Research Article Open Access
Efficient Removal of Phenol from Water Samples Using Sugarcane Bagasse Based Activated Carbon
Magda AA Akl1*, Magda B Dawy2 and Asmaa A Serage31Chemistry Department, Faculty of Science, Mansura University, Mansura, Egypt
2Chemistry Department, National Research Center, Cairo, Egypt
3MSc’s Student, Chemistry Department, Faculty of Science, Mansura University, Mansoura, Egypt
- *Corresponding Author:
- Magda A Akl
Professor of Analytical Chemistry
Faculty of Science, Mansoura University, Egypt
Tel: 0020502217833
E-mail: magdaakl@yahoo.com
Received date: March 17, 2014; Accepted date: April 19, 2014; Published date: April 22, 2014
Citation: AAM Akl, Dawy MB, Serage AA (2014) Efficient Removal of Phenol from Water Samples Using Sugarcane Bagasse Based Activated Carbon. J Anal Bioanal Tech 5:189. doi: 10.4172/2155-9872.1000189
Copyright: © 2014 AAM Akl, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sugarcane bagasse-based activated carbons (SCBACs) with different chemical characteristics appropriate for the removal of phenol in aqueous solutions have been prepared. The steam activated carbon (SCBACS) is obtained from the carbonized sugarcane bagasse residues in the presence of nitrogen in the temperature range from 700 to 900°C. The chemically activated carbon (SCBACN) was obtained from the carbonized sugarcane bagasse residues using NaOH. The structure of the SCBACs was characterized by N2 adsorption at 77 K, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The B.E.T methods are used to deduce the effective surface areas. The parameters, such as initial pH, temperature, initial phenol concentration, biosorbents dosage... etc., affecting the adsorption capacity of SCBACs toward phenol removal from aqueous solutions are investigated using batch experiments. The studies of kinetic models including pseudo first order and pseudo second-order are carried out. Adsorption isotherms are investigated. Equilibrium adsorption data fitted the Langmuir adsorption isotherm. The thermodynamic parameters including ΔG°, ΔH° and ΔS° for the adsorption processes of phenol onto the SCBACs are calculated. The negative values of ΔG° indicated the spontaneous nature of adsorption. The prepared SCBACs are successfully applied to the removal of phenol from different water samples with a recovery % more than 80.0% and a relative standard deviation (RSD) less than 4.0%. Regeneration of the activated carbons can be easily performed using 0.5 M NaOH.
Keywords
Phenol; Sugarcane bagasse; Activated carbons; Adsorption; SEM
Introduction
Organic pollution is the term used when large quantities of organic compounds contaminate the environment. It originates from domestic sewage, urban run-off, industrial effluents and agriculture wastewater, sewage treatment plants and industry including food processing, pulp and paper making, agriculture and aquaculture. During the decomposition process of organic pollutants the dissolved oxygen in the receiving water may be consumed at a greater rate than it can be replenished causing oxygen depletion and having severe consequences for the stream biota.
Wastewater with organic pollutants contains large quantities of suspended solids which reduce the light available to photosynthetic organisms and, on settling out, alter the characteristics of the river bed rendering it an unsuitable habitat for many invertebrates. Organic pollutants include pesticides, fertilizers, hydrocarbons, phenols, plasticizers, biphenyls, detergents, oils, greases, pharmaceuticals, proteins and carbohydrates [1-3].
Phenol is widely used for the commercial production of a wide variety of resins, including phenolic resins, which are used as construction materials for automobiles and appliances, epoxy resins and adhesives, and polyamide for various applications. Phenolic pollutants occur in wastewater of a number of industries such as high temperature coal conversion, petroleum refining, resin and plastics etc. Such aromatic hydroxyl compounds are considered as priority pollutants since they are harmful to organisms especially human and also to aquatic life, at low concentrations, can be toxic when present at elevated levels and are known or suspected to be carcinogens. Phenol decomposition is difficult, principally, due to its stability and its solubility in water. Phenol and substituted phenols have a half-life time between 2 and 72 days. They have a strong and unpleasant smell which makes the contaminated water totally unusable. Phenol finds its way into the environment through industrial effluents and is present in very high amounts in several industrial effluents which make its removal a must. Due to the toxic nature of phenol, several regulatory bodies all over the globe like, WHO, EPA and USEPA have listed phenol and phenolic compounds on the priority pollutants list. The World Health Organization (WHO) has given maximum permissible limit of 0.2 mg/L for chlorophenols. The limit of phenol acceptable in drinking water is 0.002 mg/L. Chronic toxic effects due to phenols reported in humans include vomiting, difficulty in swallowing, anorexia, liver and kidney damage, headache, fainting and other mental disturbances.
Efficient techniques for the removal of highly toxic organic compounds from water have drawn significant interest. A number of methods such as coagulation, filtration with coagulation, precipitation, ozonation, adsorption, ion exchange, reverse osmosis and advanced oxidation processes have been used for the removal of organic pollutants from polluted water and wastewater. These methods have been found to be limited, since they often involve high capital and operational costs [4,5].
Among the possible techniques for water treatments, the adsorption process by solid adsorbents [6-9] shows potential as one of the most efficient methods for the treatment and removal of organic contaminants in wastewater treatment. Commercial activated carbon is very costly. In recent years, the search for low-cost adsorbents that have pollutant –binding capacities has been intensified. Materials locally available [10] such as natural materials, agricultural and industrial wastes can be utilized as low-cost adsorbents. Activated carbon produced from these materials can be used as adsorbent for water and wastewater treatment.
The objectives of the present study are the preparation, characterization and modification of sugarcane bagasse based-activated carbons (SCBACs). Sugarcane bagasse residues have low cost and are considered as waste matter in the environment. Like other agricultural residues, sugarcane bagasse (SCB) is mainly composed of cellulose, hemi-cellulose and lignin [11]. This composition makes sugarcane bagasse a good precursor for the production of new adsorbents for water treatment processes.
In the present study, the sugarcane bagasse residues are modified physically by steam and chemically using NaOH. The modified sugarcane bagasse based activated carbons (SCBACs) are thoroughly investigated for the removal of phenol from aqueous solutions. The different experimental factors affecting the removal of phenol are studied viz. the initial concentration of phenol, the contact time, the dose of activated carbon and the temperature. In addition, the isotherm, kinetic and thermodynamic studies were established to expect the adsorption behaviour.
Experimental
Reagents and solutions
All chemicals and reagents used are of analytical grade and are purchased from Sigma-Aldrich. Phenol stock solution of solution was prepared by dissolving 1 gm in de-ionized water in a 1000 ml measuring flask. Known concentrations of phenol solution were prepared in order to construct the standard calibration curve at λmax 270 nm [12]. The pH of phenol solution was adjusted in the range of 2.0 -12.0 using 0.1 N NaOH and/or 0.1 N HCl.
Apparatus
UV-Vis spectrophotometer (Chrom Tech-Co., Ltd., USA) is used to analyze the phenol supernatant solutions after adsorption process. A pH meter (Hi 931401, HANNA, Portugal) was used to measure the solutions pH. All chemicals that are used in this study were weighed using analytical balance. To study the kinetics and thermodynamic parameters water bath shaker was used.
