Effects of Co-Culture of Graphene Oxide Scaffolds with Different Concentrations and Umbilical Mesenchymal Stem Cells on the Proliferation and Differentiation of Stem Cells
Received: 01-Dec-2021 / Accepted Date: 15-Dec-2021 / Published Date: 22-Dec-2021
Abstract
The main role of the scaffold materials is to enable cells to survive in the scaffold binding as while as to further promote their proliferation and differentiation ability. For mesenchymal stem cell, the scaffold could provide an environment for them to maintain their phenotype, and synthesize all necessary molecules and proteins. Generally, scaffold materials for stem cell need to possess basic characteristics such as high porosity, large surface area, surface rigidity and biodeg-radability. Thus, the two-dimensional Graphene Oxide (GO) with oxygen-containing functional groups may be suitable scaffold materials for mesenchymal stem cell culture. In this study, the effect of GO on the value-added differentiation activity of mesenchymal stem cell was systematically investigated. It was found that low concentration of GO and sufficient concentration of umbilical cord mesenchymal stem cells are suitable for the second Co-culture. Furthermore, the addition of hyaluronic acid will make this culture more evenly distributed. The adsorption of GO on umbilical cord mesenchymal stem cells can also make the two closely linked, which avoids the impact of animal joint activities on cells.
Keywords: Graphene oxide; Mesenchymal stem cell; Co-culture; Scaffolds; Knee osteoarthritis
Introduction
Recently, the significant advantages of Graphene Oxides (GO) used as cell scaffold materials have attracted tremendous interests due to their large surface areas, friendly biological compatibility and good hydrophility [1-5]. As an ideal seed cell in tissue engineering, mesenchymal stem cell (MSC) has been widely used in the field of regenerative medicine [6-11]. MSC has the effect of regulating local immunity and improving the inflammatory environment. At the same time, MSC is low in immunogenicity, which will not bring risks to transplantation [12-17]. Many early studies have con-firmed that MSC transplanted in damaged structures can promote the repair effect of damaged areas. Up to now, the possibility of GO combined with MSC as the cell scaf-fold has also been initially explored [18-22]. However, there are few related studies and bone marrow stromal cells (BMSC) are mostly used as seed cells in the research.
At present, umbilical cord mesenchymal stem cells (UCMSC) has been employed to try to replace bone marrow stromal cells (BMSC) [23-25]. On one hand, the human-derived BMSC needs to puncture the human bone marrow, which brings pain, safety risks and ethical problems to the human to some extent. On the other hand, bone marrow-derived MSCs are actually very small in content. The extremely low proportion of MSCs in bone marrow as well as the lowactivity has a great impact on the results of research. Although some studies have confirmed that in vitro cultured MSCs and GO are biocompatible, they are also limited to the observation of the effect of GO on MSC survival without further in-depth studies and specific dose-effect relationships.
The dynamic knee joint is one of the most important structures that carry the human body's movements [26-28]. The joint cavity must maintain the stability of mechanical and biochemical microenvironment. MSC inhibits the inflammatory response through paracrine effects. Meanwhile, MSC releases growth factors such as plate-let-derived growth factor (PDGF), transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF). These growth factors could improve the biochemical micro-environment of the knee joint and pro-mote repair of cartilage tissue damage.
The problems of cell flow and low cell survival rate can be effectively solved by using cell scaffolds. The GO scaffold with favorable hydrophility, biocompatibility, ductility and damping properties can increase the electrical activity of cells [29-31]. In addition to being equipped with MSC, GO scaffold can also be combined with sodium hyaluronic acid (HA) to prepare materials with lubricating effect on the joint cavity. Furthermore, GO can also improve the joint as lubricants. Herein, in this experiment, the culture effect of UCMSC with GO was initially explored in order to provide a novel strategy for the treatment of osteoarthritis.
Materials and Methods
Materials and instruments
GO is purchased from C6G6Technology Co., Ltd, China. The basic characterization of GO is in the Supporting Information (Figures 1 and 2). The 3rd or 4th generation human umbilical cord mesenchymal stem cells (Boya stem cell technology co. LTD). DMEM/F12 medium and HA injection 2.5 ml/piece (Japan, H20050370). High-throughput multi-sample tissue grinding machine (Nanjing, Xianou Instrument Manufacturing Co. Ltd). Electronic platform scale (Switzerland, Mettler Toledo). Constant temperature and humidity incubator (SHEL LAB in the United States). OLYMPUS photomicroscope (OLYMPUS in Japan). ADAM automatic cell counter (NanoEnTek, Korea).
