Efficacy of Plaqx Forte Therapy for the Maintenance of Cardiovascular, Blood Vessels and Cellular Health
Received: 22-Apr-2019 / Accepted Date: 15-May-2019 / Published Date: 22-May-2019
Abstract
Phosphatidylcholine is a polyunsaturated fatty acid compound and is an essential cell membrane phospholipid. It is important in maintaining normal membrane structure and increase cell protection against external factors. Maintenance of the integrity of the cell membrane is vital as destructive cell membrane results in the release of lowdensity lipoprotein cholesterol into the blood. Phosphatidylcholine is also effective in lowering cholesterol level by enhancing cholesterol efflux, down-regulating fatty acid synthesis and increasing the cholesterol oxidation to bile salts. It is well known to decreases the triglycerides level in blood and dissolves atherosclerotic plaques. Atherosclerosis has long been established as a major risk factor of cardiovascular disease, which develops when scar tissues form on the damage arteries wall and cause the buildup of plaques. Both hypercholesterolemia and hypertriglyceridemia contribute towards atherosclerosis as excessive cholesterol and triglycerides in blood accumulate along the wall of blood vessels and eventually cause blockage. Considering the beneficial effects of phosphatidylcholine, this work hypothesised that PlaqX Forte administration in a group of 13 volunteers may improve their blood glucose and lipid profile. A total of 25 ml of PlaqX Forte infusions diluted in 250 ml of 5% glucose were administered intravenously between three to four times per week. Blood samples were collected for the analysis of blood glucose and lipid profile before treatment and after 7 to 12 times of infusions. The results showed a significant reduction in total cholesterol, triglycerides, low-density lipoprotein cholesterol and haemoglobin A1c levels, suggesting that PlaqX Forte infusion may be used as a remedy for hypercholesterolemia and hypertriglyceridemia. However, a reduction in high-density lipoprotein cholesterol level was observed in this study. Further investigation is required following the recommended full course of 30 infusions to fully understand the efficacy of PlaqX Forte therapy in the management of cardiovascular, blood vessels and cellular health.
Keywords: Phosphatidylcholine; Atherosclerosis; Cardiovascular disease; Cholesterol; Triglycerides
Abbreviations:
PC: Phosphatidylcholine; LDL: Low-density Lipoprotein; CVD: Cardiovascular Disease; PAD: Peripheral Artery Disease; WHO: World Health Organization; TG: Triglycerides; HDL: High-density Lipoprotein; BG: Blood Glucose; HbA1c: Haemoglobin A1c; TC: Total Cholesterol; SE: Standard Error; eAG: Estimated Average Glucose; LCAT: Lecithin-cholesterol-acyl Transferase; CMC: Critical Micelle Concentration; RBC: Red Blood Cells; FFAs: Free Fatty Acids; DPPC: Dipalmitoylphosphatidylcholine; TMAO: Trimethylamine-N- oxide
Introduction
Cardiovascular disease (CVD) is a class of disorders of the heart and blood vessels. According to the World Health Organization (WHO), CVD is found to be a major cause of morbidity worldwide. In 2016, approximately 17.9 million people died from CVD, accounting for 31% of all global deaths [1]. CVD is most prevalent in people over the age of 50, with increased risk as they age. Atherosclerosis, a major underlying cause of CVD [2], often developed when the inner wall of arteries is damaged and fatty deposits (plaque) start to build up. As the plaque accumulates, coronary arteries become narrow and the shear force of the blood flow through the arteries increases. This force may ultimately cause plaque rupture and resulting in thrombus formation which in turn causes heart attack and ischemic stroke [3]. High level of lowdensity lipoprotein (LDL) in the blood is known to increase the risk of atherosclerosis as it is the primary source of artery-clogging plaque. In addition, high plasma triglycerides (TG) level has been reported to be a risk factor for CVD, independent of high-density lipoprotein (HDL) cholesterol level [4]. Many studies have shown that the risks of CVD morbidity and mortality are directly correlated with the level of atherosclerosis in coronary arteries and carotid arteries [5]. In fact, atherosclerosis is also a risk factor for peripheral artery disease (PAD), a circulatory system disorder with similar prevalence as coronary heart disease and stroke [6].
