Effectiveness of Single and Microbial Consortium of Locally Isolated Beneficial Microorganisms (LIBeM) in Bioaugmentation of Oil Sludge Contaminated Soil at Different Concentration Levels: A Laboratory Scale
Received: 24-Oct-2017 / Accepted Date: 08-Feb-2018 / Published Date: 12-Feb-2018 DOI: 10.4172/2155-6199.1000430
Abstract
An aerated static pile ASP-bioreactor system made up of acrylic material dimension (60 cm × 40 cm × 20 cm) was developed to examine the potential of single and microbial consortium of LIBeM to remediate oil sludge contaminated soil at different concentration levels. Three different strains of LIBeM namely P. aeruginosa-BAS-Cr1, S. paucimobilis-ReTOS-Cr1 and S. maltophilia-RAS-Cr1 were used in this study was obtained from Environmental Microbiology Laboratory, Universiti Malaysia Sabah. Five sets of experiment filled with 10 kg of soils contaminated with 5% and 10% of oil sludge were carried out as Treatment 1 (contaminated soil+P. aeruginosa-BAS-Cr1), Treatment 2 (contaminated soil+S. paucimobilis-ReTOS-Cr1), Treatment 3 (contaminated soil+S. maltophilia-RASCr1), Treatment 4 (contaminated soil+Microbial Consortium; P. aeruginosa-BAS-Cr1+S. paucimobilis-ReTOSCr1+ S. maltophilia-RAS-Cr1) and Treatment 5 (contaminated soil+indigenous bacteria in soil; NA). Their ability to degrade hydrocarbon in the soil was investigated during 60 days incubation periods. Physical and chemical analyses were carried out from each of the treatment and control plot on a weekly basis to check for pH, moisture content, temperatures and Total Petroleum Hydrocarbon (TPH). The results showed that single strain P. aeruginosa- BAS-Cr1 has the highest oil degrading capacity compared to microbial consortium with 80% and 85.2% at both concentration studied. The percentage of TPH removal by P. aeruginosa-BAS-Cr1 is 3-fold higher than NA, thus confirmed that the addition of oil selective degrading bacteria was much better than the control plot. High degradation of long chain alkanes were observed between the control and treatment plot suggested that bioaugmentation using single and microbial consortium had decrease the level of oil sludge in contaminated soil.
Keywords: Bioremediation; Oil sludge; Oil degrading bacteria; Different concentration levels; Single strain; Microbial consortium; LIBeM
Introduction
Contaminations of soil by petroleum hydrocarbon have become a global problem across decade’s concern by many countries. This contaminant is mostly come from oil exploration, accidental spills, transport and oil processing [1]. The release of hydrocarbons into the environment poses a significant impact especially on human health, species and groundwater system. Due to its eco-toxicity, mutagenic and carcinogenic properties, oil sludge has been characterized as hazardous waste according to Environment Protection Act and Hazardous Waste Handling Rules. Soil contamination with oil sludge can result in diminished microbial biomass, losses in soil quality, inhibition of organic matter mineralization, reduced viable bacterial population densities and decreased leaf litter decomposition [2]. Various physiochemical methods such as soil washing, soil vapour extraction, incineration, the use of oil booms and solidification are available for oil spills remediation. Nevertheless, most of these technologies are costly, energy intensive, inefficiently and not to be eco-friendly towards the environment. In order to overcome these problems, bioremediation technologies based on natural microbial population is one of the extensive attentions and have become a current research focus especially in terrestrial environment [3]. Bioremediation, the use of microorganisms specifically oil degrading bacteria can enhance and breakdown contaminants such as oil sludge into less harmful substances [4]. One commonly used bioremediation method involves the use of single and microbial consortium as bioaugmentation strategies. Previous studies by Sorkhoh showed that individual microorganism can metabolize only a limited range of hydrocarbon substances whereby mixed populations with overall broad enzymatic capacities are required to bring the rate and extent of petroleum biodegradation further. However, the utilization of oil sludge contaminated soil by mixed bacterial culture has to be more focused into synergistic and antagonistic interactions among members of the association which may lead to incomplete/complete degradation of the product. In this paper, we are trying to examine the effectiveness of single and microbial consortium of LIBeM in bioaugmentation of oil sludge contaminated soil at different concentration levels in a laboratory scale experiment.