Preparations of biosorbents
Preparation of carbonized sugarcane bagasse residue (SCBC)
Sugarcane bagasse residues (SCBR), collected from local markets in Mansoura city, Egypt, were dried in sunlight and then cut into small pieces. The dried SCBR were crushed, washed with 0.5% HCl to remove all dirt, dried in an oven at 105°C overnight, ground by a mill, sieved to mesh size of 1-4 mm and carbonized in a tabular furnace at 600°C for 2 h to yield the carbonized sugarcane bagasse residue (SCBC).
Preparation NaOH activated carbon (SCBACN)
The SCBC was soaked in sodium hydroxide solution with the ratio of (1:3 of SCBC: NaOH). Then, the impregnated sample is dehydrated in an oven at 110°C, and activated in muffle furnace at 750°C for about 2 hrs. The obtained NaOH activated carbon (SCBACN) was soaked in de-ionized water several times for half an hour with constant stirring after cooling until pH of filtrate reached to (7-8). This was followed by drying of the SCBACN overnight in an oven at 105°C.
Preparation steam activated carbon (SCBACS)
32 gm of SCBC were placed in the tube of the muffle furnace that was preheated at 750°C under steam flow of 150 ml/min for approximately 36 min. At the end of the process, the activated carbon was allowed to cool, washed with 0.1 HCl to remove the ash and then washed with water to remove the acid residues. The SCBACS samples were dried at 105°C [13].
Characterization of biosorbents
- The BET surface area (SBET) measurements of the carbons were made by nitrogen adsorption at 77K using surface Area & Size Analyzer (QUANTACHROME – NOVA 2000 Series
- The surface micrographs of the carbons were taken by using a scanning electron microscope JSE - T20 (JEOL, Japan).
- Fourier transform infrared spectra (FT-IR) were analyzed with a Nicolet FTIR spectrophotometer using KBr in a wave number range of 4000-500 cm-1.
- Surface acidity and Boehm titration technique were applied for determination the type and amount of functional groups on the carbon surfaces by neutralization with bases of various strengths [13].
Adsorption experiments
Batch sorption experiments were done by shaking a known amount of the activated carbon with 50 ml aqueous solution of phenol in 250 ml-Erlenmeyer flasks placed in a temperature controlled shaking water bath at different concentrations (between 100 and 1600 mg/l), pHs (between 2 and 12), ionic strength (between 0.002 and 0.09 mole/l), temperature (between 17 and 55°C) and sorbent doses (between 0.01 and 0. 2 g) at a constant shaking rate of 125 rpm. The amounts of phenol removed by sorbents (qe) and the removal % (R%) can be calculated using the following equations:
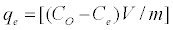 (1)
(1)
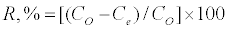 (2)
(2)
Where qe is the amount of dye adsorbed (mg/g). Co and Ce are the initial and equilibrium liquid-phase concentrations of phenol (mg/g), respectively. V is the volume of the solution (l), and m is the weight of the sorbent used (g).
Desorption studies
0.2 gm of each activated carbon was shaken with 100 ml of phenol solution of known concentration (40 mg/l). The adsorbed amount of phenol was detected spectrophotometrically. Then, the phenol loaded SCBAC activated carbons were eluted using distilled water, 0.1% N HCl and 0.5 N NaOH solutions. The desorption % was calculated from the following equation:
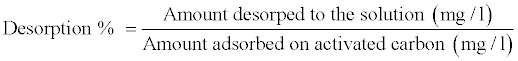 (3)
(3)
Results and Discussion
Characterization of the adsorbents
Textural properties
The BET surface area analysis: The surface area and porosity of carbons are prominent factors in determining their adsorption capacities [14]. The textural properties of solids are conventionally determined from the adsorption of nitrogen at 77 K and the adsorption data are usually analyzed by the application of the BET equation [15].
The adsorption of nitrogen by the carbons investigated was found to be relatively rapid with the equilibrium attained within 25 min indicating that the adsorption is not controlled by activated diffusion encountered in ultrafine pores and meanwhile refers to the accessibility of the entire pore structure to the nitrogen molecules.
The values of surface area of the SCBAC samples were calculated from the linear BET plots of N2 adsorption at 77 K. The results represented in Table 1 depicts:-
| Carbon sample | Moisture content % | Ash content % | pH of supernatant | Point of zero charge pHpzc | Bohem titration (mmole/g) | Surface area (m2/g) | |||
|---|---|---|---|---|---|---|---|---|---|
| Carboxylic | Lactonic | Phenolic | Total basic | ||||||
| SBR | 6.95 | .794 | 3.65 | 4.27 | - | - | - | - | - |
| SBC | 5.9 | 3.91 | 8.35 | 7.62 | - | - | - | - | 282.949 |
| SBACN | 29.62 | 4.67 | 10.46 | 9.86 | 0.038 | 0.944 | 0.314 | 1.6575 | 697.369 |
| SBACS | 13.84 | 7.21 | 10.84 | 10.25 | 0.125 | 1.472 | 0.715 | 3.125 | 347.539 |
SCBR= sugar cane bagasse residues
SCBC= carbonized sugar cane bagasse
SCBACS= steam activated sugar cane bagasse based activated carbon
SCBACN= NaOH activated sugar cane bagasse based activated carbon
Table 1: Physicochemical characters of the biosorbents.
(i) The surface area for non-activated carbon is very low compared to activated carbon due to the absorption of activating agent NaOH or steam.
(ii) The SBET values of SCBACN increased from 282.949 m2/g to 697.369 m2/g. This increase in surface area could be attributed to the change in the textural surface and formation of new pores in the surface.
(iii) The SBET of steam activated carbon increased from 282.949 m2/g to 347.539 m2/g. The increase in surface area may be attributed to the C-H2O reaction leading to removal of some carbon atoms and some of the contaminated tars causing slight increase in surface area and formation of micro pores on carbon surface [16].
Moisture and ash content: Moisture and ash content is a measure of the efficiency of the prepared activated carbon. As shown in Table 1, the moisture and ash content of SCBR (precursor of bagasse) is very low which proves the high efficiency of it in producing activated carbon.
Scanning electron microscopy (SEM): The scanning electron microscopy images gave insight into the olive stone structure with respect to its shape. The dark areas are macro pores and the pale grey areas are due to the carbon matrix. Figure 1 represents the morphology of the resulting activated carbon prepared via physical (SCBACS) and chemical (SCBACN) activation methods, respectively.
It can be clearly seen that physical and chemical activations caused some changes in the surface of the particles after activation. The different pore structures of the activated carbon prepared from either physical (using steam) or chemical (using NaOH) activation are observed. These changes depend upon different reaction mechanisms. Figure 1 depicts pictures of (a) carbonaceous precursor, (b) physically activated carbon (SCBACS) and (c) chemically activated using NaOH (SCBACN). In this concern, the sample (b) activated by steam indicated that the carbon matrix are greater than the dark areas. This is referred to the nature of the micropores resulting from the activating steam. The dark areas are greater than the carbon matrix using NaOH as activator.