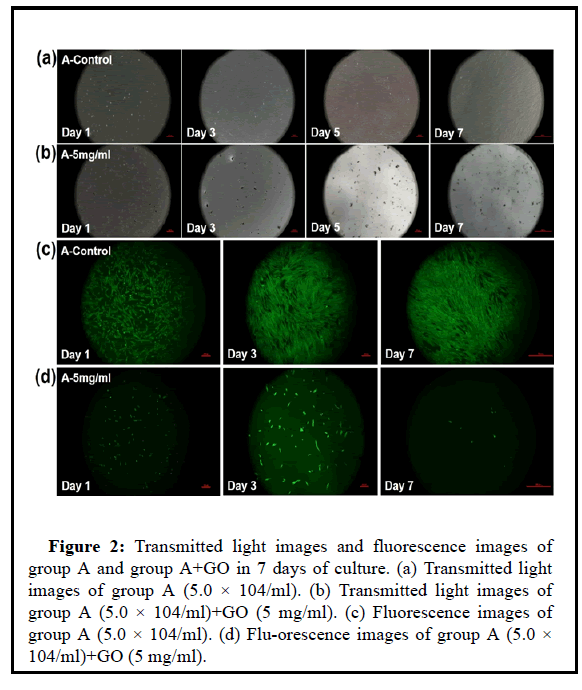
Figure 2: Transmitted light images and fluorescence images of group A and group A+GO in 7 days of culture. (a) Transmitted light images of group A (5.0 × 104/ml). (b) Transmitted light images of group A (5.0 × 104/ml)+GO (5 mg/ml). (c) Fluorescence images of group A (5.0 × 104/ml). (d) Flu-orescence images of group A (5.0 × 104/ml)+GO (5 mg/ml).
Mixture of GO and HA
GO is placed in a small reagent tube and put it into a tissue grinder. The control frequency is set as 70 Hz and the stop time is 60 s. After the machine stops running, remove the reagent tube, put it into a centrifuge, and centrifuge Operation: According to the needs of the experiment, use an electronic balance to weigh the masses of graphene oxide required by 4 groups of GO solution, and put them into four small reagent tubes labelled with groups, put them on the ultraviolet operating table, and use 1 ml without The pipette tip of the bacteria tube transfers a part of the HA injection in the unsealed syringe containing 2.5 ml of HA injection into a small reagent tube filled with GO. The GO combined HA solution is configured to two concentrations: 15 μg/ml GO+0.5% HA and 30 μg/ml GO+0.25% HA. After mixing, use the pipette Transfer all the liquid guns to the large test tube cap, and then transfer the HA injection solution in the remaining syringes to the large test tube caps of the same group. Use the pipette head to mix them thoroughly and turn on the ultraviolet light. , Perform UV sterilization for 35 min, and then use a pipette to transfer the mixture in the large test tube cap into the original syringe.
Isolation, culture and identification of human UCMSC
Take a healthy full-term fetal umbilical cord, rinse it thoroughly with PBS, remove the umbilical arteriovenous vein under sterile conditions, cut the remaining interstitial tissue (Walton's gel) into 1.0-2.0 mm size tissue blocks, and flatten the tissue blocks on In the cell culture flask, add an appropriate amount of DMEM/F12 culture solution containing 10% fetal bovine serum in volume, and place in 37ºC, 5% CO2 volume in-cubator for incubation.
Replace the culture solution according to the cell growth rate. When the cells have reached the bottom of the culture flask, remove the tissue blocks, pass them down according to the number of cells, observe with an inverted microscope and take a video. Take the 3rd or 4th generation human UCMSC, digest with 0.25% trypsin, centrifuge at 1200 r/min for 5 minutes, count the cells, and use about 2 × 10 cells per tube.
Use 0.1% sodium azide and 0.5 Wash the cells twice with PBS, resuspend the cells in PBS, add mouse anti-rat CD34, CD45, CD90, and CD105 primary antibodies (1:50 dilution), leave them at 4ºC for 30 min, wash them twice with PBS, add isocya-nate Rabbit antimouse IgG secondary antibody labeled with fluorescein thiocyanate, placed at 4℃ for 30 min, washed twice with PBS, resuspended the cells with PBS without BSA, and detected cell surface markers CD34, CD45, CD90 and CD105 expression.
Experimental grouping and processing
The experiment was divided into 4 groups. GO was mixed with HA injection after being treated with a tissue grinder and a centrifuge, and a mixture of 10 μg/ml, 20 μg/ml, 30 μg/ml and 40 μg/ml was used as a culture medium. Mark the cells separately, divide the human UCMSC into four groups of approximately equal amounts, mark the serial numbers, and transfer them to the corresponding medium with a pipette for cultivation.