Materials and Methods
Bioreactor design
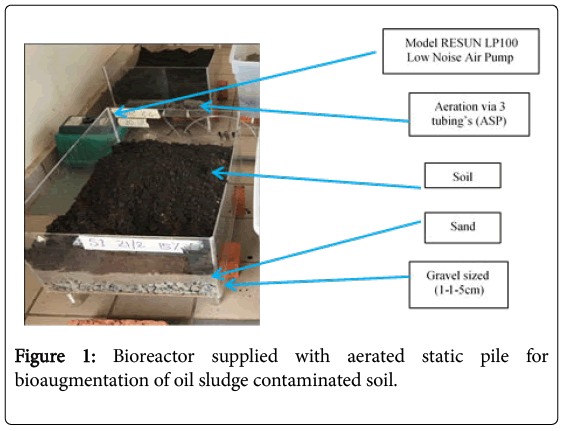
An aerated static pile (ASP)-bioreactor made up of acrylic material with dimension of 60 cm × 40 cm × 20 cm with 3 silicone tubing for aeration at the side of the reactor connected to air pump (Model RESUN LP100 Low Noise Air Pump) [5] were prepared (Figure 1). The reactor were divided into 3 parts where the base is filled with gravelsized (1-1.5 cm) followed by sand (3 cm) and soil (3 cm) on the surface. The soils used in the study were mixed thoroughly before being put in the treatment plot.
Sources of locally isolated bacteria
LIBeM strains used in this study were from Environmental Microbiology Laboratory, Faculty of Science and Natural Resources at Universiti Malaysia Sabah. These microbes (LIBeM) were previously isolated from contaminated area and had already undergone screening. All of these microorganisms have been proven to degrade oil and phenol based on the previous research done by Piakong and Nurul Huda [6,7]. The carbon source of these strains is oil sludge and MgSO4.7H2O (Figure 2).
Culture medium
Ramsay broth was used as the culture medium to supply nutrients for the microbial growth. All the components and constituents listed in Table 1 were mixed with 1.0 L of sterile distilled water. The liquid medium was transferred to Scott bottle and autoclaved for 15 minutes at 121°C.
| Ingredients | Composition (g/L) |
|---|---|
| Ammonium nitrate (NH4NO3) | 2.0 |
| Monopotassium phosphate (KH2PO4) | 0.5 |
| Dipotassium phosphate (K2HPO4) | 1.0 |
| Potassium chloride (KCl) | 0.1 |
| Calcium chloride dihydrate (CaCl2.2H2O) | 0.01 |
| Yeast extraction | 0.06 |
| Glucose | 20 |
| Magnesium sulfate heptahydrate MgSO4.7H2O | 0.5 |
Table 1: Ingredients for the preparation of Ramsay broth [24].
Inoculum preparation
Single colony and mixed cultures of LIBeM isolated were inoculated into Ramsay broth at 30°C for 24 hours in an orbital shaker at 200 rpm. A total of 10% of the cultured bacteria with OD 0.5 and above at 600 nm were used as inoculum. The dense cultures (1 × 107) were harvested for further use.
Soil preparation
10 kg soil was sieved through 0.20 mm sieve size. The physical analysis of soil (pH, temperature and moisture content) was determined according to the Alef and Nannipieri [8]. 0.5 liter (5% v/v) and 1 liter of oil sludge (10% v/v) was sprinkled over the sieved soil and allowed to get adsorbed for 30 minutes for further bioremediation studies [9].
Experiments set-up
The ability of single and microbial consortium of LIBeM isolated to remediate oil sludge contaminated soil was analyzed by carrying out the biodegradation experiment in bioreactor (60 cm × 40 cm × 20 cm) at 5% and 10% oil sludge concentration studied. Five treatments (T1, T2, T3, T4 and T5) were prepared in duplicate at open and air ventilated with following treatment combination (Table 2):
| Treatment | Content |
|---|---|
| Treatment 1: | Soil+oil sludge+(P.aeruginosa-BAS-Cr1) |
| Treatment 2: | Soil+oil sludge+(S. paucimobilis-RETOS-Cr1) |
| Treatment 3: | Soil+oil sludge+(S. maltophilia-RAS-Cr1) |
| Treatment 4: | Soil+oil sludge+(P.aeruginosa-BAS-Cr1+S. paucimobilis-RETOS-Cr1+S. maltophilia-RAS-Cr1) |
| Treatment 5: Control plot |
Soil+oilsludge+without inoculation (Control set) |
Table 2: Treatment combinations.
