Effects of a Cesarean Scar on the Flow Resistance of the Uterine Artery: Doppler Ultrasonography Performance
Received: 17-Nov-2019 / Accepted Date: 05-Jun-2020 / Published Date: 15-Jun-2020 DOI: 10.4172/2167-7964.1000318
Abstract
Purpose: Assessment of the uterine artery is becoming increasingly important in the prediction of early preeclampsia and a low birth weight. Incidents of caesarean section are gradually increasing, and it is mentioned that caesarean scar increases resistance of the uterine artery. The aim of the study was to evaluate the effects of a caesarean scar on resistance of the uterine artery.
Methods: Prospectively, a totally of 498 pregnant women were evaluated. The average of the last menstrual period of the participating pregnant women was calculated as 21 w 1, and the average age was 29.6 (±5.5). Uterine artery doppler USG examination was conducted on 250 pregnant women (one or more caesarean sections), and a control group consisted of 248 pregnant women (non-caesarean scar). The UtA-RI and UtA-PI values of the uterine artery were measured on the doppler USG examination and statistical analysis was performed.
Results: IBM SPSS (for Windows version 24) statistical software, ROC analysis, and student’s (independent) ttest methods were used for in the statistical evaluation of this study. There was no statistically significant difference between groups (p>0.05).
Conclusion: This study assessed the effects of a caesarean scar on the resistance of the uterine artery on the doppler USG examination. No statistically significant difference was observed in the UtA-RI and UtA-PI values, and only 1.9-3.5% of the variability in the UtA-RI and UtA-PI values, based on the obtained effect size, could be explained by an effect of the C/S scar.
Keywords: Caesarean scar; Uterine artery; Doppler
Introduction
Recently, there has been an increase in the number of caesarean scar (C/S), which constitutes approximately 20-25% of all deliveries [1]. Some studies mention that pregnant women with C/S may have a higher uterine artery (UtA) resistance [2]. UtA doppler USG is a noninvasive method frequently used to detect altered uteroplacental circulation and deterioration in placentation [3]. One of the major causes of maternal morbidity and mortality, preeclampsia (PE) emerges during the second-third trimesters of pregnancy because of degenerated placentation with an increase in UtA flow impedance [4]. Various studies and data have been published regarding an increase in the resistance of UtA flow, generating a prognosis for PE and low birth weight (LBW) [4-12].
Such information further increases the significance of UtA examination. Meanwhile, stress factors in pregnant women such as aneuploidy, increase risk of preeclampsia and LBW in direct proportion with the error rate in prognosis criteria. Therefore, it is important to minimize the parameters that negatively affect the factors that determine a prognosis. While UtA doppler USG examination is predominantly used during the third trimester, today it has become an inseparable part of second trimester screening [13]. Because the C/S scar region will become avascular-acellular with relatively less blood build-up as a result of inadequacy of the vessel structure of the scar tissue and the connective tissue that acts as an accessory, there will be resistance in blood flow in this region during future pregnancies. This resistance can be shown by the increase in RI and PI values in the uterine artery that feeds the uterus.
A total of 498 pregnant women were evaluated via doppler USG for to evaluate the effects of a C/S on UtA flow during the second trimester. The uterine artery resistance index (UtA-RI) and uterine artery pulsatility index (UtA-PI) statistical data were compared between with the C/S and control groups. SPSS (IBM SPSS for Windows, ver.24) statistical package programs were used for calculations. There was no significant difference (p >0.05).
Methods
Patients
Approval from our University Local Ethics Committee (Approval no: 40465587-15/04) and the permission for the study (permission no: 64960800-799) from the training and research hospital management was received. Informed consent was obtained from all individual participants included in the study. and women signed consent form. Gestational age was determined based on the mother’s last menstrual period (LMP) and confirmed with fetal biometric measurements.
Pregnant women were assessed the second trimester screening USG examination, prospectively. Those whose LMP was unknown, who had erroneous registration information, whose UtA doppler USG examination showed end diastolic incisura, those who had high risk disease such as multiple pregnancies that might negatively effects the uterine flow resistance, pregnant women who suffered from diseases like diabetes mellitus, thyroid dysfunctions, and cardiovascular disease, or obesity (with BMI >30) were excluded from this study. With the single or multiple (1-5 times) C/S, 250 pregnant women were included in the assessment and no history of C/S and healthy 248 pregnant women, constituted the control group.