Effect of the mixture of GO and HA injection on the proliferation of human UCMSC
The four concentration mixtures were placed in 96-well culture plates, and 100 μL of medium was added to each well. The inoculated 96-well culture plate was placed in a constant temperature incubator (5% CO2, 100% humidity, and 37ºC constant temperatures) for 24 hours, and observed under continuous microscope.
Results and Discussion
Biocompatibility of GO and UCMSC
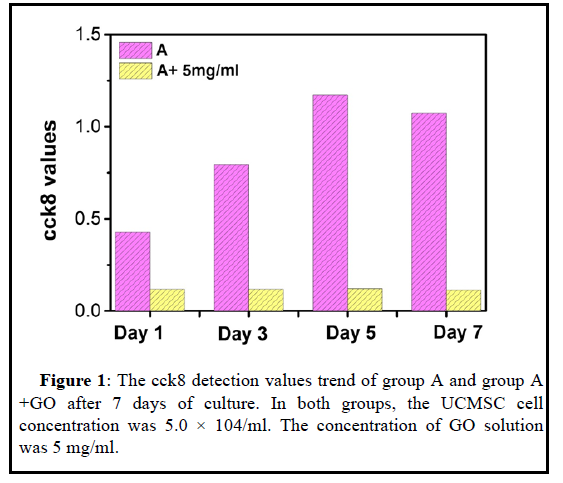
To observe the cytotoxicity of GO to UCMSC, the specific method is: take P4 generation UCMSC cells and GO solid particles, and conduct group culture observation. The control group is a pure UCMSC culture group, and the UCMSC concentration is 5.0 × 104 MSC/ml; the experimental group is In the UCMSC+GO co-culture group, the cell concentration was also 5.0 × 104 MSC/ml; the GO pellet mass was 5 mg, after relatively mixing, inoculated into a 24- well plate, inoculated 500 ul per well. Incubate overnight in a 37°C, 5% CO2 incubator, continuously observe, and take photos for recording. Among them, Day-1, Day-3, Day-5 and Day-7 are for CCK8 detection. Day-1, Day-3 and Day-7 for life and death staining.
The cck8 test results in Figures 1 and 2 showed that the cells had no proliferation within 7 days with the 5 mg/ml drug. As the GO concentration is too high, the cell survival space is compressed, and the overall culture density is too high to cause cell death. High concentrations of GO can produce toxicity to cells and mediate cell apoptosis.
In vitro culture of UCMSC and GO at different concentrations
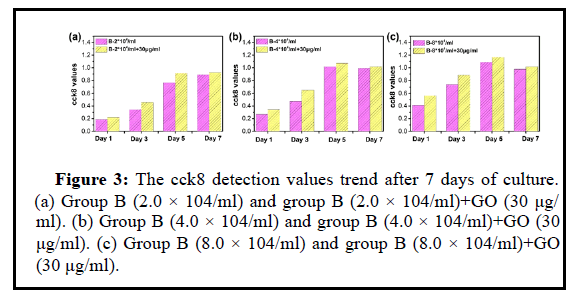
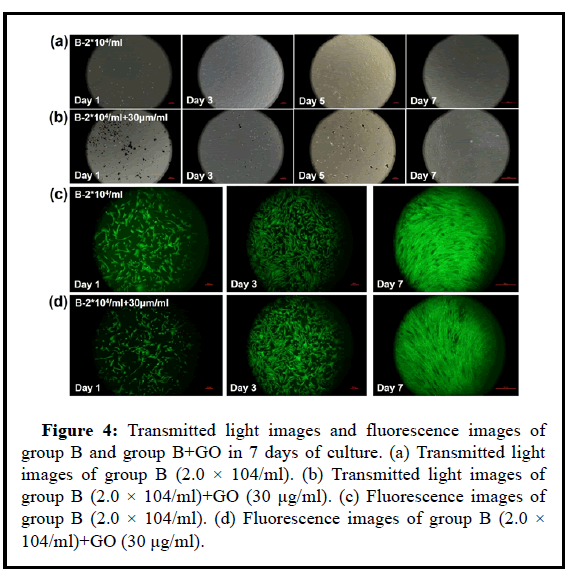
UCMSCs with concentrations of 8.0 × 104/ml, 4.0 × 104/ml, and 2.0 × 104/ml were cul-tured in vitro with GO nanoparticles at a concentration of 30 μg/ml, and P4 generation umbilical cord UCMSCs were mixed with GO Inoculate into a 24-well plate, inoculate 500 ul per well, inoculate 2 wells in parallel for each concentration. Incubate overnight in a 37°C, 5% CO2 incubator, continuously observe and take photos for recording. Among them, Day-1, Day-3, Day-5 and Day-7 are for CCK8 detection. Day-1, Day-3 and Day-7 for life and death staining.