PlaqX Forte solution is a plant-derived supplement consisting of phosphatidylcholine (PC). PC is a strong bilayer-forming lipid and the most abundant phospholipids found in the cell membrane [7]. It plays an important role in the maintenance of cell membrane integrity and function. Although PC is found naturally in the human cell, the rate of PC renewal declines with increasing age [8]. In addition, factors such as toxins, detergents, free radicals, heavy metals, chemical fumes, inflammation, allergies, immunological processes and metabolic diseases further cause damage to the cell membrane [9]. The decrease of PC in the biological membrane can lead to the excessive release of cholesterol, LDL, and plaque deposits, which eventually results in the development of various diseases including CVD and PAD.
Hence, alternative therapy is becoming increasingly important in an effort to address the prevalence of CVD, PAD and their prevention. One such approach is by replenishing PCs in the human body through PlaqX Forte infusion in order to maintain cardiovascular, blood vessels and cellular health. Therefore, the objective of this study is to investigate the efficacy of PlaqX Forte therapy in improving cardiovascular health and wellbeing.
Material and Methods
Volunteers
Volunteers consist of thirteen (8 male and 5 female) Asian aged between 36 to 68 years old, with an average weight of 79.1 ± 8.8 kg. Volunteers included in this study consisted of those with atherosclerosis and/or fatty liver disease as confirmed ultrasonographically.
Blood glucose and lipid profile studies
Blood tests including blood glucose and lipid profile studies were performed on the volunteers to evaluate their health condition prior to and after the treatment. The blood test parameters include blood glucose (BG), haemoglobin A1c (HbA1c), total cholesterol (TC), TG, HDL and LDL levels. Post-treatment blood glucose and lipid profile studies were performed following 7 to 12 infusions.
Treatment protocol
A total of 25 ml of PlaqX Forte infusion were diluted in 250 ml of 5% glucose and administered intravenously for a duration of 90 to 120 minutes. Each volunteer was given three to four infusions per week, predominantly at morning hours.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistic software (Version 25). HbA1c, TC, TG, HDL and LDL levels were analysed using paired T-tests. BG levels were statistically analysed using the Wilcoxon-signed rank test. P values less than 0.05 were considered statistically significant and data were presented as means with standard error (SE) of the means.