Chemical properties
The chemistry of the surface of a carbon is more important than its textural properties in determining its adsorption capacity of pests from aqueous solution particularly when the adsorption involves interaction with the surface functional groups via ion exchange and/or complex formation which is most probably the case in adsorption of pests on activated carbons.
FTIR spectroscopy: The chemistry of the carbon surface is attributed to the existence on the surface of carbon-oxygen functional groups of acid or basic character. The FTIR spectra of SCBR, SCBC, SCBACS and SCBACN (before and after adsorption of phenol), have been recorded (Figure 2).
For all carbon samples, a broad band can be observed in at (3430- 3380) cm-1 region which is attributed to O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials. Also, it can be observed that all samples have IR peaks in the region between (2925- 2852) cm-1 which are a result of asymmetric and symmetric vibrations of methylene group [17]. The analysis of infrared spectra for all carbon samples is shown in Table 2 [12,17-29].
| Sample | IR band n (cm-1) | Band Assignment | Reference |
|---|---|---|---|
| SCBR | 3421.726 | O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 2922.714 | asymmetric and symmetric vibrations of methylene group | [17] | |
| 1630.428 | C=O stretching vibration in quinones | [18] | |
| 1726.53 | esters of acetic, carboxyl groups, p-coumeric, ferulic and uronic acids that are the main constituents of hemi-cellulose | [19] | |
| 2128.153 | C=C stretching vibration | [20] | |
| 1513 | lignin esters that is stable band in all lignin structure | [21] | |
| 1252.11 | C-O stretching vibration of aryl group in lignin | [22] | |
| 1380.84 | in plane bending vibration of (C-H) in methyl group | [17] | |
| 1048 | C-O contribution in glycosidic linkage | [23] | |
| 604-666 | plane deformation vibration of C-H groups located at the edge of aromatic planes | [24] | |
| SCBC | 3424.137 | O-H stretching vibration in alchol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 2919.822 | asymmetric and symmetric vibrations of methylene group | [17] | |
| 1559.713 | C=O stretching vibration | [25] | |
| 1386.144 | in plane bending vibration of (C-H) in methyl group | [17] | |
| 1106.504 | C-O stretching in phenols, alcohols, acids, ethers and esters. | [26] | |
| SCBACS | 3427.994 | O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 2921.268 | asymmetric and symmetric vibrations of methylene group | [17] | |
| 1557.785 | C=O stretching vibration | [25] | |
| 1383.31 | in plane bending vibration of (C-H) in methyl group | [17] | |
| 1098.308 | C-O stretching in phenols, alcohols, acids, ethers and esters | [26] | |
| SCBACS-Ph | 3434.744 | O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 1631.522 | C=O stretching vibration in quiones | [18] | |
| SCBACN | 3427.944 | O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 2919.822-2851.358 | asymmetric and symmetric vibrations of methylene group | [17] | |
| 1578.99 | C=O stretching vibration | [27] | |
| 1384.215 | in plane bending vibration of (C-H) in methyl group | [17] | |
| 1117.11 | R-OH group | [28] | |
| 1034.184 | C-O stretching vibration of ether and alcohol | [29] | |
| SCBACN-Ph | 3430.887 | O-H stretching vibration in alcohol, phenol and adsorbed H2O in cellulosic materials | [12] |
| 1627.694 | C=O stretching vibration in quinones | [18] |
SCBR= sugar cane bagasse residues
SCBC= carbonized sugar cane bagasse
SCBACS= steam activated sugar cane bagasse based activated carbon
SCBACS-Ph= Phenol loaded steam activated sugar cane bagasse based activated carbon
SCBACN= NaOH activated sugar cane bagasse based activated carbon
SCBACS-Ph= Phenol loaded NaOH activated sugar cane bagasse based activated carbon
Table 2: Infrared spectral data of SCB, SCBC, SCBACS and SCBACN.
The surface functionality of SCBACS and SCBACN (before and after adsorption of phenol) can be characterized by FTIR spectra (Figure 2b and 2c), respectively. The difference in peaks of both spectra is attributed to the adsorption of phenol onto SCBAC activated carbons. The FTIR spectra of SCBAC after adsorption of phenol show the following changes:
(i) For SCBACS-Ph : the appearance of a peak at 1631 cm-1 due to N-H bend denoting the presence of primary amines and appearance of the peak at 919 cm-1 due to C-O stretching due to presence of alcohols, carboxylic acids , esters and ethers, and the disappearance of the peaks at 1557,1383,1098 and 879 cm-1.
(ii) For SCBACN-Ph: The disappearance of the peaks at 1578 cm-1 and peaks at 1384 cm-1. This indicates the absence of –C≡C– stretch of alkyne groups and absence of C-C stretch (in- ring) of aromatic rings, respectively. The appearance of peaks at 1627 cm-1 due to N-H bending denoting the presence of primary amines.
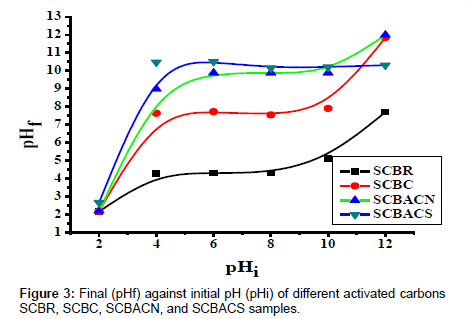
Surface acidity and Boehm titration: The pH of the aqueous slurry of the carbon material provides a convenient indicator of the type and concentration of the chemical parameters of the ACs investigated. Table 1 reveals that:-
- (i) the surface pH of carbon SCBC is (8.35) indicating its surface basicity, i.e the basic functional groups on the surface of non-oxidized carbon are more dominating compared with those of the acid type.
- (ii) The same is also true for steam activated ACPNS of surface pH= 8.2 and 10.84. Steam activation at 950°C usually leads to the formation on the carbon surface of C-O groups of basic character [29].
- (iii) Treatment with NaOH increased the surface pH from 8.35 to 10.48.
The pH of zero point charge pHpzc (Table 1 and Figure 3) is 10.84 and 10.46 for SCBACS and SCBACN, respectively.
The surface acidic groups could be determined in (m.eq/g) by the selective neutralization with a series of bases of varying strength, viz. NaHCO3, Na2CO3, NaOH and NaOC2H5. NaHCO3 neutralizes carboxylic groups where as those neutralized by Na2CO3 but not by NaHCO3 are lactones. The weak acid groups neutralized by NaOH but not by Na2CO3 were postulated as phenols. The groups reacting with NaOC2H5, but not with NaOH, were suggested to be carbonyl groups [23].