The cck8 test results from Figures 3-8 showed that the proliferation degree of 8.0 × 104/m, 4.0 × 104/ml, 2.0 × 104/ml plus 30 μg/ml GO was lower than that of the control group. With the increase of stem cell concentration, 30 μg/ml GO was the effect of MSC proliferation is reduced.
Figure 4: Transmitted light images and fluorescence images of group B and group B+GO in 7 days of culture. (a) Transmitted light images of group B (2.0 × 104/ml). (b) Transmitted light images of group B (2.0 × 104/ml)+GO (30 μg/ml). (c) Fluorescence images of group B (2.0 × 104/ml). (d) Fluorescence images of group B (2.0 × 104/ml)+GO (30 μg/ml).
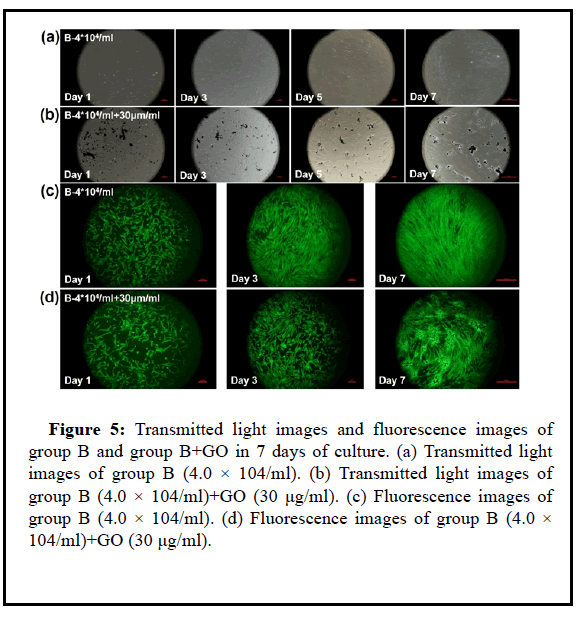
Figure 5: Transmitted light images and fluorescence images of group B and group B+GO in 7 days of culture. (a) Transmitted light images of group B (4.0 × 104/ml). (b) Transmitted light images of group B (4.0 × 104/ml)+GO (30 μg/ml). (c) Fluorescence images of group B (4.0 × 104/ml). (d) Fluorescence images of group B (4.0 × 104/ml)+GO (30 μg/ml).
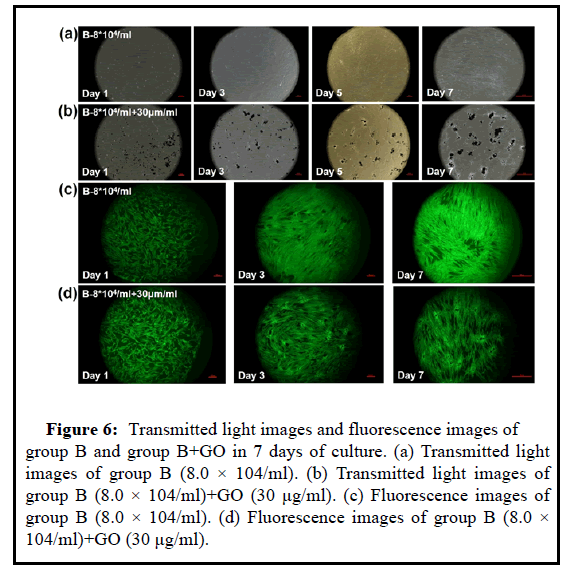
Figure 6: Transmitted light images and fluorescence images of group B and group B+GO in 7 days of culture. (a) Transmitted light images of group B (8.0 × 104/ml). (b) Transmitted light images of group B (8.0 × 104/ml)+GO (30 μg/ml). (c) Fluorescence images of group B (8.0 × 104/ml). (d) Fluorescence images of group B (8.0 × 104/ml)+GO (30 μg/ml).