Results
The effects of PlaqX Forte therapy on blood glucose and lipid profile for all 13 volunteers after 7 to 12 times of infusions are given in Table 1 and Figure 1. A total of 25 ml of PlaqX Forte infusion diluted in 250 ml of 5% glucose was given intravenously to all the volunteers between three to four times weekly as described under the treatment protocol.
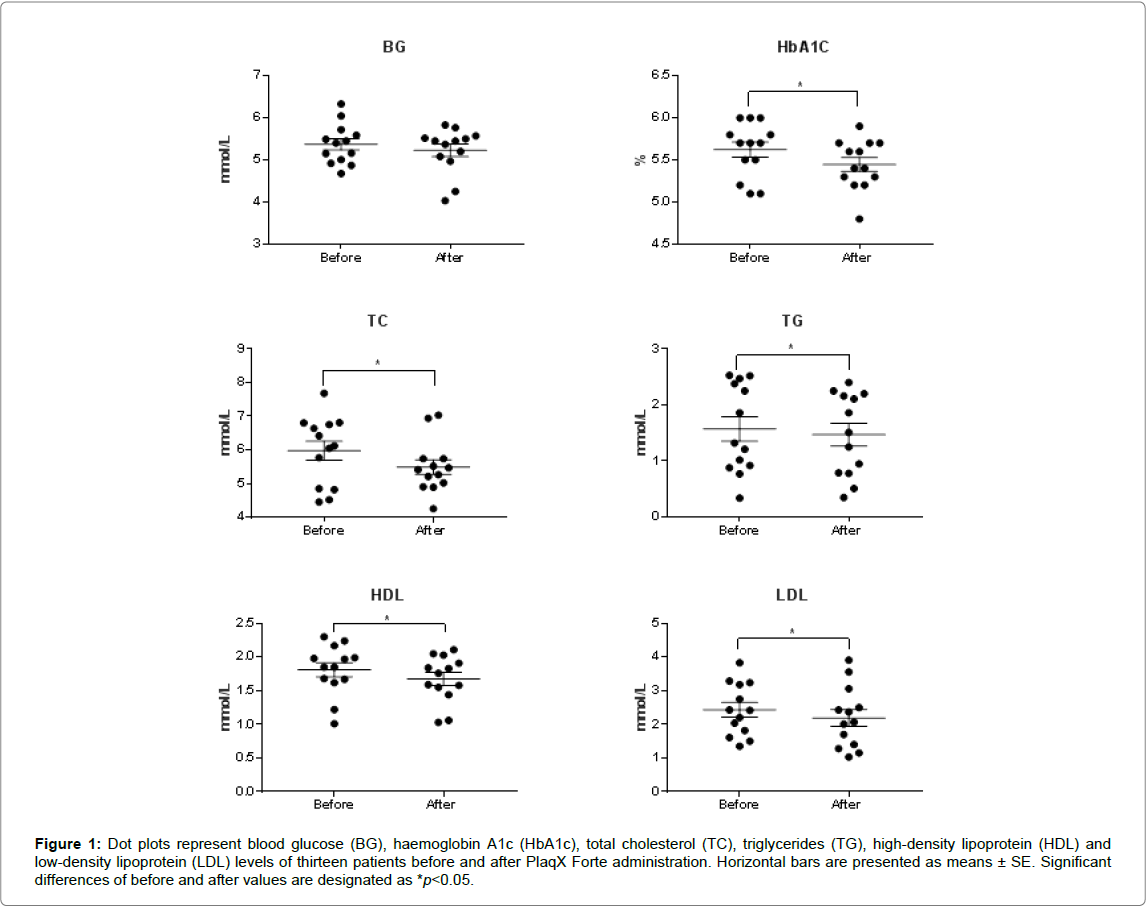
After receiving the partial course of PlaqX Forte therapy, an overall reduction of BG and HbA1c levels by 0.14 mmol/L and 0.17%, respectively, were observed as recorded in Table 1. However significant difference is observed only in the lowering of HbA1c level but not in the BG level. Furthermore, lipid profiles of the volunteers showed overall significant improvement where the plasma TC, TG, and LDL levels decreased by 0.49 mmol/L, 0.10 mmol/L and 0.25 mmol/L, respectively Table 1 and Figure 1. However, the HDL level was decreased significantly by 0.13 mmol/L compared with the initial value. The PlaqX Forte therapy was well tolerated by all the volunteers without any undesirable side-effects.
Figure 1: Dot plots represent blood glucose (BG), haemoglobin A1c (HbA1c), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels of thirteen patients before and after PlaqX Forte administration. Horizontal bars are presented as means ± SE. Significant differences of before and after values are designated as *p< 0.05.
| Test Parameter | Before | After | Reference Range |
|---|---|---|---|
| Blood glucose (BG) | 5.37 ± 0.13 | 5.23 ± 0.15 | 3.89-6.11 mmol/L |
| Haemoglobin A1c (HbA1c) | 5.62 ± 0.09 | 5.45 ± 0.29* | 3.6-6% |
| Total cholesterol (TC) | 5.98 ± 0.28 | 5.49 ± 0.21* | 3.12-6.24 mmol/L |
| Triglycerides (TG) | 1.57 ± 0.22 | 1.47 ± 0.20* | 0.34-1.70 mmol/L |
| High-density lipoprotein (HDL) | 1.81 ± 0.10 | 1.68 ± 0.10* | ≥ 0.9 mmol/L |
| Low-density lipoprotein (LDL) | 2.44 ± 0.22 | 2.19 ± 0.25* | ≤ 4.13 mmol/L |
Table 1: Blood glucose and lipid profile before and after PlaqX Forte treatment. Values with significant differences post-treatment are designated as *p< 0.05. Results are expressed as mean ± standard error.