Table 1 shows the surface acidity and the abundance of different acidic groups on the surface of non-activated and activated carbon samples. Inspection of the data in Table 1 reveals (i) the total surface of basic sites are more than the acidic sites which confirm the basic nature of their surfaces. This finding totally agreed with the results of pHpzc and pHsup. (ii) The order of the acidic groups on the surface of all samples is
Lactones >> phenolic > carboxylic
Adsorption of phenol
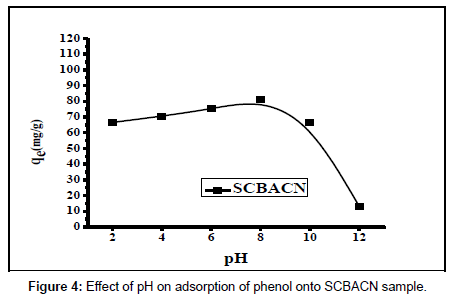
Effect of pH on the adsorption of phenol: The solution pH is one of the most important factors that can affect the adsorption process of phenol in aqueous solution. Figure 4 shows the effect of pH on phenol adsorptiononto SCBACN. It can be noticed that phenol uptake increases with increase of pH from pH 2 till it reaches its maximum at pH 7.5-8 and then sharply decreases at higher pH from 8.5-12.0.
The lower phenol removal at acidic (pH<2) is probably due to the presence of excess H+ ions com¬peting with phenol molecules for the sorption sites of sorbents. Furthermore, at lower pH values (below the pKa of carboxylic groups, approximate 4.6), the -COO- groups in SCBACN are protonated to -COOH groups and the hydrogen bonds between -COOH and –NH2 groups are formed resulting in a decrease of phenol uptake. On the other hand, at higher pH (>8), the OH- ions concentration increase and these ions repulse with the negative active sites on the sorbent adsorbents leading to decrease in phenol adsorption and hence its uptake [19]. Also, at higher pH, phenol dissociates to phenolate anions that form negative charges in adsorbate solution resulting in high repulsion forces between adsorbate and adsorbent which decrease the phenol uptake and hence its removal from aqueous solution [21].
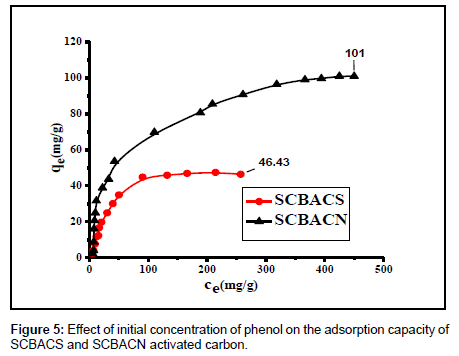
The effect of initial phenol concentration: The effect of initial concentration on the removal of phenol by SCBAC samples was studied at pH 7.0. The results are graphed in Figure 5. It is seen that the equilibrium sorption capacities of the two sorbents increase with an increase in initial phenol concentration and the adsorption is rapid in the initial stages and gradually increases with the progress of adsorption until the equilibrium is reached. This may be attributed to the fact that, the higher the initial phenol concentration, the greater the driving force of the concentration gradient at solid-liquid interface which cause an increase of the amount of phenol adsorbed on the adsorbent [30].
At higher phenol concentrations adsorption capacity reached a plateau indicating saturation of the available binding sites on the adsorbent. The steep slope at initial phenol concentrations is a desirable feature of the sorption system and the results indicates that the SCBACs are efficient adsorbents for phenol.
Effect of adsorbent dosage: An experiment was made to study the effect of adsorbent dosage on phenol adsorption. Various quantities of SCBACS as selected sample were added to a fixed initial phenol concentration. The uptake of phenol after a contact time of 24 h increased from 15 to 85% when SCBACS dose was increased from 1.0 to 4.0 g/L, respectively. This is due to the fact that as the adsorbent dosage is increased, more adsorption sites are available for adsorbate enhancing the phenol uptake. Also, with increasing adsorbent loading, the quantity of phenol adsorbed on to the unit weight of the adsorbent is reduced causing a decrease in qe (mg/g) values with increasing activated carbon loading.
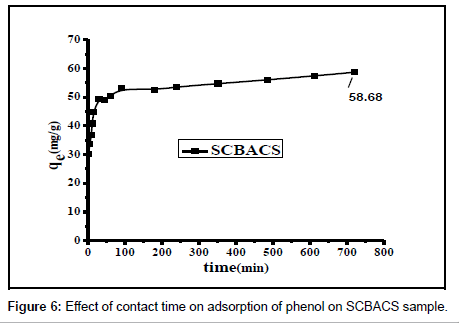
Effect of contact time: Figure 6 shows the effect of contact time on the adsorptioncapacity of SCBACS, as an example, for phenol. The adsorption capacity of SCBACS increased rapidly with the increase of contact time from 0 to 20 min and more than 90% of the equilib¬rium adsorption capacity for phenol occurred within 15 min. After 100 min, the adsorption capacity became constant and the adsorp¬tion reached equilibrium. Therefore, 100 min was selected as the contact time for the adsorption of phenol onto the SCBAC under our experimental conditions.
As shown, the adsorp¬tion process was divided into three stages: (1) an initial stage with adsorption occurring instantly, (2) subsequently, slow adsorption, and (3) a final stage with adsorption reaching equilibrium and remaining constant. The first stage can be attributed to the rapid attachment of phenol to the surface of the SCBAC by surface mass trans¬fer. At this stage, more than 80% of phenol adsorption was found in all cases. The second stage was slower, possibly because many of the available external sites was already occupied and because of the slow diffusion of phenol molecules into the network of the SCBAC. An asymptotic trend was found after approximately 20 min regardless of the initial phenol concentration applied to the adsorption system. The amount adsorbed did not vary significantly at contact times longer than the equilibrium time (100 min). The kinetics of the adsorption process indicates that the adsorptionof phenol onto SCBAC can be considered as a fast adsorption process because more than 80% of phenol was adsorbed within 20 min especially at phenol concentration lower than the maximum adsorbed. Such findings reveal the ben¬efits of using this low-cost adsorbent or so-called eco-adsorbent for the treatment of aqueous solutions rich in pesticides in general and phenol in particular.
Effect of interferents: Phenol rich wastewaters usually contain ions such as sulphide, phosphate, sulphate and chloride [31]. The effect of these ions on the adsorption of phenol onto SCBACs was investigated and the results are presented in Table 3, which showed that the adsorption percentage of phenol from solution was 94.6 and 96.6 for SCBACS and SCBACN respectively in the absence of any of the ions studied. The adsorption percentage decreases as the concentration of interferents increases in all cases. This already shows that the presence of chloride, phosphate, sulphate and sulphide ions interfere in the removal of phenol from solutions.
| Interferent | Concentration, mmol/l | adsorption | |
|---|---|---|---|
| SCBACN | SCBACS | ||
| Chloride | 0 | 95.8 | 94.5 |
| 0.5 | 94.8 | 92.5 | |
| 1.0 | 93.2 | 91.0 | |
| 1.5 | 92.6 | 90.8 | |
| 2.0 | 90.3 | 88.4 | |
| phosphate | 0 | 95.8 | 94.5 |
| 0.5 | 91.8 | 92.8 | |
| 1.0 | 90.0 | 90.2 | |
| 1.5 | 86.6 | 85.6 | |
| 2.0 | 83.3 | 82.3 | |
| Sulphate | 0.0 | 95.8 | 94.5 |
| 0.5 | 93.2 | 93.2 | |
| 1.0 | 92.6 | 91.6 | |
| 1.5 | 90.0 | 90.3 | |
| 2.0 | 87.6 | 85.5 | |
| Hydrogen sulphide | 0 | 95.8 | 94.5 |
| 0.5 | 93.0 | 92.2 | |
| 1.0 | 92.1 | 90.6 | |
| 1.5 | 90.2 | 89.3 | |
| 2.0 | 86.6 | 83.5 | |
SCBACS= steam activated sugar cane bagasse based activated carbon
SCBACN= NaOH activated sugar cane bagasse based activated carbon
Table 3: Effect of interferents on the adsorption % of phenol.