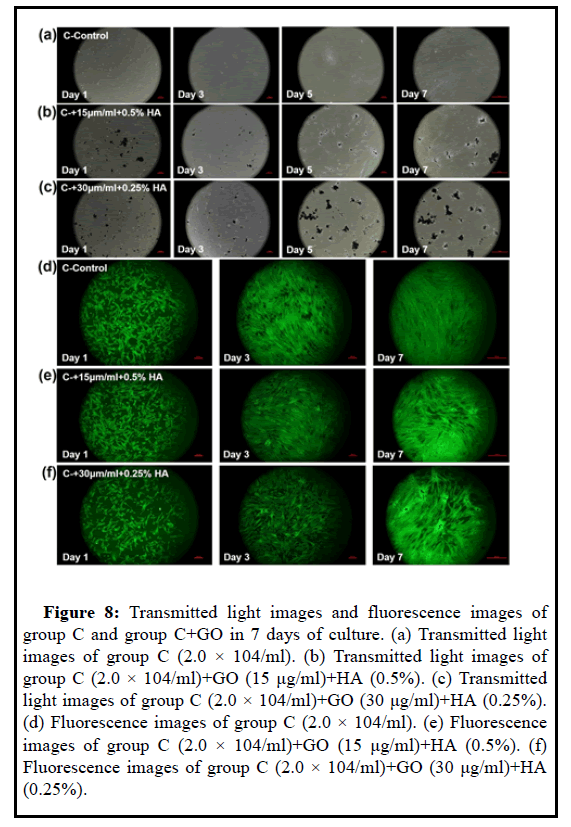
Figure 8: Transmitted light images and fluorescence images of group C and group C+GO in 7 days of culture. (a) Transmitted light images of group C (2.0 × 104/ml). (b) Transmitted light images of
group C (2.0 × 104/ml)+GO (15 μg/ml)+HA (0.5%). (c) Transmitted light images of group C (2.0 × 104/ml)+GO (30 μg/ml)+HA (0.25%). (d) Fluorescence images of group C (2.0 × 104/ml). (e) Fluorescence images of group C (2.0 × 104/ml)+GO (15 μg/ml)+HA (0.5%). (f) Fluorescence images of group C (2.0 × 104/ml)+GO (30 μg/ml)+HA (0.25%).
Combining the images under the magnification lens and the stained images of life and death, the low concentration of UCMSC cells cannot well reflect the migration and proliferation effects in the low concentration of GO environment. It is considered that the effect of GO particles on the cells is limited to a certain distance If the cell density is too low, the distance between GO and the cells is too far, so GO and UCMSC can be cultured separately, which cannot form a cocultivation environment. However, it does not mean that the higher the density of UCMSC, the better, and the higher the seeding density will cause cell death. Therefore, in general, GO particles need a lowconcentration environment to ensure their safety, while UCMSC must at least ensure a certain concentration to form a co-cultivation system.
In vitro co-culture of UCMSC and GO granular lubricant
To observe the in vitro culture of UCMSC and GO granular lubricant. The inoculation density of UCMSC is 2.0 × 104/ml. The mixed concentration of GO granular lubricant is 15 μg/ml GO+0.5% HA and 30 μg/mlGO+0.25% HA; Inoculate 0.5 ml into each well, inoculate 2 wells in parallel at each concentration; incubate overnight in a 37℃, 5% CO2 incubator, observe continuously and take pictures for recording.
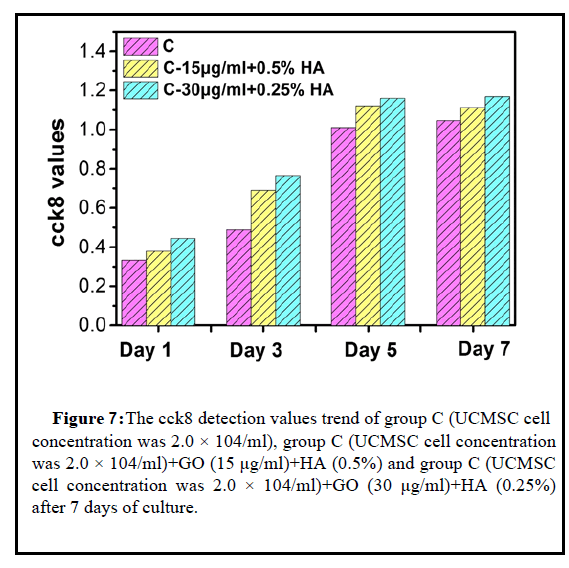
The cck8 test results in Figures 7 and 8 showed that within 72 hours, the proliferation of 15 μg/ml GO+0.5% HA and 30 μg/ml GO +0.25% HA was lower than that of the control group, and with the increase of GO concentration, the degree of MSC proliferation decreased. On the 5th day, the stem cell proliferation of the 15 μg/ml GO+0.5% HA group was higher than that of the control group. The CCK8 value of the 15 μg/ml GO+0.5% HA group was 1.160 and that of the control group was 1.121; the proliferation degree of 30 μg/ml GO+0.25% HA was higher than that of the control Group is low, as the GO concentration decreases, the degree of MSC proliferation increases. On the 7th day, the stem cell proliferation of the 15 μg/ml GO+0.5% HA group was higher than that of the control group. The cck8 value of the 15 μg/ml GO+0.5% HA group was 1.169 and that of the control group was 1.111; the proliferation degree of 30 μg/mlGO +0.25% HA was higher than that of the control group, as the GO concentration decreases, the degree of MSC proliferation increases. The proliferation of stem cells in the 15 μg/ml GO+0.5% HA group in group C was higher than that in the con-trol group. It may be that the stem cells in the control group have proliferated to the maximum value and have been lost. Therefore, the cck8 value decreased from 1.121 to 1.111 at 5th day. The adsorption effect of GO on stem cells, the growth surface area in the well plate is larger than that of the control group, so the degree of stem cell proliferation is higher than that of the control group. The ratio of the mixed lubricant of GO and HA is also related to the cultivation of UCMSC. First of all, because sodium hyaluronate injection is a kind of macromolecular structure, it has high viscosity, theoretically, it will have a certain impact on the migration of MSC and hinder its aggregation to GO particles, so the concentration of HA should not be too high. However, HA will also hinder the aggregation of GO particles, which is conducive to the uniform distribution of GO particles. The results also show this. Small particles of GO will be safer and less toxic than large particles of GO. Therefore, the mixing of HA is conducive to the longterm uniform distribution of UCMSC+GO, and from the results, the low concentration of HA is more suitable for co-cultivation of the two.