Discussion
PlaqX Forte (LabRMS) is a plant-derived supplement made up of PC compound which has been formulated to repair cell membrane, restore cellular metabolism and clear excess plaque deposits from the human system. PC derived from soybeans have been used worldwide for more than 40 years to improve cellular function and treat various diseases including CVD, myocardial ischemia, and fatty liver-induced liver dysfunction due to its ability to inhibit fatty acid accumulation and prevent plaque build-up [9,10]. Several studies have been done to prove that PC can significantly lower TC, LDL and TG levels while increasing HDL level in patients with primary hyperlipidaemia [11-13]. This may also be partially attributed to the presence of deoxycholic acid, a solvent that not only solubilises PC, but also influences TG metabolism and prevents the formation of plaques [10,14].
In this study, volunteers were administered with PlaqX Forte infusion between three to four times weekly following initial blood glucose and lipid profile study. Even though the initial blood test results of the majority of the volunteers fell within the normal range, further overall improvement was observed following PlaqX Forte treatment despite having undergone the treatment for only 7 to 12 times, which is less than half the recommended complete course 30 infusions in total.
Although there was improvement in BG and HbA1c tests as shown in the result, the estimated average glucose (eAG) level derived from HbA1c is considered to be more reliable than the fasting BG test as it reflects the average blood glucose for the past 8 to 12 weeks, thereby eliminating the issue of variability and fluctuation that occurs in daily blood glucose concentration [15]. Unlike HbA1c, fasting BG level is easily affected by other short-term factors anxiety, depression, sleep deprivation and other physiological reactions [16,17]. Furthermore, fasting BG level in the human body can also be affected if the individual fasted for less than 8 hours or had taken a heavy meal on the night before the test. Considering insulin resistance is one of the main factors in the pathogenesis of diabetes and is associated with an increased risk of CVD [18], PC plays an important role in increasing cells’ sensitivity towards insulin, promoting body cells to absorb sugar from the blood and keep BG levels in the normal range [19].
Besides the improvement in terms of HbA1c, the results also revealed improvement in TC, TG and LDL levels of volunteers, indicative of lower CVD risk. Kirsten et al. reported a similar effect of PC in improving the lipoprotein profile of diabetic patients [20]. With PlaqX Forte, lipid peroxidation is inhibited [9]. Lipid peroxidation has been found to be an important consequence of oxidative stress in the injured arterial wall, which in turn contributes to the development of atherosclerotic lesions [21]. PC increases cell membrane stability and protects cell membrane against free radicals by maintaining and restoring the normal membrane structure where lecithin-cholesterolacyl transferase (LCAT) is activated and the esterification of free cholesterol in plasma is accelerated [9]. Free cholesterol on the surface of lipoprotein, erythrocyte membranes or in cells can then be taken up by HDL, esterified and finally being eliminated from plasma. PC circulating in the bloodstream as a result of LCAT activity can also be taken up by the cell for re-acylation to form membrane phospholipid [22]. Besides its capability to esterify cholesterol in the plasma, LCAT also possesses antioxidant properties which help to prevent the accumulation of oxidised lipid in plasma lipoproteins [23].
According to Brown [24], critical micelle concentration (CMC) can be achieved with a high concentration of PC, resulting in the formation of micelles with cores that are capable of solubilising and transporting TG and free fatty acid. Hence, oxidative modification of LDL in the blood vessels caused by free radicals that leads to plaque accumulation and atherosclerosis [25] can be bound into micelles by PC and subsequently transported to the liver to be excreted with gall fluid. This results in the normalisation of plasma LDL cholesterol level.