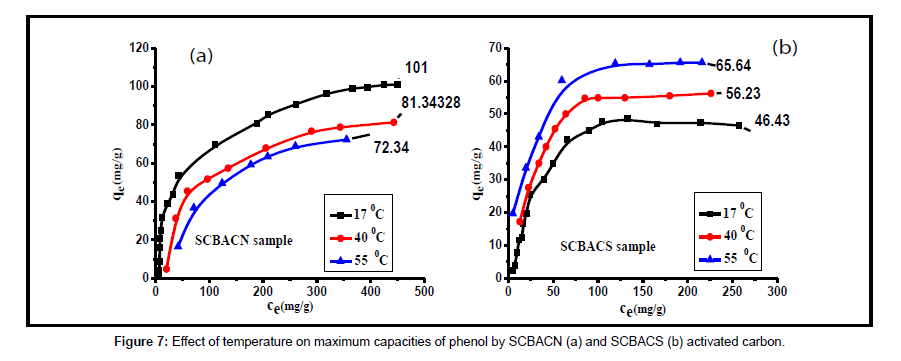
Effect of temperature: Table 4 shows the relationship between the temperature and the adsorption capacity of phenol by the SCBACs. For SCBACS, the adsorprtion capacity increased with increasing the temperature from 17 to 55°C, as shown in Figure 7. This can be attributed to the increase in the rate of diffusion of phenol molecules across the external boundary layer and in the internal pores in the SCBACS with the increase in temperature leading to an in increase in the amount of phenol uptake. When the temperature increased from 17°C to 55°C the maximum amounts of phenol removed by SCBACS increased from 46.43 to 65.64 mg/g. For SCBACN, on the other hand, the adsorp¬tion capacity decreased with increasing of the temperature from 17 to 55°C. When the temperature increased from 17°C to 55°C the maximum amounts of phenol removed by SCBACN decreased from 101.0 to 72.34 mg/g, as shown in Figure 7.
| Carbon sample | Qe(mg/g) | ||
|---|---|---|---|
| 17°C | 40°C | 55°C | |
| SCBACN | 101 | 81.34 | 72.34 |
| SCBACS | 46.43 | 56.23 | 65.64 |
SCBACS= steam activated sugar cane bagasse based activated carbon
SCBACN= NaOH activated sugar cane bagasse based activated carbon
Table 4: Effect of temperature on adsorption capacities of phenol by SCBACN and SCBACS activated carbon.
Adsorption isotherms
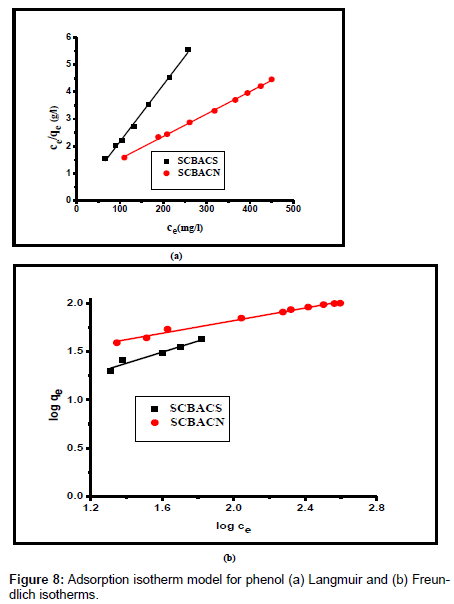
In equilibrium analysis, Langmuir [13] and Freundlich [32] models are the most frequently employed isotherm models for studying the behavior and system of adsorption.
Langmuir isotherm [13] can be expressed in its linear formula as:
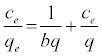 (4)
(4)
Where Ce is the concentration of phenol at equilibrium (mg/l), qe the amount of the phenol adsorbed per unit weight of the adsorbent for monolayer capacity (mg/g) and b is the Langmuir equilibrium constant (l/mg). Both qe and b can be determined from a linear plot of Ce/qe versus Ce, as shown in Figure 8a.
The favorable nature of adsorption can be evaluated by determination of the dimensionless separation factor of equilibrium parameter RL which is expressed as:
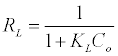 (5)
(5)
Where Co in this case is the highest initial solute concentration and KL is Langmuir constant. The values of RL indicates the type of isotherm to be irreversible where (RL=0), favorable (0< RL<1), linear (RL=1) or unfavorable (Rl>1) [14].
The Freundlich isotherm is often used for heterogeneous surface energy systems [14]. Its linear formula can be expressed as:
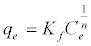 (6)
(6)
Where KF is the Freundlich constant which is a comparative measure of the adsorption capacity of the adsorbent, and n is an empirical constant which gives valuable information about the isotherm shape. 1/n values indicate the type of isotherm to be irreversible (1/n = 0), favorable (0< 1/n< 1) and unfavorable (1/n> 1).
The Freundlich parameters can be obtained from the following linearized equation:
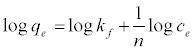 (7)
(7)
By linear plotting of log qe as the function of log Ce, the values of Kf and n can be obtained from the slope and the intercept of the plot (Figure 8b).
The model parameters obtained by applying Langmuir and Freundlich models to the experimental data are given in Table 5. Inspection of Table 3 reveals: (i) the regression coefficients R2 obtained from Langmuir model is closer to 1 than that of the Freundlich model, suggesting that the Langmuir isotherm fits better with the adsorption of phenol on SCBACs; (ii) the RL values obtained are in all cases lie between 0 and 1 confirming that the adsorption is a favorable process. Hence, it can be concluded that the monolayer Langmuir adsorption isotherm is more suitable to explain the adsorption of phenol onto SCBACs; (iii) the values of 1/n are less than 1 indicating that adsorption of phenol on the surface of activated carbons is a favorable process.
| Carbon samples | SCBACN | SCBACS | |
|---|---|---|---|
| Langmuir | qm (mg/g) | 121.36 0.01153 0.136 0.99839 |
47.92 0.045 0.0526 0.99604 |
| b (l\mg) | |||
| RL | |||
| R2 | |||
| Freundlich | K | 14.65 0.32634 0.98871 |
3.76 0.57316 0.93986 |
| 1/n | |||
| R2 |
Table 5: Langmuir and Freundlich isotherm parameters.
