Case Report Open Access
Effects of a Hardness Discrimination Task in Failed Back Surgery Syndrome with Severe Low Back Pain and Disturbed Body Image: Case study
| Tomohiko Nishigami1,2*, Hiroyuki Okuno3, Hideki Nakano4, Yutaka Omura3, Michihiro Osumi3,4, Shimizu Michele Eisemann1, Motohiro tsujishita1, Akira Mibu5and Takahiro Ushida2 | |
| 1Department of Physical Therapy, Konan Woman’s University, Japan | |
| 2Multidisciplinary Pain Center, Aichi Medical University, Japan | |
| 3Neurocognitive Center, Setsunan General Hospital, Japan | |
| 4Department of Neurorehabilitation, Graduate School of Health Sciences, Kio University, Japan | |
| 5Rehablitation Center, Tanabe Orthopaedics, Japan | |
| Corresponding Author : | Tomohiko Nishigami Department of Physical Therapy Konan Woman’s University; 6-2-23 Morikita-machi, Higashinada-ku Kobe, 658-0001, Japan Tel: +81 78 413 3648 Fax: +81 78 413 3742 E-mail: t-nishi@konan-wu.ac.jp |
| Received September 04, 2012; Accepted September 27, 2012; Published September 30, 2012 | |
| Citation: Nishigami T, Okuno H, Nakano H, Omura Y, Osumi M, et al. (2012) Effects of a Hardness Discrimination Task in Failed Back Surgery Syndrome with Severe Low Back Pain and Disturbed Body Image: Case study. J Nov Physiother S1:008. doi: 10.4172/2165-7025.S1-008 | |
| Copyright: © 2012 Nishigami T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
A 20-year-old woman began experiencing low back pain (LBP) in September 2008 and dysesthesia, pain in the left leg, muscle weakness, and gait disturbance in January 2009. Three low back surgery were performed in April, May and July 2009, respectively. However, her symptoms were relieved for only a few days, eventually re-emerging and intensifying. On our initial examination, on physical contact, a sharp increase in pain was experienced in the left lower back (numerical rating scale: NRS = 10). Loss of body image in the left lower back with severe pain was presented. Lying in the supine position, independent upright sitting, and trunk flexion to the left beyond a certain point were impossible. Motor imagery and tactile discrimination training were performed. However the training was not effective. Next, the patient was asked to determine the various degrees of hardness of the sponge material that was placed on the left lower back of another person; she was simultaneously instructed to imagine it being placed on her own left lower back. Hardness discrimination training was performed for 20 min a day, 6 days a week for 4 weeks. EEG was performed to determine the cortical activation in the somatosensory cortex during motor imagery and the hardness discrimination task. Four weeks after hardness discrimination training, on contact with the left lower back, left LBP decreased from 10/10 to 5/10 on the subjective NRS. In addition, perception of body image in the left lower back improved. Lying in the supine position, independent upright sitting, and trunk flexion to the left became possible. Neural activity was observed in the right somatosensory cortex in the hardness discrimination task compared with the control task. These results raised the possibility that hardness discrimination training decreased pain through reorganization of the somatosensory cortex.