Data collection procedure
All doppler USG examinations were conducted trans-abdominally (GE Medical Systems, Logiq E9, 2013). Each assessment was performed by an experienced radiology. UtA doppler USG examination was performed unilaterally on the inguinal region by vertically setting up the probe, and giving it a mild medial angle, where the right UtA crosses the external iliac artery. The insonation angle was adjusted to keep it below 30 degrees. Once at least three similar consecutive waveforms were obtained following the application of the correct angle, measurements were taken. The UtA-RI and UtA-PI values were added (Table 1). New Zealand obstetric doppler guideline was used for the UtA-RI and UtA-PI reference range in the doppler USG examination [14].
| C/S number | Patient Number | Mean | Standart. Deviation | Min. | Max | ||
|---|---|---|---|---|---|---|---|
| UA RI | Non-C/S | 0 | 248 | 0,556 | 0,105 | ,29 | ,81 |
| C/S | 1 | 164 | 0,563 | 0,103 | ,28 | ,79 | |
| 2 | 68 | 0,562 | 0,100 | ,27 | ,77 | ||
| 3 | 16 | 0,568 | 0,116 | ,36 | ,74 | ||
| 4 | 1 | 0,420 | . | ,42 | ,42 | ||
| 5 | 1 | 0,630 | . | ,63 | ,63 | ||
| Totally | 498 | 0,559 | 0,104 | ,27 | ,81 | ||
| UA PI | Non-C/S | 0 | 248 | 0,913 | 0,320 | ,03 | 1,96 |
| C/S | 1 | 164 | 0,935 | 0,303 | ,03 | 1,91 | |
| 2 | 68 | 0,934 | 0,321 | ,08 | 1,80 | ||
| 3 | 16 | 0,911 | 0,326 | ,47 | 1,52 | ||
| 4 | 1 | 0,550 | . | ,55 | ,55 | ||
| 5 | 1 | 1,170 | . | 1,17 | 1,17 | ||
| Totally | 498 | 0,923 | 0,314 | ,03 | 1,96 | ||
| AGE | Non-C/S | 0 | 248 | 28,39 | 5,821 | 16 | 43 |
| C/S | 1 | 164 | 29,49 | 4,363 | 19 | 40 | |
| 2 | 68 | 33,15 | 4,379 | 23 | 44 | ||
| 3 | 16 | 34,56 | 6,293 | 26 | 49 | ||
| 4 | 1 | 37,00 | . | 37 | 37 | ||
| 5 | 1 | 25,00 | . | 25 | 25 | ||
| Totally | 498 | 29,61 | 5,502 | 16 | 49 |
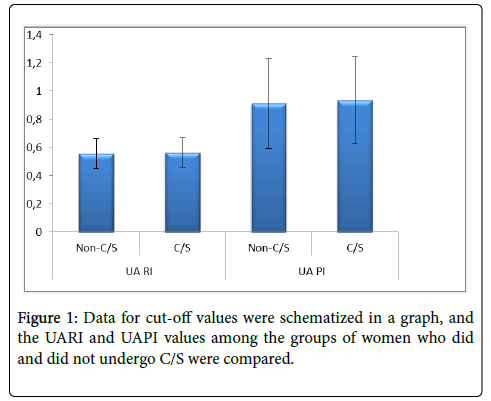
Table 1: General definitive statistics of the UARI, UAPI, and AGE variables, which are divided into groups of women who did and did not undergo C/S.
Statistical analysis
H_0: There was a difference between the UtA-RI and/or UtA-PI averages in patients who did not undergo caesarean section, and in those who underwent one or more C/S. H_1: 2 hypotheses were formed stating that there is a difference between the UtA-RI and/or UtA-PI averages of patients who did not undergo caesarean section and those who did undergo 1 or 2 C/S . In calculating the sample wideness of this study groups, Power (the power of the test) was determined for each variable as at least 0.80 and by taking a type 1 error as 0.05.
While the descriptive statistics for numerical variables in the study were expressed as average standard deviation, and minimum and maximum values, the categoric variables were stated in numbers and percentages. The Kolmogorov-Smirnov test was employed to determine whether continuous variables were normally distributed. The student ’ s (independent) t-test was used in comparing group averages. In determining the relationship among variables, Pearson correlation coefficients were calculated separately in groups. The cutoff value of UtA-RI and UtA-PI values based on groups were determined via ROC analysis. The statistical significance level in calculations was taken as 5%, and SPSS (IBM SPSS for Windows, ver. 24) statistical package programs were used for calculations.