Technical procedures protocol
For each of the experimental unit the soils were tillage daily and watering with sterile distilled water at an interval 2 days to maintain the water holding capacity of soil. The bioreactors were supplied with 3 silicone tubing for continuous aeration using air pump (Model RESUN LP100 Low Noise Air Pump). 10% (v/v) of inoculants of each single and microbial consortium of LIBeM were added into the plot containing 10 kg of soil contaminated with 5% and 10% of oil sludge. The inoculation of single and microbial consortium of LIBeM into each plot was done for every 2 weeks during 60 days treatment periods. Initial readings were taken immediately after the inoculation of the bacterial culture and further. The soil samples were drawn after every 7th day from the respective experimental unit and were analyzed up to 60 days incubation periods.
Physiochemical analysis of soil
pH: Analysis pH was carried out as according to ASTM [10]. 20 g of soil were weighed and mixed in a beaker contain 40 ml of distilled water. Analysis was done by taking three reading for accuracy using the pH meter.
Temperature: The temperature of each plot was measured by using thermometer in three different places at each bioreactor treatment. The readings were recorded, and the averages were obtained.
Soil moisture content: The soil moisture was analysed by using gravimetric method as described by Gardner [11]. An empty crucible was weighed and record as (M1). Then, 10 g of soil sample were grained with mortar and pestle and recorded as (M2). The soil samples were dry at 110°C for 24 hours in an oven. Let the soil being cooled in desiccator and weighed the crucible as (M3). The soil moisture content was measured by using the formula below:
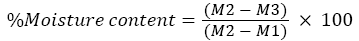
Soil microbial population: The growths LIBeM in each plot soil were determined according to method APHA 9215. Analyses were carried out weekly in each plot study. 10 g of soil sample was mixed with 100 ml sterile distilled water by diluting 1 ml of soil sample into 9 ml of sterile distilled water (dilution plate technique). The dilution series from 10-3 until 10-5 were chosen to calculate the biological population. 0.1 ml of culture of LIBeM was spreader into Nutrient agar and incubated at 30°C for 24 hours. The total CFU were calculated based on the colony growth in the agar.
Total Petroleum Hydrocarbon (TPH) analysis
Total petroleum hydrocarbon was carried out based on gravimetric method (Soxhlet extraction) (USEPA 3540C) (USEPA, 1996). 20 g of soil sample was grained and placed in thimble and extracted with dichloromethane (DCM). Then the thimble was placed in soxhlet extractor. 175 ml of dichloromethane was added into round bottomed flask (RBC). After that the soxhlet extraction were arranged and mixed with enough dichloromethane until it covers the thimble. The cooling temperature and mantel heater was set at 17°C and 4°C respectively. The extraction process takes places for less than 24 hours. Then the content was cooled and let the DCM flow to the round bottomed flask from the extractor. The total solvent was cleared completely with the vacuum evaporator at 40-50°C. By using the rotary pump, let DCM left the sample. The RBC together with the extract was cooled in desiccator after being dried in oven at 40°C. The RBC was measured until constant weighed obtained. The percentage of total petroleum hydrocarbon (TPH) was calculated using the formula below:
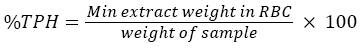
Determination of TPH-(alkane)
Gas chromatography–mass spectrometry (GC-MS) was used to measure the carbon profile of the soil samples in the bioreactor. Total petroleum hydrocarbon was extracted by soxhlet apparatus using dichloromethane as a solvent. The degradation of petroleum hydrocarbon was determined by gas chromatography GCMS (Perkin Elmer). Column Elite 5MS (30 m × 0.25 mm × 0.25 μm) was used together with helium gas for hydrocarbon analysis. 1 μL of sample will be injected into GCMS analysis at 35°C [12].