| Keywords |
| Pain; Low back pain; Failed back surgery syndrome; Body image; Discrimination; Somatosensory cortex; Electroencephalography |
| Introduction |
| Failed back surgery syndrome (FBSS) is defined as persistent chronic low back pain and/or leg pain for more than 1 year, despite treatment with 1 or more surgical procedures. Patients with FBSS often do not experience lasting relief even after administration of therapies such as repeat surgery, medication, and neuromodulation techniques. However, the effectiveness of physical therapy for FBSS has not been reported. |
| Extensive evidence shows that chronic back pain is associated with cortical dysfunction [1-3] and disrupted tactile processing [4,5]. In one study, disturbance in body image and decreased tactile acuity coincided with the distribution of chronic low back pain (LBP) [6]. In addition, Moseley et al. [7] demonstrated an association between neglect-like tactile dysfunction and persistent musculoskeletal disorders in patients with chronic unilateral back pain. |
| Complex regional pain syndrome (CRPS) can develop after tissue trauma and is accompanied with observable decreased tactile acuity [8] and altered body perception [9] as well as chronic back pain. Mosely [10] demonstrated that a motor image program comprising mental rotation, motor imagery, and mirror therapy decreased pain and disability in patients with CRPS [11]. More recently, sensory discrimination training to the affected limb was shown to decrease pain, increase tactile acuity, and speed recovery, and might be associated with reorganization in the somatosensory cortex [12-14]. However, no studies have investigated the effectiveness of sensory discrimination training in patients with severe pain and loss of body image in the lower back. |
| We report the case of a patient with FBSS with severe LBP and distorted body image. Trunk movement imaging and contact with the patient’s lower back was difficult. This patient was successfully treated with hardness discrimination training using sponges in contact with the lower back of another person. In addition, this study describes the result of cortical activation during the hardness discrimination task using multichannel electroencephalography (EEG). |
| Case Description |
| Patient history |
| A 20-year-old woman presented with lumbar disc hernia at the L4–L5 level. The patient began experiencing LBP in September 2008 and dysesthesia, pain in the left leg, muscle weakness, and gait disturbance in January 2009. Percutaneous nuclectomy was performed from L4–L5 at another institution in April 2009 to alleviate the severe LBP and pain in the left leg. However, her symptoms were relieved for only 4 days, eventually re-emerging and intensifying. Microendoscopic discectomy (MED) and posterior lumbar interbody fusion at the L4–L5 level were performed in the same institution in May and July 2009, respectively. However, neither LBP nor leg pain was alleviated. In addition, walking became difficult 2 weeks after MED. The patient underwent implantation of a spinal cord stimulator (SCS) with bipolar leads at a different hospital in May 2011. SCS was effective in alleviating her LBP, which was indicated using a subjective numerical rating scale (NRS: unbearable pain intensity=10, 0=no pain at all). The score was reduced from 10/10 to 6/10 following SCS, but leg pain remained unaffected. Physical therapy was administered 5 days a week for 1 hour from April 2009 to December 2011. Although the physical therapy incorporated lower extremity strengthening exercises and stretching, the pain intensified. Therefore, the patient was admitted to our hospital for treatment of LBP and leg pain in January 2012. |
| Examinations and medication |
| On our initial examination, constant pain in the lower back and leg was rated 6/10 on NRS in a resting state. On physical contact, a sharp increase in pain was experienced in the left lower back (NRS=10). Analgesics including nonsteroidal anti-inflammatory drugs (NSAIDs, once daily), clonazepam (4 times daily), and tramadol hydrochloride/acetaminophen (4 times daily) were administered. The stimulation system was in continuous use 24 h/day at the following settings: amplitude 10.5 V, frequency 50 Hz, and pulse width 200. |
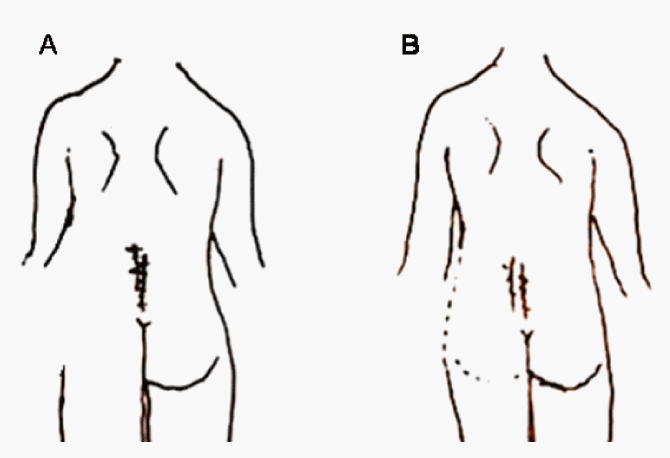