Results
The study included 498 pregnant women. 248 (49.8%) of these pregnant women were those who did not undergo C/S, and 250 (50.2%) did undergo C/S (164 women once, 68 women twice, 18 women thrice or more) (Table 1). The average LMP of pregnant women has been observed between the 17-week (w) 5-day (d)-24 w 5 d range; the average age and the age range was 29.61 (±5.5) years old and 16-49 years, respectively.
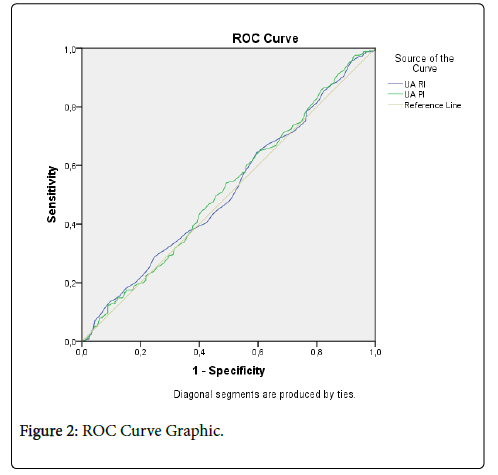
The cut-off value of the UtA-RI and UtA-PI values based on groups were determined via ROC analysis. Sensitivity; the separation level for those who had C/S, specificity; when considered as the separation level for those without C/S, the cut-off values that show the optimum sensitivity and specificity values of UtA-RI and UtA-PI values were given. These are separative values that could separate groups. Accordingly, the sensitivity and specificity values, 0.555 for UtA-RI (Sensitivity: 52.8%; Specificity: 46.8%), and 0.885 for UtA-PI (Sensitivity: 51.2%; Specificity: 52.0%) have been determined as the optimum cut-off values. When the areas of UtA-RI and UtA-PI values that remained under the curve were examined, both values seemed to have an average-level similar selectivity (51.5%-51.9%) (Table 2).
| Cut Off | Sensitivity | Specificity | |
|---|---|---|---|
| UA RI | 0,535 | ,604 | 0,427 |
| 0,545 | ,568 | 0,452 | |
| 0,555 | ,528 | 0,468 | |
| 0,565 | ,480 | 0,496 | |
| 0,575 | ,440 | 0,548 | |
| UA PI | 0,865 | ,540 | 0,508 |
| 0,875 | ,528 | 0,512 | |
| 0,885 | ,512 | 0,520 | |
| 0,895 | ,496 | 0,544 | |
| 0,905 | ,476 | 0,552 |
Table 2: The cut-off values that show the optimum sensitivity and specificity values of UARI and UAPI values are given.
The optimum cut-off value was determined as 0.5550 (Sensitivity: 52.8%; Specificity: 46.8%) for UARI, and 0.8850 (Sensitivity: %51.2; Specificity: %52.0) for UAPI.
Once the cut-off values were determined, the student ’ s (independent) t-test was used to compare group averages. There was no statistically significant difference between groups based on the UtARI and UtA-PI averages (p>0.05). In other words, the presence or absence of C/S did not have a significant effect on the variables (UtARI and UtA-PI) (Table 3 and Figure 1).
| C/S | Patient Number | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| UA RI | No | 248 | ,5561 | ,10545 | ,00670 |
| Yes | 250 | ,5633 | ,10334 | ,00654 | |
| UA PI | No | 248 | ,9131 | ,32071 | ,02037 |
| Yes | 250 | ,9330 | ,30891 | ,01954 | |
| AGE | No | 248 | 28,39 | 5,821 | ,370 |
| Yes | 250 | 30,82 | 4,883 | ,309 |
Table 3: Independent t-test definitive statistics were calculated.
Moreover, both the UtA-RI and UtA-PI values of pregnant women who did and did not undergo C/S produced similar results, and a statistically distinctive characteristic (Figure 2) was not found. In the groups that did and did not undergo C/S, correlation coefficients were examined between the UtA-RI and UtA-PI values and pregnancy and age. Accordingly, between the groups did and did not undergo C/S, a meaningful relationship between the UtA-RI and UtA-PI values (P>0.05) was not found. An effect of 0.019 was obtained for UtA-RI (exceptionally low effect) and 0.035 for UtA-PI (extremely low effect). Accordingly, only 1.9-3.5% of the variability in the UtA-RI and UtA-PI measurements could be explained by an effect of the C/S scar.
It shows the region of UARI and UAPI values that stayed below the reference line. Accordingly, when the area that stayed under the reference line is examined, both values seemed to have average-level similar selectivity (51.5-51.9%). Both the UARI and UAPI values among the groups have demonstrated similar results, and no significant difference was observed between women who did and did not undergo C/S.