PC is also a significant component of HDL and plays an essential role in enhancing HDL metabolism. HDL system is enhanced through promoting cholesterol efflux from cells and reverse cholesterol transport [26]. Furthermore, PC is also effective in platelet aggregation and improves the viscosity and rheology of the blood [9]. A study conducted by Ehrly & Blendin [27] suggested that PC improves the capillary flow rate by affecting the flow properties and deformability of red blood cells (RBC) and thus facilitate the passage of RBC through narrow capillaries. PC has been proven to significantly reduce the TG synthesis and levels, raise the HDLs in cholesterol metabolism and inhibit the formation of atherosclerotic plaques in blood vessels [28], thereby reducing the risk of developing CVD and PAD.
Interestingly, a significant reduction in HDL level was observed from the result of this study. This may be explained by the role of PC in inducing lipolysis, coupled with a series of events that consequently results in a lower level of HDL. According to Hasenschwandtner [29], PC treatment is known to induce fat cell lipolysis, which results in the release of free fatty acids (FFAs) to the blood circulation [30].
FFAs are presented to the liver for the production of very-lowdensity lipoprotein TG, which are subsequently transferred to HDL and causing TG enrichment of the HDL [31]. Hepatic lipase then catabolises the TG-rich HDL more rapidly than native HDL which ultimately lead to the reduction of HDL [32]. Furthermore, although HDL is useful in assessing catabolises CVD risk, its significant correlation with CVD risk has been found to decrease in patients with lower LDL concentration [33].
It is, however, important to acknowledge that diverging opinions on the effects of PC on the risk of CVD incidence and mortality are still prevalent in the current literature. While many of the earlier studies associated PC with a lower risk of CVD, several recent studies seem to suggest the opposite. Notably, several studies associate increased risk of CVD incidence and mortality with dietary PC, particularly those derived from egg yolk or dipalmitoylphosphatidylcholine (DPPC)-d9 lecithin [34,35]. These studies suggest that metabolism of PC by intestinal microbial results in the production and elevation of trimethylamine-N- oxide (TMAO), a pro-atherosclerotic metabolite, that increases the risk of CVD. The rise of these bodies of literature has prompted Meyer and Shea [36] to conduct a systematic review and meta-analysis that scrutinised several prospective studies which have implicated choline metabolite TMAO with CVD risk. However, they found no association between dietary choline with CVD [36]. Interestingly, one of the studies that suggested a positive association between PC and CVD mortality by Zheng et al. actually found no association between PC and CVD incidence [37]. Furthermore, this association with CVD mortality was found to be significantly stronger in patients with underlying diabetic issues compared to non-diabetic patients [37].
Coincidently, based on their systematic review and metaanalysis, Meyer and Shea [36] also argued that “studies that show a positive association between TMAO and CVD risk have generally been conducted in clinic-based samples at high risk for CVD risk or in disease-based cohorts”. Many other earlier studies, including a large-scale prospective cohort study of over 16 000 healthy women [38], further reiterate the absence of an association between choline (including those derived from PC) and CVD mortality risk, with perhaps the exception of sphingomyelin-derived choline [39]. Further to that, the PC in this study is administered through infusion. Hence, they completely bypass the intestine and are not metabolised by TMAO-producing intestinal microbial.
Besides restoring cellular membranes and enhancing cholesterol efflux to keep cholesterol and plaque levels low in the blood as discussed above, PC also improves the transportation of metabolic products and nutritious elements between cells by improving the cellular function. In cases of PC deficiency, the exchange of biological components between cells becomes more difficult due to the hardening of the cell wall. This will eventually affect cell functions and leads to premature cell ageing [29]. Furthermore, PC also possesses hepato-protective properties which help in liver repair and cholesterol reduction. To date, several studies have proven that PC allow successful improvement of specific indicators of liver damage, speed up the structural and functional recovery of liver tissue and promote the restoration of subjects’ overall well-being [40]. The current body of evidence also shows that PC has significant antioxidant properties [41,42]. In addition, PC contributes to the synthesis of acetylcholine, which helps in transmitting nerve signal and creating memories. Hence, PC acts as a memory enhancer and is known to be useful in treating Alzheimer’s disease [43].