| A subjective mental representation of lower back perception was captured in a pencil drawing. Loss of body image in the left lower back with severe pain was presented (Figure 1A). The two-point discriminating threshold was not measured because her severe pain resulted in guarding. Lying in the supine position, independent upright sitting, and trunk flexion to the left beyond a certain point were impossible. Although the movement during trunk flexion to the left was very mild, the patient felt that she had reached the maximum limit. |
| Rehabilitation intervention |
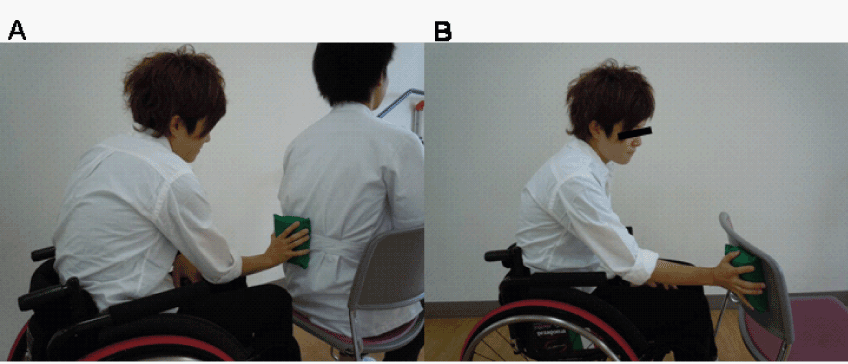
| Physical therapy was performed by three physical therapists (H.O., H.N., Y.O.). First, the patient was instructed to imagine independent trunk movement; however she was unable to do so. Second, tactile training with discrimination of the location and type of tactile stimuli was attempted in the left lower back, which was unsuccessful because of guarding. Finally, the patient was asked to lightly push on the left lower back of another person using a sponge. The patient was asked to discriminate the hardness of the sponge according to a modified version of the method described by Morioka et al. [15,16] (Figure 2A). Three sponges (INOAC Co. , Aichi, Japan; 7.5×8.0×4.0 cm) with varying degrees of hardness (58.8, 78.5, and 107.9 N), which were measured by an automatic hardness tester (type JIS K6400, Asker JA, Kyoto, Japan), were used. All sponges were made of the same material and were similar in sharpness and size. The patient was asked to determine the various degrees of hardness of the sponge material that was placed on the left lower back of another person; she was simultaneously instructed to imagine her own left lower back. No pain was reported in the left lower back during this hardness discrimination task. Body perception of the left lower back returned during this hardness discrimination task. Therefore, hardness discrimination training was performed for 20 min a day, 6 days a week for 4 weeks. |
| EEG measurement |
| EEG was performed to determine the cortical activation during the hardness discrimination task while the patient was at rest in a quiet, air-conditioned room. The Discovery 24E (BrainMaster Technologies, Inc., Bedford, OH, USA) was used with 19 electrodes arranged according to the 10 to 20 international conventions with FPz as a reference. EEG signals were obtained at 256 samples/s. During measurements, the impedance of all electrodes was maintained at <5 kΩ. |
| Experimental task |
| The experiment comprised two sessions: one motor imagery task session and one hardness discrimination task session. |
| Motor imagery task (motor imagery vs. eyes closed) |
| In the motor imagery task, the patient was instructed to sit in a chair and imagine trunk flexion and extension alternately with her eyes closed. In a block design, a baseline session (30 s) was followed by motor imagery (30 s) alternating with rest (30 s), and this procedure was repeated 3 times for a total of 3 min. To differentiate from the motor imagery condition, the patient was instructed to sit in the chair with her eyes closed for 3 min. |
| Hardness discrimination task (hardness discrimination vs. control task) |
| In the hardness discrimination task, the patient was asked to push lightly on the left lower back of another person using the sponge while discriminating its hardness. She was also instructed to imagine her own left lower back simultaneously, similar to the rehabilitation intervention (Figure 2A). To differentiate from the hardness discrimination task, the patient was asked to push lightly on the back of a chair in the control task (Figure 2B). In a block design, the baseline session (30 s) was followed by the hardness discrimination or control tasks alternating with 30 s of rest, and this procedure were repeated 3 times for a total of 3 min. |
| Data analysis |
| Both split-half and test–retest reliability tests were conducted on the edited, artifact-free, and EEG segments. Records with >90% splithalf reliability, >90% test–retest reliability, and a total measurement time of >30 sec were subjected to the low-resolution electrical topographic analysis (LORETA). LORETA is a discrete, threedimensional (3D), distributed, linear, inverse solution. LORETA inverse solution corresponds to the 3D distribution of electrical neuronal activity that features maximum similarity between neuronal populations in adjacent voxels in terms of orientation and strength. |
