10,11]. |
| Likewise, Hammouda et al. [26] showed that Lac and glucose responses to a Yo-Yo intermittent recovery test were higher in the evening than the morning which could indicate a greater anaerobic contribution to energy production (i.e., higher mobilization of glucose metabolism at this time-of-day). Moreover, diurnal variations in Lac responses have been identified during mild [85] and maximal [86,87] aerobic exercises that would be indicative of raised anaerobic metabolic activity and muscle fatigue in the evening compared with the morning. Additionally, Hammouda et al. [26] showed that LDH and CK increase during the Yo-Yo test was higher in the evening than the morning. As previously shown, this increase in muscle damage enzyme and Lac levels could be indicative of higher muscle damage in the evening [10,11]. |
| Hence, biochemical markers of muscle damage and antioxidant status could explain, at least in part, the metabolic origins of the diurnal fluctuation in muscle fatigue. However, further research is imperative to clarify the conflicts regarding the diurnal variation in muscle fatigue during high-intensity exercises. |
| Conclusion |
| Short-term maximal performances vary with time-of-day, with morning nadirs, afternoon acrophases, and amplitudes ranging from 3 to 22%. However, the literature concerning the effect of time-ofday on muscle fatigue presents inconclusive results. Indeed, although some investigations did not show any time-of-day effect, most studies reported higher muscle fatigue (decrease in muscle power or strength during short-term maximal exercises) in the evening compared with the morning. Moreover, to explain, in part, the diurnal variation in muscle fatigue, recent studies showed that some biomarkers of cellular damage were higher in the evening compared with the morning, while markers of antioxidant status were higher in the morning. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 17050
- [From(publication date):
May-2013 - Sep 01, 2025] - Breakdown by view type
- HTML page views : 12347
- PDF downloads : 4703