Results And Discussion
Biodegradation of oil sludge by single and microbial consortium of LIBeM
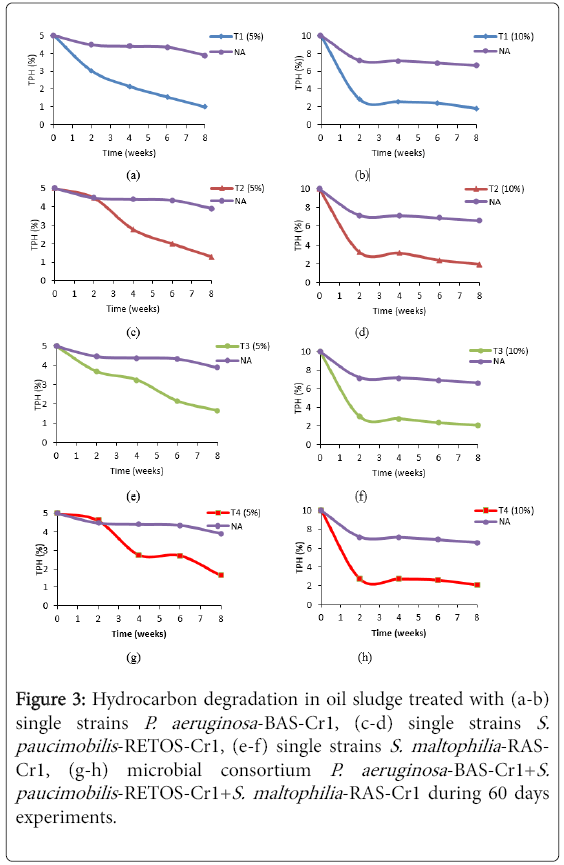
The results for bioaugmentation of oil sludge at different concentration levels showed that TPH decreased by 74%-85% in single strain treatment and 67-79% in microbial consortium of LIBeM. At the same time, activation of indigenous microorganisms in soil (control plots) decreased the TPH by 11%-34% respectively. The inoculation of the single strains and microbial cosortium of LIBeM into the soil rapidly adapted to the environment efficiently degrading hydrocarbon compounds.
This can be proven by the same trend profile in Figures 3a-3h. It is clear to note that all sets treatments studied achieved 50% degradation of TPH after 30 days incubation periods except for control plot. T1 treated with single strain P. aeruginosa -BAS-Cr1 showed the highest degradation TPH with 80% and 85% at both concentrations studied. This followed by Treatment 2>4>3>5 which 74%, 67%, 66% and 22% respectively for 5% oil sludge treated and Treatment 3>2>4>5 which 81%, 80%, 79% and 34% for 10% oil sludge studied. These results confirmed that single strain P. aeruginosa -BAS-Cr1 showed the great potential in oil degrading as compared to microbial consortium.
Figure 3: Hydrocarbon degradation in oil sludge treated with (a-b) single strains P. aeruginosa -BAS-Cr1, (c-d) single strains S. paucimobilis -RETOS-Cr1, (e-f) single strains S. maltophilia -RASCr1, (g-h) microbial consortium P. aeruginosa -BAS-Cr1+S. paucimobilis -RETOS-Cr1+S. maltophilia -RAS-Cr1 during 60 days experiments.
Previous studied by Heinaru et al. [13] reported that microbial consortium were more effective than single strain by the fact that intermediates of a catabolic pathway of one strain may be further degraded by other possessing suitable catabolic pathway. However, this study was not to be significant with Heinaru et al. [13]. The inter/intraspecific interaction between assembles microbial consortium P. aeruginosa -BAS-Cr1+S. paucimobilis -RETOS-Cr1+S. maltophilia - RAS-Cr has influences the rate of biodegradation of oil sludge. The inefficiency of microbial consortium (T4) might be due to the nutrient stress and competition between three single strains thus this phenomenon is commonly known as antagonistic effect [14]. However, as compared to previous researches by Mishra et al. [15], our microbial consortium of P. aeruginosa -BAS-Cr1+S. paucimobilis -RETOS-Cr1+S. maltophilia -RAS-Cr was much better in degrading oil sludge. Mishra et al. [15] reported that the reduction of TPH in oily-sludgecontaminated soil with bacterial consortia of A. baumannii S30, B. cepacia , and nutrients was 38.1% in 120 days while the reduction of TPH in plots treated with A. baumannii S30 and B. cepacia was only 35.7%. This finding also concluded that the percentage of TPH degradation was increased with increasing of oil sludge in the soil. This is due to the fact that oil sludge acted as carbon source and nutrients for the bacteria to consume [1].