| Quantitative neuroanatomy (including Brodmann areas) was determined using the probabilistic Talairach atlas [17]. Anatomical labels such as Brodmann areas were also reported using the MNI (Montreal Neurological Institue) space, with correction to the Talairach space [18]. |
| The removal of artifacts and calculation of the statistical properties of the segments were performed using NeuroGuide software (http:// www.appliedneuroscience.com). Artifacts were removed using the automatic algorithms in the NeuroGuide software and by visual inspection. |
| To avoid multiple statistical comparisons, this study focused on one spectral band: the absolute alpha band (8–12 Hz). Alpha band activity has previously been reported to play a key role in engagement and disengagement of the somatosensory cortex depending on task demand [19]. Since most quantitative EEG norms are measured with the linked-ears montage, it was used as the standard in this study. |
| Statistical analysis |
| As stated previously, only absolute alpha values were analyzed to avoid multiple comparisons. The paired t-test was applied for data comparison using LORETA. T >2.1 denoted an alpha level set at 0.05. |
| Outcome measurement |
| Four weeks after hardness discrimination training, left LBP decreased from 6/10 to 4/10 on the subjective NRS in the resting state. On contact with the left lower back, left LBP decreased from 10/10 to 5/10 on the subjective NRS. Analgesics administered at this time included NSAIDs (3 times daily), clonazepam (3 times daily), opioids (3 times daily), and eperisone hydrochloride (3 times daily) ; SCS was gradually discontinued. In addition, perception of body image in the left lower back improved, although it remained ambiguous (Figure 1B). Lying in the supine position, independent upright sitting, and trunk flexion to the left became possible. |
| Eeg measurement outcome |
| No statistically significant differences were noted between the motor imagery and eyes closed conditions for the alpha band activity (8–12 Hz) in the right somatosensory cortex. Lower alpha band activity (12 Hz) was observed in the right somatosensory cortex (including Broadman areas 2 and 3) in the hardness discrimination task compared with the control task (Figure 3). |
| Discussion |
| The outcome of the hardness discrimination training in the present case indicated that cortical reorganization may have been induced. As a result, pathological pain improved and severe LBP and disturbed body image were alleviated. |
| Severe pain, loss of body perception in the left lower back, and disability after lower back surgery have also been reported in patients with CRPS. Difficulty with anatomical identification without visual feedback has been demonstrated in CRPS patients [20]. Several studies of cortical reorganization in CRPS have noted a decrease in the cortical representation of the affected hand in the primary and secondary somatosensory cortices [21-24]. These changes support the hypothesis that body perception is distorted in patients with CRPS. Individual decrease in hand representation contralateral to the CRPSaffected limb was significantly correlated with subjective pain levels experienced over the previous 4 weeks [25]. Loss of body image in the left lower back and mismatch in flexion perception in this case may indicate a reduction in left lower back representation. In contrast, in CRPS patients who received treatment, including physical therapy and NSAIDs, significant pain alleviation was reported and cortical reorganization of the somatosensory cortex was largely reversed after approximately 1 year [26]. More recently, Benedict et al. reported that a sensorimotor retraining approach decreased pain intensity and disability in patients with chronic nonspecific LBP [27]. These studies indicate that normalization of cortical representation is required to decrease pathological pain in patients experiencing chronic pain. |
| Moseley et al. reported that motor imagery and a tactile discrimination task were effective in patients with LBP [10,11,13,14]. However, these tasks were ineffective in the present patient because the patient was unable to imagine trunk movement, and the tactile discrimination task induced severe pain. EEG revealed no somatosensory cortex activation during motor imagery task. The motor imagery task may have been difficult because trunk movement and related imagery had been limited for approximately two years and a half. |