Adsorption kinetics
The pseudo-first order and the pseudo-second order kinetic models
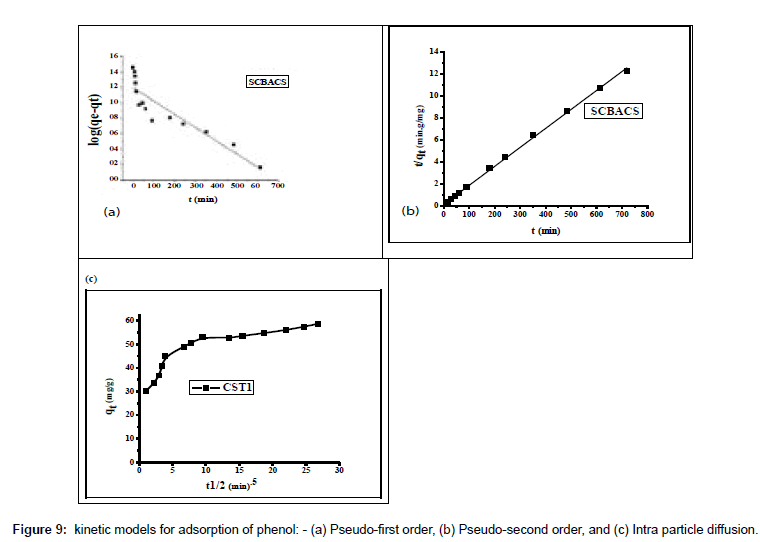
In order to investigate the controlling mechanism of adsorption process of phenol onto SCBACs, the pseudo-first order and the pseudosecond order kinetic models were cited to evaluate the experimental data obtained from batch phenol removal experiments. The linear form of pseudo-first order kinetic model can be formulated as:
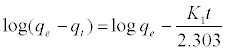 (8)
(8)
Where qe is the adsorption uptake of phenol at time t (mol g-1) and k1 (min-1) is the rate constant of the pseudo-first-order adsorption. K1 and qe were determined from the slope and intercept of linear plot of log (qe-qt) versus t as shown in Figure 9a.
The kinetic data were further analyzed using Ho’s pseudo-sec¬ondorder kinetic model [33]. It can be expressed as
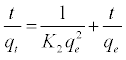 (9)
(9)
Where k2 (g mol-1 min-1) is the rate constant of pseudo-secondor ¬der adsorption. Both K2 and qe can be obtained from the linear plot of t/qt against t as shown in (Figure 9b). The parameters of the pseudo-first and pseudo-second order kinetic models are represented in Table 6. From Table 6, it can be noticed that: (i) the correlation coeffi¬cient (R12) is very low (0.81102) for the pseudo-first order kinetic model and there is large difference between the experimental qe values (qe,exp) and the calculated qe values (qe,cal). These findings indicated that the pseudofirst order kinetic model was poor fit for the adsorption processes of phenol onto SCBACs. (ii) the R2 for the pseudo-second order kinetic model is 0.99883 and the qe,cal value for the pseudo-second order kinetic model is consistent with the qe,exp value. These findings suggested that the adsorption processes of phenol onto SCBACs can be well described by the pseudo-second order kinetic model.
| Carbon sample | SCBACS | |
|---|---|---|
| qe exp(mg/g) | 58.68 | |
| First-order kinetic equation. | q1 (mg/g) | 15.59 |
| k1 (1/min) | 0.00393 | |
| R12 | 0.81102 | |
| Second-order kinetic equation. | q2 (mg/g) | 58.0046 |
| k2 [g/(mg min)] | 2.074x10-3 | |
| R22 | 0.99883 | |
| Intra-particle diffusion equation. | kint [mg/(g min1/2)] | 1.45194 |
| C | 38.96171 | |
| Rint2 | 0.98501 |
Table 6: Kinetic parameters for the adsorption of phenol onto SCBACS.
Intra-particle diffusion model
The intra-particle diffusion parameter, Kp (mmol.g-1 h-0.5) is defined by equation
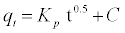 (10)
(10)
Where kp is the intra-particle diffusion rate constant (mmol.g-1 h-0.5) and C is a constant. According to this model qt versus t0.5 should be linear if intra-particle diffusion is involved in the adsorption process [34]. From Equation. (10), if pore diffusion is the rate limiting step, then a plot of qt against t0.5 must give a straight line with a slope that equals kp and the intercept value C represents the resistance to mass transfer in the external liquid film.
The results obtained indicated that the plots of qt against t0.5 of the adsorption processes of phenol onto SCBACS are multilinear (Figure 9c) containing at least three linear segments which indicate that three steps occur during adsorption process. The first sharper portion is transport of phenol molecules from the bulk solution to the adsorbent external surface by diffusion through the boundary layer (film diffusion). The second portion is the diffusion of phenol molecules from the external surface into the pores of the adsorbent. The third portion is the final equilibrium stage, where the phenol molecules were adsorbed on the active sites on the internal surface of the pores and the intra-particle diffusion starts to slow down due to the solute concentration getting lower and lower in solution [35]. Also, the linear portions of curves did not pass through the origin, suggesting that pore diffusion is not the step controlling the overall rate of mass transfer at beginning of adsorption. In Table 6, the correlation coefficients (R2) for the linear segment intra-particle diffusion model is 0.9850, indicating that the intra-particle diffusion was not the only rate controlling step; other process could control the rate of adsorption.
| Carbon samples | SCBACN | SCBACS | ||||
|---|---|---|---|---|---|---|
| T (k) | 290.15 | 313.15 | 328.15 | 290.15 | 313.15 | 328.15 |
| ΔG° (KJ/mol) | -18.76 | -20.11 | -20.98 | -22.054 | -24.305 | -25.71 |
| ΔH° (KJ/mol) | -1.796 | 5.928 | ||||
| ΔS° (J/mol/k) | 58.482 | 96.47 | ||||
Table 7: Thermodynamic parameters for the adsorption of phenol on SCBACN and SCBACS.
Thermodynamic studies
The adsorption mechanism can be determined through estimation of thermodynamic parameters such as change in free energy (ΔG°), change in enthalpy (ΔH°) and change in entropy (ΔS°). Change in the Gibbs free energy (ΔG°) indicates the degree of the spontaneity. It can be calculated from the following equation:
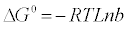 (11)
(11)
Where T is the temperature in (K), R is the universal gas constant (8.314 × 10−3 kJ/mol K) and b is Langmuir constants (l/mole). Change in enthalpy (ΔH°) and change in entropy (ΔS°) can be estimated from Van’t Hoff equation [31]:
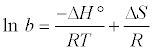 (12)
(12)
ΔH° and ΔS° can be determined from linear plot of ln b versus 1/T from slope and intercept, respectively. Where the slope and intercept of Van’t Hoff plot, Figure 10, are equal to 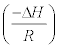 and
and 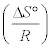 respectively.
respectively.