Analysis of hydrocarbon content by GCMS
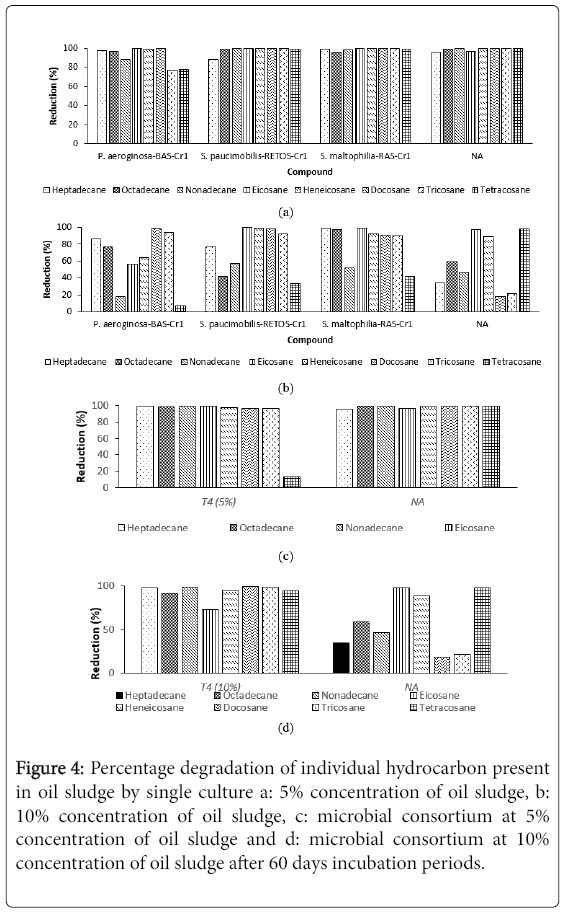
Figures 4a-4d showed the GCMS profile for the hydrocarbon components in single, microbial consortium and control plot after 60 days experiments set. There were eight types of hydrocarbon compounds being observed, namely Heptadecane (C17H36), Octadecane (C18H38), Nonadecane (C19H40), Eicosane (C20H42), Heneicosane (C21H44), Docosane (C22H46), Tricosane (C23H48) and Tetracosane (C24H50). It can be observed that the degradation percentage of individual hydrocarbon in 5% (v/v) degraded more efficiently compared to 10% (v/v) oil sludge. This may due to the high concentration of oil sludge which influences the degradation. Treatment with single strain P. aeruginosa -BAS-Cr1, S. paucimobilis - RETOS-Cr 1 and S. maltophilia -RAS-Cr1 at 5% and 10% concentration of oil sludge showed that the highest degradation (98%) were recorded in Octadecane, Heneicosane and Docosane. Nonadecane experienced the highest degradation (99%) in microbial consortium for both concentration studied. At the same time, treatment in control plot at 10% concentration of oil sludge showed the small degradation of hydrocarbon range from 17.64%-58.75% except for Eicosane and Heneicosane. Vinas et al. reported the reduction of hydrocarbon peak heights and depletion of total area is strong evidence that selected microbes have the ability to utilize and degrade the hydrocarbon as sole carbon sources. By comparing reduction of concentration of alkanes after 60 days, biodegradation of oil sludge in (a and c) 5% (v/v) and (b and d) 10% (v/v) of oil sludge, it was suspected that there was incomplete degradation of oil sludge in 10% (v/v) as the percentage reduction is low. This finding suggested that the time constraint and the accumulation of recalcitrant hydrocarbon are the reasons contribute the problem. Singh et al. suggested conducting longer degradation time with aeration for better oil sludge mineralization. With longer degradation time, the production of biosurfactants and enzyme will be increased and enhanced the degradation process.