The overall result of this study supports the beneficial effects of PC which include the regulation of lipid homeostasis, reduction in fatty acid synthesis and oxidation, increase in cholesterol oxidation into bile salts, cell membrane maintenance, restoration of cellular metabolism and clearance of excess plaque from the human system. Therefore, administration of PlaqX Forte infusion is believed to be able to bring significant improvement towards the blood glucose and lipid profile and may serve as a potential therapy in improving and maintaining cardiovascular, blood vessels and cellular health.
Conclusion
This work suggests that despite volunteers having only undergone a partial course of the treatment, PC has a great influence on the regulation of lipid homeostasis and blood glucose level. Hence, this warrants further investigation with a larger number of volunteers completing the recommended full course treatment of 30 infusions.
Acknowledgements
The authors would like to thank the volunteers, who granted us consent for this publication and also EW European Wellness Academy GmbH, Germany for supporting this research article.
References
- Cardiovascular diseases (CVDs). World Health Organization 2017, May 17.
- Frostegård J (2013) Immunity, atherosclerosis and cardiovascular disease. BMC Med 11: 117.
- Moreno PR (2010) Vulnerable plaque: definition, diagnosis, and treatment. Cardiol Clin 28: 1-30.
- Hokanson JE, Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A metaanalysis of population-based prospective studies. Eur J Prev Cardiovasc 3: 213-219.
- Kuller L, Borthani N, Furberg C, Gardin J, Manolio T, et al. (1994) Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol 139: 1164-1179.
- Kalbaugh CA, Kucharska-Newton A, Wruck L, Lund JL, Selvin E, et al. (2017) Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among Medicare fee-for-service beneficiaries in the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 6.
- Marsh D (1990) Lipid-protein interactions in membranes. FEBS Letters 268: 371-375.
- Wood WG, Strong R, Williamson LS, Wise RW (1984) Changes in lipid composition of cortical synaptosomes from different age groups of mice. Life Sci 35: 1947-1952.
- Noh Y, Heo CY (2012) The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: An experimental study with a murine model. Arch Plast Surg 39: 452-456.
- Wojcicki J, Pawlik A, Samochowiec L, Kaldonska M, Mybliwiec Z (1995) Clinical evaluation of lecithin as a lipid-lowering agent. Phytotherapy Research 9: 597-599.
- Simons LA, Hickie JB, Buys J (1977) Treatment of hypercholesterolemia with oral lecithin. Aust New Zealand J Med Banner 7: 262-266.
- Klimov AN, Konstantinov VO, Lipovetsky BM, Kuznetsov AS, Lozovsky VT, et al. (1995) “Essential†phospholipids versus nicotinic acid in the treatment of patients with type IIb hyperlipoproteinemia and ischemic heart disease. Cardiovasc Drugs Ther 9: 779-784.
- El Kamshoushy A, Abel Maksoud R, El Mahdy N (2012) Evaluation of the efficacy of injection lipolysis using phosphatidylcholine/deoxycholate versus deoxycholate alone in treatment of localized fat deposits. J Clin Exp Dermatol Res 3: 2.
- Little RR, Sacks DB (2009) HbA1c: How do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes 16: 113-118.
- Facco F, Bradley M, Twedt R, Deiseroth D, Althouse A (2014) Sleep duration and fasting blood glucose in gestational diabetics. Am J Obstetrics and Gynecology 210: S169.
- Park C, Phillips D, Pagnini F, Langer E, Reece A (2016) Blood sugar level follows perceived time rather than actual time in people with type 2 diabetes. Proc Natl Acad Sci 113: 8168-8170.
- Shepherd PR, Kahn BB (1999) Glucose transporter and insulin action - implications for insulin resistance and diabetes mellitus. N Engl J Med 341: 248-257.
- Cantafora A, Masella R, Angelico M, Gandin C, Blount RJ, et al. (1992) Effect of intravenous polyunsaturated phosphatidylcholine infusion on insulin receptor processing and lipid composition of erythrocytes in patients with liver cirrhosis. Eur J Clin Invest 22: 777-782.