Discussion
This study has to the largest sample which it is assessed the effects of a caesarean scar on the resistance of the uterine artery. Based on the doppler USG examination, no statistically significant difference was observed in the UtA-RI and UtA-PI values.
The increase in the cesarean index increased the C/S scar rate. C/S scar can affect local anatomy and it becomes increasingly important in obstetric outcomes. Recently, the cesarean section declaration published by WHO summarizes the results of the systematic reviews and analyzes performed for this purpose [15]. Caesarean birth rates are increasing worldwide according to the recently published rates (2016), (24.5% in Western Europe, 32% in Northern America, and 41% in South America) [16].
The uterine arteries originate from the anterior branches of the iliac artery on both sides, and extend to the fundus from the corpocervical intersection region, and form a network towards the inside of the myometrium, which are called the arcuate arteries [17]. To evaluate uteroplacental resistance, uterine artery doppler USG is frequently performed in the second trimester [18]. During pregnancy, various doppler indexes were identified for to evaluate the uterine artery speed waveform. The PI and RI has been the most frequently used index on its own or in combination with the presence of early diastolic incisura [19].
During incisional wound healing after section, there is no vessel source in the wound center, the feeding is from blood vessels on the wound edge. Microvascular network consisting of capillaries around the wound occurs within a few days [20]. While the wound heals, the intensity of fibroblasts and macrophages are minimized via apoptosis [21]. Over time, capillary vessels stop growing, as the blood flow and metabolic activity on the wound region decrease [21]. In the final stage, the number of blood vessels and cells decreases, and scar tissue is formed [22]. As a result of caesarean wound healing, scar tissue develops in the uterus, which has a weak regeneration power, and the scar replaces normal uterine tissue. As a result, C/S scar; due to the vascular structure of the scar tissue and inadequate connective tissue, it becomes an avascular-acellular feature. Therefore, in a new pregnancy, there will be a resistance in the blood stream in this region. This resistance increase can be shown by the RI and PI values in the uterine artery with doppler USG examination. Based on the doppler USG examination, no statistically significant difference was observed in the UtA-RI and UtA-PI values in this study. When compared to the size of the uterus that was subject to hypertrophy and hyperplasia, and a placenta that utilizes 85% of the blood flow, changes in uterus due to C/S scar do not make a significant change in uterine artery resistance. Our results support this assumption. Therefore, our study, the scar volumes in the uterus of pregnant women and interacting factors were unknown. The sample group was comprised of only second trimester pregnant women.
Flo et al. compared uterine artery doppler USG results. gestational week in a group of pregnant women with a history of C/S and a control group and found a significant decrease in uterine artery blood flow and increased uterine artery resistance [3]. Contrary to that, Filho et al. have not found a significant difference between UtA-PI values in the study they conducted evaluating 45 pregnant women who underwent C/S [23]. This is significant regarding doppler assessment for the patient group with a larger C/S scar, which may cause changes in uterine flow volume, and bring resolution to the handicaps that occur, regarding the use of this data in the prognosis of preeclampsia, LBW, and IUGR. UtA-PI is frequently used in daily practice to evaluate vascular resistance; however, it does not always fully reflect resistance. Therefore, it is mentioned that the measurement of blood flow volume in the uterine artery and vascular resistance will provide additional information regarding fine hemodynamic changes that may occur in pregnancies with a history of C/S [24].
Conclusion
The main characteristic of the present study is that this is the first comparative research study with the widest series in cases of C/S. The UtA-RI and UtA-PI values statistical data comparison was conducted between the C/S and non-C/S control groups, and no statistically significant difference was observed in the UARI and UAPI values, and only 1.9-3.5% of the variability in the UtA-RI and UtA-PI values, based on the obtained effect size, could be explained by an effect of the C/S scar.
Limitations
• The uterine artery was unilaterally evaluated.
• The sample group was comprised of only second trimester pregnant women.
• The lesser number of interacting factors (such as age).
• Not knowing the uterine scar dimension or volume.
• The study was single-centered.
References
- Daltveit AK, Hofoss D, Ha RME (2004) Complications of cesarean deliveries : Rates and risk factors. American Journal of Obstetrics and Gynecology 190: 428-434.
- Flo K, Widnes C, Acharya G (2014) Blood flow to the scarred gravid uterus at 22-24 weeks of gestation. BJOG An Int J Obstet Gynaecol 121: 210-215.