Figure 4: Percentage degradation of individual hydrocarbon present in oil sludge by single culture a: 5% concentration of oil sludge, b: 10% concentration of oil sludge, c: microbial consortium at 5% concentration of oil sludge and d: microbial consortium at 10% concentration of oil sludge after 60 days incubation periods.
To successfully exploit the microbial degradation of oil sludge, it is imperative to understand and known the mechanism involved in order to manipulate the microbial activities in the soil. In this study, the metabolic pathway involves in oil sludge degradation is known as multi-enzymes complex process. The initial step in this mechanism is the catabolism of oil sludge by single and microbial consortium of LIBeM (P. aeruginosa -BAS-Cr1, S. paucimobilis -RETOS-Cr 1 and S. maltophilia -RAS-Cr1) which involves the oxidation of the substrate by oxygenases enzymes, in the present of molecular oxygen. The product of the oxidation will be transported inside the cell wall and resulting in hydroxylated hydrocarbon (alcohol). The metabolism pathway continued to degrade hydroxylated hydrocarbon (alcohol) into aldehyde group by oxidation process thus finally form carboxylic acid which similar to fatty acid. Later the process decomposed the carboxylic acid to acetyl-CoA by β-oxidation that easily to degrade.
Soil microbial activity
The data on CFU counts of microorganisms in all treatment soil are shown in Figures 5a-5d. It can be observed that microbial count in all treatment augmented with single and microbial consortium of LIBeM showed a highest cell counts range from 3.1 × 105 to 6.6 × 107 CFU g-1 as compared to control plot. The highest CFU can be observed in T1 treated using P. aeruginosa -BAS-Cr1 proven that these strains have highest adaptation to the oil sludge and thus can reproduce highest population of CFU. The indigenous microorganism in the control plot showed fluctuated to 1.0 × 105 CFU g-1 at T5 (5%) during week 6 incubation periods. Natural attenuation typically depends on the adaptation of available microorganisms for growth and reproduction without human disturbance. Low microbial population in natural attenuation was probably because of less available beneficial microorganisms to degrade the contaminant. It is important to note that the increase and fluctuation of the CFU g-1 per week in all set treatment studied are explained by the impact on the bacteria caused by the oil sludge addition to the medium and the adaptability period or the competition among the augmented strains and indigenous microorganism in the soil [16]. Thus, these findings indicated that the viable cell counts in the inoculated soil with single and microbial consortium of LIBeM are higher than natural attenuation condition. This study highlighted that the inoculations of LIBeM in the treatment plot were done every 2 weeks along the treatment period. This is to ensure that the soil is adequate with mineral nutrient and to avoid the microbial population in the soil drop below 103 CFU g-1 during the biodegradation process. As reported by Sihag [1], for successful biodegradation the soil inoculation numbers must be in range 104 to 107 CFU g-1 . Microorganisms number lower than 103 CFU g-1 indicate the presence of toxic concentrations of organic or inorganic contaminants [17].
Changes of pH during bioaugmentation treatments
pH is one of the key factor for microbial metabolism. Most microorganism are known to grow best in a pH range between 6 to 8. Too much acidic or base pH in the soil will disrupt the cellular activities of the bacteria. Figures 6a-6d indicate the pH profile of treatment single and microbial consortium of LIBeM at 5% and 10% concentration of oil sludge with respect to the control plot during 60 days experiment set. The result showed that treatment in soil augmented with single and microbial consortium has reached the optimum pH for hydrocarbon biodegradation where the range are recorded within 6.02-6.73. At this pH condition, the soils are suitable for hydrocarbon degradation. Otherwise in control plot, the pH value fluctuated in a very small range 4.85-5.81. The pH showed slightly acidic which may result in slower growth of microbe. The reason why the pH was acidic may be due to the by-products produced by the indigenous microbes while surviving in the oil sludge [18]. The variation of pH value fluctuated and increase in the augmented bioreactor was synchronized with the time of the microbe preparation which inoculation of microbe proving that the preparation had an effect on decontamination of oil sludge. Slightly deviation of pH from the optimum range may result in reduced microbial population, because most of the microbes can grow well in optimum growth at a particular pH.