- Kirsten R, Heintz B, Nelson K, Hesse K, Schneider E, et al. (1994)Polyenylphosphatidylcholine improves the lipoprotein profile in diabetic patients. Int J Clin Pharmacol Ther 32: 53-56.
- Halliwell B, Susanna C (1993) Lipid peroxidation : its mechanism, measurement, and significance. Am J Clin Nutr 57: 715S-725S.
- Taylor LA, Pletschen L, Arends J, Unger C, Massing U (2010) Marine phospholipids — a promising new dietary approach to tumor-associated weight loss. Supportive Care in Cancer 18: 159-170.
- Vohl MC, Neville TAM, Kumarathasan R, Braschi S, Sparks DL (1999) A novel lecithin-cholesterol acyltransferase antioxidant activity prevents the formation of oxidized lipids during lipoprotein oxidation. Biochemistry 38: 5976-5981.
- Brown SA (2006) The science of mesotherapy: chemical anarchy. Aesthetic Surg J 26: 95-98.
- Heinecke JW (1998) Oxidants and antioxidants in the pathogenesis of atherosclerosis: Implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 141: 1-15.
- Schaefer EJ (1997) Effects of dietary fatty acids on lipoproteins and cardiovascular disease risk : summary. The Am J Clin Nutri 65: 1655S-1656S.
- Ehrly AM, Blendin R (1976) Influence of essential phospholipids on the flow properties of the blood. In: Phosphatidylcholine Springer Pp: 228-236.
- Brook JG, Linn S, Aviram M (1986) Dietary soya lecithin decreases plasma triglyceride levels and inhibits collagen- and ADP-induced platelet aggregation. Biochem Med Metab Biol 35: 31-39.
- Hasengschwandtner F (2005) Phosphatidylcholine treatment to induce lipolysis. J Cosmet Dermatol 4: 308-313.
- Ebbert JO, Jensen MD (2013) Fat depots, free fatty acids, and dyslipidemia. Nutrients 5: 495-508.
- Rydén M, Arner P (2017) Subcutaneous adipocyte lipolysis contributes to circulating lipid levels. Arterioscler Thromb Vasc Biol 37: 1782-1787.
- Rashid S, Uffelman KD, Lewis GF (2002) The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J Diabetes Complications 16: 24-28.
- Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, et al. (2010) HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy : an analysis from the JUPITER trial. Lancet 376: 333-339.
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, et al. (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New Engl J Med 368: 1575-1584.
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, et al. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57-63.
- Meyer KA, Shea JW (2017) Dietary choline and betaine and risk of cvd: A systematic review and meta-analysis of prospective studies. Nutrients 9: 711.
- Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, et al. (2016) Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am J Clin Nutr 104: 173-180.
- Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, Van der Schouw YT (2007) Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr 62: 386-394.
- Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, et al. (2015) Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr 145: 1787-1792.
- Kidd PM (1996) Phosphatidylcholine: A superior protectant against liver damage. Altern Med Rev 1: 258-274.
- Jayaraman T, Kannappan S, Ravichandran MK, Anuradha C V (2008) Impact of Essentiale L on ethanol-induced changes in rat brain and erythrocytes. Singapore Med J 49: 320-327.
- Nwosu CV, Boyd LC, Sheldon B (1997) Effect of fatty acid composition of phospholipids on their antioxidant properties and activity index. J Am Oil Chem Soc 74: 293-297.
- Little A, Levy R, Chuaqui-Kidd P, Handt D (1985) A double-blind, placebo controlled trial of high-dose lecithin in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 48: 736-742.
Citation: Nalapko YI, Chan MKS, Yee HR, Chia YC, Klokol D, et al. (2019) Efficacy of Plaqx Forte Therapy for the Maintenance of Cardiovascular, Blood Vessels and Cellular Health. J Nutr Sci Res 4: 136.
Copyright: © 2019 Nalapko YI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 9036
- [From(publication date): 0-2019 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 8036
- PDF downloads: 1000