- Torabi S, Sheikh M, Fattahi Masrour F (2018) Uterine artery Doppler ultrasound in second pregnancy with previous elective cesarean section. J Matern Neonatal Medic 0: 1-7.
- Gallo DM, Poon LC, Akolekar R (2013) Prediction of preeclampsia by uterine artery doppler at 20-24 weeks’ gestation. Fetal Diagn Ther 34: 241-247.
- Ghi T, Youssef A, Piva M (2009) The prognostic role of uterine artery Doppler studies in patients with late-onset preeclampsia. Am J Obstet Gynecol 201.
- Kafkaslı A, Turkcuoglu I, Turhan U (2013) Maternal, fetal and perinatal characteristics of preeclampsia cases with and without abnormalities in uterine artery Doppler indexes. J Matern Neonatal Medic 26: 936-940.
- Khong SL, Kane SC, Brennecke SP (2015) First-trimester uterine artery doppler analysis in the prediction of later pregnancy complications. Dis Markers.
- Kleinrouweler CE, Bossuyt PM, Thilaganathan B (2013) Value of adding second-trimester uterine artery Doppler to patient characteristics in identiï¬cation of nulliparous women at increased risk for pre-eclampsia: an individual patient data meta-analysis. Ultrasound Obstet Gynecol 42: 257-267.
- Lovgren TRM, Dugoff LM, Galan HLM (2010) Uterine Artery Doppler and Prediction of Preeclampsia. Clin Obstet Gynecol 53: 888-898.
- Madazli R, Kuseyrioglu B, Uzun H, Uludag S, Ocak V (2005) Prediction of preeclampsia with maternal mid-trimester placental growth factor, activin A, fibronectin and uterine artery Doppler velocimetry. Int J Gynecol Obstetric 89: 251-257.
- Myatt L, Clifton RG, Roberts JM (2012) The Utility of Uterine Artery Doppler Velocimetry in Prediction of Preeclampsia in a Low-Risk Population. Obstet Gynecol 120: 815-822.
- Pedrosa AC, Matias A (2011) Screening for pre-eclampsia: A systematic review of tests combining uterine artery Doppler with other markers. J Perinat Med 39: 619-635.
- Albaiges G, Missfelder-Lobos H, Lees C (2000) One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet Gynecol 96: 559-564.
- Zealand N, Mccowan L, Necas M (2013) Which Doppler Test When? New Zealand Obstetric Doppler Guideline 1-17.
- Betran AP, Torloni MR, Zhang JJ (2016) WHO statement on caesarean section rates. BJOG An Int J Obstet Gynaecol 123: 667-670.
- Keag OE, Norman JE, Stock SJ (2018) Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLOS Med 15: e1002494.
- Dane B (2010) İkinci Trimester Uterin Arter Doppler Bulguları Gebelik Komplikasyonlarını Öngörmede Etkin midir ? Turkiye Klinikleri J Gynecol Obst 20: 211-217.
- Contro E, Maroni E, Cera E (2010) Unilaterally increased uterine artery resistance, placental location, and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 153: 143-147.
- Gómez O, Figueras F, Fernández S (2008) Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet Gynecol 32: 128-132.
- Greenhalgh DG (1998) The role of apoptosis in wound healing. Int J Biochem Cell Biol 30: 1019-1030.
- Muhammet YT, Nilufer SC (2016) Cellular and Molecular Mechanism of Wound Healing. SDU Tıp Fakultesi Dergisi 23: 140-146.
- Hart J (2002) Inflammation 1 its role in the healing of acute wounds. J Wound Care 11: 205-209.
- Marcondes L, Nardozza M, Camano L (2011) Repercussions of previous cesarean uterine scar at uterine arteries Doppler velocimetry between the 26th and 32nd. Radiol Bras 44: 163-166.
- Elena C, Elisa M, Emanuela C (2010) Unilaterally increased uterine artery resistance, placental location, and pregnancy outcome. European Journal of Obstetrics & Gynecology and Reproductive Biology, 143-147.
Citation: Turan A, ÇELIKER FB, ÇÖLÇIMEN N, Ural UM, Inecikli MF, et al. (2020) Effects of a Cesarean Scar on the Flow Resistance of the Uterine Artery: Doppler Ultrasonography Performance. OMICS J Radiol 9: 1000318. DOI: 10.4172/2167-7964.1000318
Copyright: © 2020 Turan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3103
- [From(publication date): 0-2020 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 2233
- PDF downloads: 870