Soil moisture content during bioremediation process
Figure 7 showed variation of soil moisture content (%) in treatment with single strain and microbial consortium of LIBeM (a-d) during 60 days experiment set. The soil moisture (%) of treated soil in both concentrations studied were range in 5.21%-23.15%. Treated soil using single and microbial consortium at 5% oil sludge showed lowest percentage of moisture content by 3.05 fold as compared to 10% concentration studied. According to Testa and Winegardener [19] soil moisture content lower than 10% (v/v) of the holding capacity will affect the microbial activity in the soil. Due to the fact that water is one of the important nutrients to microorganism the watering process has to be done regularly in order to avoid dehydration in the soil. In this study, the microbial activity in single strain and microbial consortium at 5% concentration of oil sludge is lower than 10% concentration. The loss of moisture content in the soil may due to several reasons such as evaporation due to hot weather, uptake of water for microbial activity and insufficient frequency of watering into the soil [1]. Water provides moisture for microbial activity and enables the diffusion of nutrients and by-products during the degradation process. If water content in soil is oversaturated, oxygen transfer to the microorganisms will be resisted by the soil and the rate of degradation of hydrocarbon will decrease. However, if water content is too low, microbial activity will be inhibited. Thus, optimal water content in soil is important for microorganisms. Our finding suggested that treated soil need to be done every day in order to achieve the optimum soil moisture content for hydrocarbon degradation process.
Variation of soil temperature
Temparature is one of the most environmental factors affecting the biodegradation of oil sludge. Temperature influences hydrocarbon degradation by its effect on the physical nature and chemical composition of the oil, rate of hydrocarbon metabolism by microorganism and composition of the microbial community [20,21]. Figures 8a-8d showed the range of temperature in all set treatment and control plot. The results showed that the range of temperature during 60 days treatment do not pronounce a large deviation as both within in the range 27-35°C.
According to Parr et al. [22], most microbes in soil are adapted in the optimum temperature range 20°C to 35°C. Within these range, majority of organisms degrade petroleum products actively. Degradation rates are usually performs well in the range of 30-40°C in soil environments [23]. It was concluded that the range of temperature are dependent on the average daily air temperature. Sihag et al. [14] have stated that the optimum temperature for microbial growth ranges from 10°C to 45°C. Since all temperature measured daily within these range, thus, it is suitable for oil sludge degradation in all set of treatment.
Conclusion and Recommendation
This study concluded that TPH degradation in oil sludge contaminated soil was enhanced by bioaugmentation of LIBeM namely P. aeruginosa -BAS-Cr1, S. paucimobilis -RETOS-Cr1 and S. maltophilia -RAS-Cr. It was observed that treatment using single strain S. paucimobilis -RETOS-Cr1 into oil sludge contaminated soil at 5% and 10% concentration performed higher hydrocarbon degradation by 1.1-fold as compared to microbial consortium. Analysis of hydrocarbon compounds confirmed that most of the alkanes have been degrade up to 99% especially in 5% concentration studied. This study also demonstrated the potential of single LIBeM in hydrocarbon degradation with further research can be exploited in the different delivery technique such as powder and capsule form. Their effectiveness can be improved by monitoring the bioremediation parameters such as pH, temperature, soil moisture content and substrate concentration.
Acknowledgements
This study was financially supported by a grant (GUG0035-SGP- 1/2016) from the Center of Research and Innovation, Universiti Malaysia Sabah. We are indebted to Department of Environment (DOE) Malaysia and Labuan Shipyard and Engineering Sdn. Bhd. for granting us permission to use and treat the oil sludge for this research.
References
- Sihag S, Pathak H, Jaroli DP (2014) Factors Affecting the Rate of Biodegradation of Polyaromatic Hydrocarbons. International Journal of Pure & Applied Bioscience 2: 185-202.
- Coyne MS (1999) Soil microbiology: an exploratory approach. Delmar, New York, USA.
- Jayashree R, Nithya SE, Prasanna PR, Krshnaraju M (2012) Biodegradation capability of bacterial species isolated from oil contaminated soil. J Academia Indust Res 1: 140-143.
- Kriipsalu M, Marques M, Maastik A (2008) Characterization of oily sludge from a wastewater treatment plant flocculation–flotation unit in a petroleum refinery and its treatment implications. Journal of Material Cycles and Waste Management 10: 79-86.