| The hardness discrimination training task employed in this case was a modification of that described by Morioka et al. [15,16]. It involved activation of the somatosensory cortex by representation of the trunk without lower back contact. Morioka et al. demonstrated in randomized controlled trials that plantar perceptual training using a hardness discrimination task improved stable standing posture in cerebral stroke patients and elderly people living in housing facilities [15,16]. They speculated that the hardness discrimination task resulted in an improvement in perception capability of the foot sole in their patients. In addition, active touch during surface exploration induced greater activation in the primary somatosensory cortex than passive touch [28]. |
| In the present case, EEG confirmed a decrease in alpha band activity (12 Hz) in the right somatosensory cortex due to hardness discrimination training using sponges on the lower back of another person. This result indicated that an increase in neural activation in the somatosensory cortex was induced by this task. The somatosensory cortex is known to be responsible for body schema [29,30]. Improvement of body image perception in the left lower back during the hardness discrimination task may have occurred because of the activation of the somatosensory cortex. Reorganization of the primary somatosensory cortex in adult owl monkeys by controlled tactile stimulation and greater representation of the primary somatosensory cortex in the digits of the left hand of string players than that in controls indicated that plastic changes in the somatosensory cortex may be induced by the frequency of use [31,32]. These results raised the possibility that hardness discrimination training decreased pain through reorganization of the somatosensory cortex. In the present case, the improved body imagery through reorganization of the somatosensory cortex may have improved independent sitting upright and trunk movement to the left. |
| Several limitations should be considered when interpreting the results from this case report. First, and most importantly, the lack of a control group and the multiple-baseline approach may have caused changes in the outcome measures to occur spontaneously rather than as a result of the intervention. However, we believe that improvement in patients with severe pain and distorted body image over two and half years do not happen spontaneously. Second, although the hardness discrimination task resulted in substantial alleviation of symptoms and disability, it was not completely curative. During the assessment following the hardness discrimination training for 4 weeks, moderate pain and incomplete body perception persisted in the left lower back. However, the improvement achieved through the intervention was significant, quality of life was largely improved, sleep in the supine position was resumed, and independent sitting upright became possible. Finally, Hardness discrimination task was not continued because this treatment over the 4-week training period was not an effective. Moderate pain after 4-week training period might be due to injury of peripheral tissue including muscle, ligament, joint or nerve in low back than not disturbed body perception. |
| Conclusion |
| This is the first report of decreased pain and somatosensory cortex activation induced by a hardness discrimination task in a patient with distorted body image and severe LBP. These changes were associated with improved movement and alleviated disability. Future investigations will investigate the implications of these findings in a larger group of subjects with distorted body image and severe LBP. |
References
- Flor H, Braun C, Elbert T, Birbaumer N (1997) Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224: 5-8.
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463-484.
- Tsao H, Galea MP, Hodges PW (2008) Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131: 2161-2171.
- Bray H, Moseley GL (2011) Disrupted working body schema of the trunk in people with back pain. Br J Sports Med 45: 168-173.
- Luomajoki H, Moseley GL (2011) Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br J Sports Med 45: 437-440.