The values of ΔG°, ΔH° and ΔS° are listed in Table 7. The Gibbs free energy change (ΔG°) was negative as expected for a spontaneous process under the applied conditions. For SCBACS sorbents, the positive value of ΔH° suggested the endothermic nature of the adsorption process. This finding indicates that the sorption capacity would increase because of inter molecular pore diffusion [36].
| Eluent | Desorption % | |
|---|---|---|
| SCBACS | SCBACN | |
| Distilled water | 5.00 | 4.57 |
| HCl, 0.1 M | 10.5 | 9.74 |
| NaOH, 0.5 M | 91.98 | 84.77 |
Table 8: Desorption % of phenol from spent SCBACs.
The negative value of ΔH°, on the other hand, suggested the exothermic nature of the adsorption process oh phenol onto SCBACN sorbents. At high temperature kinetic energy of adsorbate phenol is so high that they do not bind with the active sites available on the SCBACN surface [37]. Moreover, the positive values of ΔS° point out the increased randomness at the solid/liquid interface during the sorption of phenol onto SCBACs [38].
Desorption studies
Desorption studies help recycling the adsorbent and recovery of adsorbate. To remove the adsorbed phenol the spent adsorbent was treated with distilled H2O, 0.5 M NaOH and 0.1 M HCl, equilibrated for 8 hr and filtered. After filtration, the filtrate was removed, diluted as required and analyzed spectrophotometrically for phenol. Table 8 shows the elution profiles of phenol. The desorption percentage of phenol followed the sequence as:
| Sample | Phenol added, ppm | SCBACS | SCBACN | ||
|---|---|---|---|---|---|
| R,% | RSD, % | R,% | RSD,% | ||
| Bi-distilled water (Our lap) |
15 30 45 |
83.96 81.05 80.035 |
0.2841 0.2165 0.6489 |
86.622 83.179 83.107 |
3.887 0.5608 0.294 |
| Tap water (Mansoura city) |
30 35 40 |
105.73 101.078 100.98 |
1.880 0.026 0.347 |
101.07 100.9 98.89 |
0.5427 0.5239 0.6311 |
| Sea water (Alexandria) | 30 35 40 |
99.52 98.75 97.045 |
0.480 | 100.396 98.950 95.045 |
0.1346 0.4900 0.2129 |
| 0.162 | |||||
| 0.154 | |||||
R, % = Removal of phenol
RSD, % = Relative standard deviation
Table 9: Recovery (R, %) of phenol from dif ferent water samples using SCBACS and SCBACN activated carbons (n=3).
0.5 M NaOH>0.M HCl>distilled H2O.
This observed trend implies that by converting phenol molecules into phenolate anions with NaOH, the adsorbed phenol would be easily desorbed. In conclusion, sodium hydroxide should be a more suitable reagent for elution than acid or simple water.
Analytical applications
The applicability of the SCBACs for removal of phenol from different water samples was investigated by spiking known amounts of phenol. The recovery % is more than 80.0 % with a relative standard deviation (RSD, %,) less than 4% as shown in Table 9.
The uptake of phenol using such SCBACs activated carbons is very comparable to the commercial adsorbents previously used for removal of phenols [39-46] as shown in Table 10. The prepared low cost sugar cane bagasse-based activated carbon is advantageous to the high cost commercial activated carbon.
| Ref. | Adsorption Maximum, | Adsorbent | Organic Pollutants |
|---|---|---|---|
| Present study | 46.43 mg/g | Sugarcane bagasse-steam activated carbon | phenol |
| Present study | 101 mg/g | Sugarcane bagasse-sodium hydroxide activated carbon | phenol |
| [39] | 14.5 mg/g | Porous Clay | Phenol |
| [39] | 45.5 mg/g | Porous Clay | 2,5-dichlorophenol |
| [39] | 48.7 mg/g | Porous Clay | 3,4-dichlorophenol |
| [40] | 97.2 mg/g | (cetyl-pyridinium-Al PILC) | 3,5-dichlorophenol |
| [41] | 370 ppm | Hemidesmus Indicus Carbon(HIC) | phenol |
| [41] | 294 ppm | Commercial Activated Carbon(CAC) | phenol |
| [42] | 74.07 mg/g | NORIT Granular Activated Carbon (NAC 1240) | phenol |
| [42] | 166.6 mg/g | NORIT Granular Activated Carbon 1010 | phenol |
| [43] | 257 mg/g | Active carbon | phenol |
| [43] | 347 mg/g | Mesoporous carbon CMK-3-100°C | phenol |
| [43] | 428 mg/g | Mesoporous carbon CMK-3-130°C | phenol |
| [44] | 473 mg/g | Mesoporous carbon CMK-3-150°C | phenol |
| [43] | 76% | Leaf litter of Shorea Robusta | phenol |
| [45] | 83.34 mg/g | activated phosphate rock (1M HNO3) | phenol |
| [39] | 15 mg/g | Natural clay | phenol |
| [46] | 232.56 mg/g | activated carbon derived from oil palm empty fruit bunch (EFB) | 2,4-dichlorophenol |
Table 10: Adsorption capacities of different adsorbents previously reported for the removal of phenols.
Conclusion
The search for low-cost adsorbents that have pollutant-binding capacities is extremely meaningful for efficient water treatment. The results of the present study reveal that Sugar Cane Bagasse based Activated Carbons (SCBACs) may be extremely viable adsorbents for application in the removal of phenol from aqueous solutions. Modification of SCBACs by steam and NaOH significantly increased the oxygen-containing groups on the surface of activated carbons with noticeable change in the surface morphology and textural properties. The adsorption of phenol was dependent on initial concentration, reaction temperature and pH. The phenol adsorption capacity increased with the increase of pH in the range of 2-8, where ionization of carboxylic group occurs. The adsorption of phenol onto the activated carbons reached equilibrium within about 60 min. The adsorption equilibrium could be well described by Langmuir adsorption isotherms, namely monolayer adsorption on a homogenous surface. The adsorption kinetics followed a pseudo-second order kinetic model and intra-particle diffusion was involved in the adsorption process. Thermodynamic results indicated that The Gibbs free energy change (ΔG°) was negative as expected for a spontaneous process under the applied conditions. The prepared SCBACs were successfully applied to the removal of phenol from natural water samples. Desorption of phenol from SCBACs could easily be carried out using 0.5 M NaOH. Finally, the present work demonstrated well the potential of this technique for more wide applications.
References
- Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manage 113: 170-183.
- Imran Ali, Hassan Y Aboul-Enein (2004) Pollutants: Distribution, Toxicity and Analysis by Chromatography and Capillary Electrophoresis. John Wiley & Sons, Chichester, USA.
- Damià B (2005) Emerging Organic Pollutants in Waste Waters and Sludge. Springer, Berlin.
- Ravindran V, Stevens MR, Badriyha BN, Pirbazari M (1999) Modeling the sorption of toxic metals on chelant-impregnated adsorbent. AIChE journal 45: 1135-1146.
- Toles CA, Marshall WE (2002) Copper ion removal by almond shell carbons and commercial carbons: batch and column studies. Separation science and technology 37: 2369-2383.