Serum NO test results
The difference between GO+MSC group and blank group in group A was statistically significant (P<0.01), and there was no significant difference between MSC group, GO group and blank group (P>0.05). The difference was not statistically significant (P>0.05). The difference between GO+MSC group and blank group in group B was statistically significant (P<0.01), the difference between MSC group, GO group and blank group was statistically significant (P<0.05). The difference between GO+MSC group and MSC group was It has statistical significance (P<0.05). Compared between groups, the difference between GO+MSC group, MSC group and GO group was not statistically significant (P>0.05), and the difference between blank group was statistically significant (P<0.05) (Table 1).
| Group | Blank | GO | MSC | GO+MSC |
|---|---|---|---|---|
| Group A | 22.097 ± 0.352 | 21.436 ± 0.0311) | 21.020 ± 0.0262) | 17.624 ± 0.1273) |
| Group B | 23.662 ± 0.056 | 20.544 ± 0.0854) | 19.424 ± 0.0465) | 17.799 ± 0.0496) |
| P value | 0.029 | 0.43 | 0.254 | 0.725 |
Table 1: Serum NO results after treatment (± s ng/ml).
Serum COL-II test results
Within group comparison, the difference between GO+MSC group, MSC group and blank group in group A was statistically significant (P<0.01), and the difference between GO group and blank group was not statistically significant (P>0.05); GO+MSC The difference between the group and the MSC group was statistically significant (P<0.01). In group B, the difference between GO+MSC group and blank group was statistically significant (P<0.01), the difference between MSC group, GO group and blank group was not statistically significant (P>0.05); GO+MSC group and MSC group. The difference was statistically significant (P<0.01).
Compared between groups, the difference between GO+MSC group and MSC group was statistically significant (P<0.01), and the difference between GO group and blank group was not statistically significant (P>0.05) (Table 2).
| Group | Blank | GO | MSC | GO+MSC |
|---|---|---|---|---|
| Group A | 13.475 ± 0.342 | 14.127 ± 0.1021) | 15.589 ± 0.0632) | 19.372 ± 0.0633) |
| Group B | 12.253 ± 0.147 | 13.644 ± 0.0284) | 14.429 ± 0.0925 | 16.257 ± 0.4166) |
| P value | 0.315 | 0.249 | 0.009 | 0 |
Table 2: Serum COL-II results after treatment ( ± s,ng/ml).
Serum GAG test results
In group A, the difference between GO+MSC group and blank group was statistically significant (P<0.01), the difference between MSC group and blank group was statistically significant (P<0.05), and the difference between GO group and blank group was not statistically significant (P>0.05). The difference between GO+MSC group and MSC group was statistically significant (P<0.01). In group B, the difference between GO+MSC group, MSC group and blank group was statistically significant (P<0.01), the difference between GO group and blank group was statistically significant (P<0.05); comparison between GO+MSC group and MSC group. The difference was statistically significant (P<0.05).
Compared between groups, the difference between the blank group and the GO+MSC group was statistically significant (P<0.01), and the difference between the MSC group and the GO group was not statistically significant (P>0.05) (Table 3).
| Group | Blank | GO | MSC | GO+MSC |
|---|---|---|---|---|
| Group A | 23.832 ± 0.891 | 26.342 ± 1.0421) | 29.022 ± 0.9732) | 37.439 ± 2.1553) |
| Group B | 18.709 ± 0.552 | 22.689 ± 0.6414) | 24.028 ± 0.6755) | 26.554 ± 0.4506) |
| P value | 0.002 | 0.096 | 0.051 | 0 |
Table 3: Serum GAG results after treatment ( ± s,ng/ml).