- Moseley GL (2008) I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain 140: 239-243.
- Moseley GL, Gallagher L, Gallace A (2012) Neglect-like tactile dysfunction in chronic back pain. Neurology 79: 327-332.
- Lewis JS, Schweinhardt P (2012) Perceptions of the painful body: The relationship between body perception disturbance, pain and tactile discrimination in complex regional pain syndrome. Eur J Pain 16: 1320-1330.
- Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR (2007) Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS). Pain 133: 111-119.
- Moseley GL (2004) Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108: 192-198.
- Moseley GL (2005) Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain 114: 54-61.
- Maihöfner C, DeCol R (2007) Decreased perceptual learning ability in complex regional pain syndrome. Eur J Pain 11: 903-909.
- Moseley GL, Zalucki NM, Wiech K (2008) Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137: 600-608.
- Moseley GL, Wiech K (2009) The effect of tactile discrimination training is enhanced when patients watch the reflected image of their unaffected limb during training. Pain 144: 314-319.
- Morioka S, Yagi F (2003) Effects of perceptual learning exercises on standing balance using a hardness discrimination task in hemiplegic patients following stroke: a randomized controlled pilot trial. Clin Rehabil 17: 600-607.
- Morioka S, Fujita H, Hiyamizu M, Maeoka H, Matsuo A (2011) Effects of plantar perception training on standing posture balance in the old old and the very old living in nursing facilities: a randomized controlled trial. Clin Rehabil 25: 1011-1020.
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, et al. (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120-131.
- Brett M, Johnsrude IS, Owen AM (2002) The problem of functional localization in the human brain. Nat Rev Neurosci 3: 243-249.
- Haegens S, Händel BF, Jensen O (2011) Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31: 5197-5204.
- McCabe CS, Cohen H, Hall J, Lewis J, Rodham K, et al. (2009) Somatosensory conflicts in complex regional pain syndrome type 1 and fibromyalgia syndrome. Curr Rheumatol Rep 11: 461-465.
- Juottonen K, Gockel M, Silén T, Hurri H, Hari R, et al. (2002) Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 98: 315-323.
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F (2004) Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63: 693-701.
- Pleger B, Tegenthoff M, Ragert P, Förster AF, Dinse HR, et al. (2005) Sensorimotor retuning in complex regional pain syndrome parallels pain reduction. Ann Neurol 57: 425-429.
- Pleger B, Ragert P, Schwenkreis P, Förster AF, Wilimzig C, et al. (2006) Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 32: 503-510.
- Pleger B, Tegenthoff M, Schwenkreis P, Janssen F, Ragert P, et al. (2004) Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome I. Exp Brain Res 155: 115-119.
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F (2003) Patterns of cortical reorganization in complex regional pain syndrome. Neurology 61: 1707-1715.
- Wand BM, O'Connell NE, Di Pietro F, Bulsara M (2011) Managing chronic nonspecific low back pain with a sensorimotor retraining approach: exploratory multiple-baseline study of 3 participants. Phys Ther 91: 535-546.
- Simões-Franklin C, Whitaker TA, Newell FN (2011) Active and passive touch differentially activate somatosensory cortex in texture perception. Hum Brain Mapp 32: 1067-1080.
- Haggard P and Wolpert DM (2005) Disorders of body scheme. In: Leiguarda R editor. Higher-order motor disorders: from neuroanatomy and neurobiology to clinical neurology. Oxford University Press: 261-271.
- Graziano MSA and Botvinick MM (2002) How the brain represents the body: insights from neurophysiology and psychology. Common Mechanisms in Perception and Action. Oxford University Press 19: 136-157.
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guíc-Robles E (1990) Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82-104.
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995) Increased cortical representation of the fingers of the left hand in string players. Science 270: 305-307.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 14153
- [From(publication date):
specialissue-2012 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 9604
- PDF downloads : 4549