- Vasudevan N, Rajaram P (2001) Bioremediation of oil sludge contaminated soil. Environ Int 26: 409-411.
- Piakong MTH, Jamaluddin S, Hasila R, Haron A, Yahya M, et al. (2004) Biodegradation of phenol by locally isolated strains from petrochemical wastewater treatment plants. Water and Environmental Management Series pp: 109-114.
- Nurulhuda Z, Piakong MT (2008) Isolation, characterization and screening of hydrocarbon-degrading bacteria from environmental samples for treatment of oil-sludge. Proceeding International Conference on Environmental Research and Technology. UniversitiSains Malaysia, Malaysia pp: 521-525.
- Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London, UK, p: 576.
- Sharma A, Rehman MB (2009) Laboratory scale bioremediation of diesel hydrocarbon in soil by indigenous bacterial consortium. Indian J ExpBiol 47: 766-769.
- American Society of Testing and Materials (ASTM) (1995) Annual Book of ASTM Standards. Designation D4972 - 95a: Standard Test Method for pH of Soils.
- Gardner CMK, Robinson DA, Blyth K, Cooper JD (2001) Soil water content. In: Soil & Environmental Analysis: Physical Methods, Marcel Dekker, New York, USA, pp: 1-64.
- United States Environmental Protection Agency (USEPA) (1996) Method 3540C: Soxhlet Extraction. Washington, USA.
- Heinaru E, Merimaa M, Viggor S, Lehiste M, Leito I, et al. (2005) Biodegradation efficiency of functionally important populations selected for bioaugmentation in phenol- and oil-polluted area. FEMS MicrobiolEcol 51: 363-373.
- McGenity TJ, Folwell BD, McKew BA, Sanni GO (2012) Marine crude-oil biodegradation: a central role for interspecies interactions. Aquatic Biosystems 8: 1-19.
- Mishra S, Jyot J, Kuhad RC, Lal B (2001) Evaluation of inoculum addition to stimulate in situ bioremediation of oily-sludge-contaminated soil. Applied and Environmental Microbiology 67: 1675-1681.
- Cerqueira VS, Holllenbach EB, Maboni F, Vainstein MH, Camargo FAO, et al. (2011) Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresource Technology 102: 11003-11010.
- Margesin R, Labble D, Schninner F, Greer CW, Whyte LG (2003) Characteristics of hydrocarbon-Degrading Microbial Population in Contaminated and Pristine Contaminated Soils. Applied and Environmental Microbiology 69: 3985-3092.
- Alkhatib MF, Alam MZ, Muyibi SA, Husain IAF (2011) An isolated bacterial consortia for crude oil biodegradation. African Journal of Biotechnology 10: 18763-18767.
- Testa SM, Winegardner DL (1991) Restoration of petroleum contaminated aquifers. Lewis Publishers, London, UK.
- Atlas RM (1981) Microbial degradation of petroleum hydrocarbon. An Environment Perspectives, Microbiological Reviews 45: 180-209.
- Alexander M (1999) Biodegradation and bioremediation. Academic Press, London, UK.
- Parr JF, Papendick RI, Hornick SB, Meyer RE (1992) Soil quality:attributes and relationship to alternative and sustainable agriculture. Am J Altern Agric 7: 5-11.
- Das N, Chandran P (2010) Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnology Research International 2011: 1-13.
- Ramsay BA, Cooper DG, Margaritis A, Zajic JE (1983) Rhodochorous bacteria: Biosurfactant production and demulsifying ability. Microbial Enhanced Oil Recovery pp: 61-65.
Citation: Zaida NZ, Piakong MT (2018) Effectiveness of Single and Microbial Consortium of Locally Isolated Beneficial Microorganisms (LIBeM) in Bioaugmentation of Oil Sludge Contaminated Soil at Different Concentration Levels: A Laboratory Scale. J Bioremediat Biodegrad 9: 430. DOI: 10.4172/2155-6199.1000430
Copyright: © 2018 Zaida NZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7147
- [From(publication date): 0-2018 - Sep 02, 2025]
- Breakdown by view type
- HTML page views: 6094
- PDF downloads: 1053