Serum IL-6 test results
In group A, the difference between GO+MSC group, MSC group, GO group and blank group was statistically significant (P<0.01); the difference between GO+MSC group and MSC group was statistically significant (P<0.01). In group B, the difference between GO+MSC group, MSC group, GO group and blank group was statistically significant (P<0.01); the difference between GO+MSC group and MSC group was statistically significant (P<0.05).
Compared between different groups, the difference between GO +MSC group was statistically significant (P<0.01), the difference between blank group was statistically significant (P<0.05), the difference between MSC group and GO group was not statistically significant (P>0.05) (Table 4).
| Group | Blank | GO | MSC | GO+MSC |
|---|---|---|---|---|
| Group A | 16.082 ± 0.323 | 10.957 ± 0.3431) | 9.668 ± 0.1942) | 7.426 ± 0.2943) |
| Group B | 18.367 ± 0.861 | 10.002 ± 0.1914) | 9.506 ± 0.1235) | 8.680 ± 0.2426) |
| P value | 0.024 | 0.169 | 0.565 | 0.009 |
Table 4: Result of serum IL-6 after treatment (± s,ng/ml).
Serum TNF-α test results
In group A, the difference between GO+MSC group, MSC group, GO group and blank group was statistically significant (P<0.01); the difference between GO+MSC group and MSC group was statistically significant (P<0.01). In group B, the difference between GO+MSC group, MSC group, GO group and blank group was statistically significant (P<0.01); the difference between GO+MSC group and MSC group was statistically significant (P<0.05).
Compared between different groups, the difference between GO +MSC group was statistically significant (P<0.01), the difference between blank group was statistically significant (P<0.05), the difference between MSC group and GO group was not statistically significant (P>0.05) (Table 5).
| Group | Blank | GO | MSC | GO+MSC |
|---|---|---|---|---|
| Group A | 9.466 ± 0.177 | 8.447 ± 0.1131) | 6.109 ± 0.0442) | 5.139 ± 0.1833) |
| Group B | 10.013 ± 0.197 | 8.891 ± 0.1884) | 6.856 ± 0.1605) | 6.210 ± 0.0586) |
| P value | 0.037 | 0.087 | 0.146 | 0.006 |
Table 5: Result of serum TNF-α after treatment ( ± s,ng/ml).
It was found that low concentration of GO and sufficient concentration of UCMSC are suitable for the second Co-culture. Furthermore, the addition of HA will make this culture more evenly distributed. The adsorption of GO on UCMSC can also make the two closely linked, which avoids the impact of animal joint activities on cells. Meanwhile, two subsequent knee osteoarthritis (KOA) animal models were selected in this experiment. The modified Hulth +cartilage defect model in group A is the main sports injury model. For the current multiple ligament tears and meniscus destruction, this injury will cause the intra-articular inflammation to gradually develop into sub-sequent KOA. The results show that the role of the threedimensional mesh scaffold of this kind of GO particles is more important, which makes UCMSC better adhere to the cartilage defect area, so as to grow and proliferate.
Conclusion
The model of group B is a model of cartilage degeneration induced by chemical factors. It can be seen that cartilage has different degrees of necrosis, which is relatively close to the pathogenesis of degenerative osteoarthritis in clinic. GAG, IL-6, and TNF-α have a statistically significant difference compared with the blank group, which also shows that the use of GO particle lubricants to carry UCMSC has a better therapeutic effect. In summary, we have confirmed that GO can coexist with UCMSC in vitro and play the role of adsorption and promotion. Cell survival and growth under different concentrations of environment were preliminary discussed. UCMSC loaded with graphene oxide can promote the chondrocyte secretion of two knee osteoarthritis animal models, reduce the level of inflammation in the joints, and play a role in cartilage repair. Our findings may provide some help for the efficacy of KOA animal models exploration.
Declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of First Teaching Hospital of Tianjin University of Traditional Chinese Medicine. Written informed consent was obtained from individual participants.
Consent for publication
Not applicable.
Availability of data and materials
The data that supports the findings of this study are available within the manuscript and its supplementary material.
Abbreviations
Not applicable
Competing interests
All the authors declare no conflicts on financial interests of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (81873316 and 81673994), and Higher Education Science and Technology Research Project of Hebei Province (ZD2019087).