- Nakagawa K, Namba A, Mukai SR, Tamon H, Ariyadejwanich P, et al. (2004) Adsorption of phenol and reactive dye from aqueous solution on activated carbons derived from solid wastes. Water Res 38: 1791-1798.
- Førland GM, Blokhus AM (2007) Adsorption of phenol and benzyl alcohol onto surfactant modified silica. J Colloid Interface Sci 310: 431-435.
- Lazo-Cannata JC, Nieto-Márquez A, Jacoby A, Paredes-Doig AL, Romero A, et al. (2011) Adsorption of phenol and nitrophenols by carbon nanospheres: Effect of pH and ionic strength. Separation and Purification Technology 80: 217-224.
- Navarro AE, Cuizano NA, Lazo JC, Sun-Kou MR, Llanos BP (2009) Comparative study of the removal of phenolic compounds by biological and non-biological adsorbents. J Hazard Mater 164: 1439-1446.
- Kennedy LJ, Vijaya JJ, Sekaran G (2004) Effect of two-stage process on the preparation and characterization of porous carbon composite from rice husk by phosphoric acid activation. Industrial & engineering chemistry research 43: 1832-1838.
- Walford S (2008) Sugarcane bagasse: how easy is it to measure its constituents? in Proceedings of the 81st. Annual Congress of the South African Sugar Technologists' Association, Durban, South Africa, 29-31 July 2008, South African Sugar Technologists' Association.
- Molva M (2004) Removal of phenol from industrial wastewaters using lignitic coals. Izmir Institute of Technology, Turkey.
- Akl MA, Yousef AM, Abd Elnasser S (2013) Removal of Iron and Manganese in Water Samples Using Activated Carbon Derived from Local Agro-Residues. J Chem Eng Process Tech 4: 154.
- Akl MA, Atta A, Youssef AM, Ibraheim MA (2013) The Utility of Novel Superabsorbent Core Shell Magnetic Nanocomposites for Efficient Removal of Basic Dyes from Aqueous Solutions. J Chrom Separation Tech 4: 185.
- Salame II, Bagreev A, Bandosz TJ (1999) Revisiting the effect of surface chemistry on adsorption of water on activated carbons. J Phy Chem B 103: 3877-3884.
- Tsai WT, Chang CY, Wang SY, Chang CF, Chien SF, et al. (2001) Utilization of agricultural waste corn cob for the preparation of carbon adsorbent. J Environ Sci Health B 36: 677-686.
- Gerçel Ö, Özcan A, Özcan AS, Ferdi GH (2007) Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Applied surface science 253: 4843-4852.
- Macías-García A, Díaz-Díez MA, Cuerda-Correa EM, Olivares-Marín M, Gañan-Gómez J (2006) Study of the pore size distribution and fractal dimension of HNO3-treated activated carbons. Applied surface science 252: 5972-5975.
- Sain M, Panthapulakkal S (2006) Bioprocess preparation of wheat straw fibers and their characterization. Industrial Crops and Products 23: 1-8.
- Stuart BH (2004) Infrared spectroscopy: fundamentals and applications. Wiley.com.
- Southichak B, Nakano K, Nomura M, Chiba N, Nishimura O (2006) Phragmites australis: a novel biosorbent for the removal of heavy metals from aqueous solution. Water Res 40: 2295-2302.
- Mandal A, Chakrabarty D (2011) Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydrate Polymers 86: 1291-1299.
- Ren JL, Sun RC, Liu CF, Lin L, He BH (2007) Synthesis and characterization of novel cationic SCB hemicelluloses with a low degree of substitution. Carbohydrate polymers 67: 347-357.
- Neel PI, Viswanathan B, Varadarajan T (2009) Methods of Activation and Specific Applications of Carbon Materials. India.
- Marsh H, Reinoso FR (2006) Activated carbon. Elsevier.
- Panumati S, Chudecha K, Vankhaew P, Choolert V, Chuenchom L, et al. (2008) Adsorption of phenol from diluted aqueous solutions by activated carbons obtained from bagasse, oil palm shell and pericarp of rubber fruit. Songklanakarin J Sci Technol 30: 185-189.
- Tsai WT, Chang CY, Lin MC, Chien SF, Sun HF, et al. (2001) Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere 45: 51-58.
- Yang T, Lua AC (2006) Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Materials chemistry and physics 100: 438-444.
- Martín-Lara MÁ, Rico ILR, Vicente ICA, García GB, Martín-Lara MC (2010) Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination 256: 58-63.
- Ho YS, Chiang TH, Hsueh YM (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40: 119.
- Sactry CA, Hashim MA, Agamuthu P (1995) Waste water Treatment Plants (Narosa Publishing House, New Delhi, 1995).
- Akl MA, Youssef AM, Al-Awadhi MM (2013) Adsorption of Acid Dyes onto Bentonite and Surfactant-modified Bentonite. J Anal Bioanal Tech 4: 174.
- Ho Y, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Transactions of the Institution of Chemical Engineers, part B 76: 332-340.
- Kiran I, Akar T, Ozcan AS, Ozcan A, Tunali S (2006) Biosorption kinetics and isotherm studies of Acid Red 57 by dried Cephalosporium aphidicola cells from aqueous solutions. Biochem Eng J 31: 197.
- Sun Q, Yang L (2003) The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res 37: 1535-1544.
- Duong D (1998) Pure component adsorption in microporous solids. Adsorption Analysis: Equilibria and Kinetics, Series on Chemical Engineering, Imperial College Press, London.
- QIU H, LV L, Bing-cai PAN, ZHANG Q, ZHANG W, et al. (2009) Critical review in adsorption kinetic models. Journal of Zhejiang University SCIENCE A 10: 716-724.
- Weber WJ (1972) Physicochemical processes for water quality control. Wiley Interscience.
- Rashed MN (2013) Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater.
- Matthes W, Kahr G (2000) Sorption of organic compounds by Al and Zr-hydroxy-intercalated and pillared bentonite. Clays and clay minerals 48: 593-602.
- Srihari V, Das A (2009) Adsorption of phenol from aqueous media by an agro-waste (Hemidesmus indicus) based activated carbon. Appl Ecol Environ Res 7: 13-23.
- Maarof HI, Hameed BH, Ahmad AL (2004) Adsorption isotherms for phenol onto activated carbon. ASEAN J Chem Eng 4: 70-76.
- Haque E, Khan NA, Talapaneni SN, Vinu A, JeGal J, et al. (2010) Adsorption of phenol on mesoporous carbon CMK-3: effect of textural properties. Bull. Korean Chem Soc 31: 1638-1642.
- Mishra S, Bhattacharya J (2006) Potential of leaf litter for phenol adsorption-A kinetic study. Indian J Chem Technol 13: 298.
- Alzaydien, AS, Manasreh W (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto activated phosphate rock. Int J Phys Sci 4: 172-181.
- Shaarani, F, Hameed B (2011) Ammonia-modified activated carbon for the adsorption of 2, 4-dichlorophenol. Chemical Engineering Journal 169: 180-185.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18850
- [From(publication date):
May-2014 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 13647
- PDF downloads : 5203