Authors' contributions
Aifeng Liu came up the basic ideas and analyzed the data of this manuscript. Jixin Chen conducted this experiment. Shuwei Gong also conducted this experiment. Qiang Wei supervised this research work. Ye Yuan were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Corresponding authors: Qiang Wei and Ye Yuan
References
- Holt BD, Wright ZM, Arnold AM, Sydlik SA (2017) Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9(3).
- Shuai C, Zeng Z, Yang Y, Qi F, Peng S, et al. (2020) Graphene oxide assists polyvinylidene fluoride scaffold to reconstruct electrical microenvironment of bone tissue. Mater Des 190: 108564.
- Serrano MC, Patiño J, GarcÃa-Rama C, Ferrer ML, Fierro JLG, et al. (2014) 3D free-standing porous scaffolds made of graphene oxide as substrates for neural cell growth. J Mater Chem B 2(34): 5698-5706.
- Rostami F, Tamjid E, Behmanesh M (2020) Drug-eluting PCL/graphene oxide nanocomposite scaffolds for enhanced osteogenic differentiation of mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl 115: 111102.
- Guo W, Qiu J, Liu J, Liu H (2017) Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep 7(5678).
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R et al.(1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411): 143-147.
- Dominici M, Blanc KL, Mueller I, Slapercortenbach I, Marini FC. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4): 315-317.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. (2002) Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Bio Cell 13(12): 4279-4295.
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, et al. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41-49.
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4): 704-715.
- Prockop DJ (1997) Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 276(5309): 71-74.
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4): 1815-1822.
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5): 1294-1301.
- Blanc KL, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, et al. (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363(9419): 1439-1441.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 3(3): 301-313.
- Sato T, Vries RGJ, Snippert HJ, De Wetering MV, Barker N, et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262-265.
- Lee WC, Lim CHYX, Shi H, Tang LAL, Wang Y. (2011) Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 5(9): 7334-7341.
- Tatavarty R, Ding H, Lu G, Taylor R, Bi X. (2014) Synergistic acceleration in the osteogenesis of human mesenchymal stem cells by graphene oxide–calcium phosphate nanocomposites. Chem Commun 50: 8484-8487.
- Kim J, Kim HD, Park J, Lee E, Kim E, et al. (2018) Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater Res 22: 1-9.
- Wang Z, Shen H, Song S, Zhang L, Chen W, et al. (2018) Graphene Oxide Incorporated PLGA Nanofibrous Scaffold for Solid Phase Gene Delivery into Mesenchymal Stem Cells. J Nanosci Nanotechnol 18(4): 2286-2293.
- Yang J, Hsieh KY, Kumar PV, Cheng S, Lin Y, et al. (2018) Enhanced Osteogenic Differentiation of Stem Cells on Phase-Engineered Graphene Oxide. ACS Appl Mater Interfaces 10(15): 12497-12503.
- Kim S, Han H, Chae G, Lee S, Bo S.(2006) Successful Stem Cell Therapy Using Umbilical Cord Blood-Derived Multipotent Stem Cells for Buerger's Disease and Ischemic Limb Disease Animal Model. Stem Cells 24(6): 1620-1626.
- Nicola MD, Carlostella C, Magni M, Milanesi M, Longoni PM. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10): 3838-3843.
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, et al. (1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382(6592): 635-638.
- Ewald FC (1989) The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 248: 9-12.
- Girgis FG, Marshall JL, Monajem A (1975) The Cruciate Ligaments of the Knee Joint: Anatomical. Functional and Experimental Analysis. Clin Orthop Relat Res 106: 216-231.
- Insall JN, Salvati EA (1971) Patella position in the normal knee joint. Radiology 101(1): 101-104.
- Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, et al. (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3(9): 563-568.
- Elias DC, Nair R, Mohiuddin T, Morozov SV, Blake P, et al. (2009) Control of Graphene's Properties by Reversible Hydrogenation: Evidence for Graphane. Science 323(5914): 610-613.
- Kudin KN, Ozbas B, Schniepp HC, Prudhomme RK, Aksay IA. (2008) Raman spectra of graphite oxide and func-tionalized graphene sheets. Nano Lett 8(1): 36-41.
Citation: Wei Q, Liu A, Chen J, Gong S, Yuan Y (2021) Effects of Co-Culture of Graphene Oxide Scaffolds with Different Concentrations and Umbilical Mesenchymal Stem Cells on the Proliferation and Differentiation of Stem Cells. J Bioremediat Biodegrad 12:013.
Copyright: © 2021 Wei Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6851
- [From(publication date): 0-2021 - Nov 26, 2025]
- Breakdown by view type
- HTML page views: 5986
- PDF downloads: 865