Review Article Open Access
Enzymatic Glucose Biofuel Cell and its Application
| Gymama Slaughter* and Tanmay Kulkarni | |
| Department of Computer Science and Electrical Engineering, University of Maryland Baltimore County, USA | |
| Corresponding Author : | Gymama Slaughter Bioelectronics Laboratory Department of Computer Science and Electrical Engineering University of Maryland Baltimore County Baltimore, MD 21250, USA Tel: +1 410 455 8483 Fax: +1 410 455 3969 E-mail: gslaught@umbc.edu |
| Received: July 02, 2015; Accepted: July 14, 2015; Published: July 16, 2015 | |
| Citation: Slaughter G, Kulkarni T (2015) Enzymatic Glucose Biofuel Cell and its Application. J Biochip Tissue Chip 5:110. doi:10.4172/2153-0777.1000110 | |
| Copyright: © 2015 Slaughter and Kulkarni. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
Biofuel cells have received significant attention in the last few decades due to its potential application as alternative energy sources and advantages over conventional fuel cells. This review summarizes different types of glucose biofuel cells with emphasis on enzymatic glucose biofuel cells. Unlike conventional fuel cells, which use fuel such as ethanol, methanol, formic acid, etc. to generate electricity, enzymatic glucose biofuel cells convert chemical energy stored in glucose into electricity. Energy generating from complex sugar is now possible due to the most common glucose selective enzymes, Glucose Oxidase (GOx) and Pyroquinoline Quinone Glucose Dehydrogenase (PQQ-GDH). Glucose as a fuel source is cost-efficient because it is readily abundant and offers a clean source of power. The micro-power generated from the selective glucose/ O2 redox reactions can be used to power ultra-low powered bioelectronic devices. In addition, in vivo glucose biofuel cell implantations and potential applications are highlighted.
| Keywords |
| Enzymatic biofuel cells; Glucose oxidase; Laccase; Pyroquinoline quinone glucose dehydrogenase; Bioelectricity |
| Introduction: Electric Energy Generation |
| The idea of energy conversion from organic fuel sources other than fossil fuels comes from the very basic reaction of photosynthesis. In this environmental friendly energy conversion reaction, the light energy is converted into chemical energy by plants and microorganisms [1]. This stored chemical energy can then be further utilized as a fuel source for other purposes. The fundamental sugar producing reaction in photosynthesis involves the utilization of atmospheric carbon dioxide and light dependent products, Adenosine Triphosphate (ATP) and Nicotinamide Adenine Dinucleotide Phosphate (NADPH), to produce sugar and release oxygen back into the environment [2,3]. |
| The large amount of glucose produced by the above reaction can serve as a fuel in glucose biofuel cells. Furthermore, one molecule of glucose upon complete oxidation to CO2 during the aerobic metabolic pathway, generates 24 electrons to produce electrical energy. |
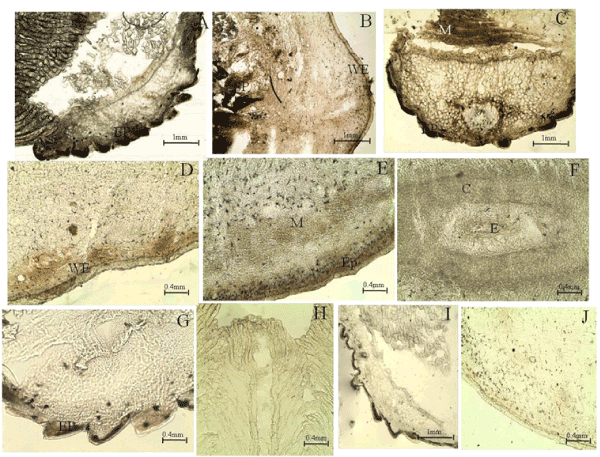
| Following the invention of the gas batteries by William Grove [4], Leonard Neidrach and Thomas Grubb from General Electric, introduced the first fuel cell for NASA in 1959 [5]. This simple and efficient fuel cell is the hydrogen fuel cell, which uses hydrogen gas as the fuel (Figure 1). This fuel cell consist of platinum electrocatalyst, which was used to oxidize hydrogen and reduce oxygen. Hydrogen is the simplest element and abundant in nature. In a hydrogen fuel cell, hydrogen is oxidized by the platinum electrocatalyst to produce protons and electrons which travel through the electrolyte and the external circuit respectively. The flow of electrons results in the generation of electricity. At the cathode, the electrons recombine with oxygen in the presence of protons, thereby resulting in the reduction of oxygen to produce water as the only by-product. Although platinum electrocatalyst is very efficient in oxidizing hydrogen, it is very expensive and not abundant in nature, thereby making hydrogen fuel cells cost-ineffective in regards to energy production. |
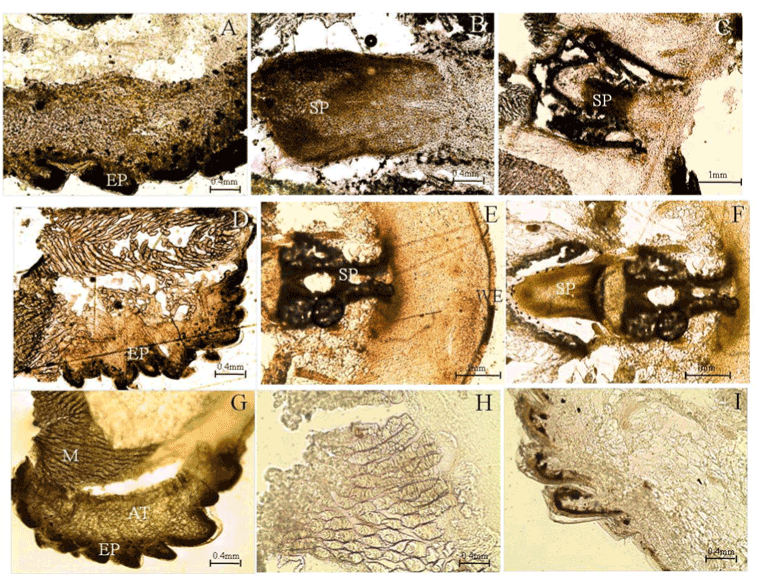
| The motivation for the extensive research into glucose biofuel cell technology is attributed to the search for an alternative sustainable ‘green’ fuel source that is cost-effective and can meet the increasing global energy demands and the recent advancements in microelectronics. Since glucose is an essential energy source in many living organisms, glucose biofuel cells have found applications in powering implantable bioelectronic devices used to diagnose and treat a variety of conditions ranging from metabolic disorders to neurological disorders. Conventional fuel cell assembly consists of an anode, a cathode and an electrolyte and relies on the conversion of the chemical energy of the fuel in the electrolyte, into electrical energy. Two types of reactions occur in a fuel cell: oxidation and reduction reaction also known as redox reaction. The oxidation reaction occurs at the interface of the anode and electrolyte; whereas the reduction reaction occurs at the interface of the cathode and electrolyte. Oxidation of fuel releases electrons, which travel through an external circuit towards the cathode thus producing electricity. Glucose biofuel cell is a subclass of conventional fuel cells, wherein glucose is utilized as the fuel in the presence of oxygen and is then oxidize to form gluconolactone and release electrons at high voltages enabling oxygen to be reduced to provide bioelectricity. The experimental set up for energy generation is as shown in (Figure 2). |
| Although glucose biofuel cells are classified as electrochemical power sources, they are different from batteries and can be differentiated based on the anodic catalysts used for the oxidation of the fuel. Batteries convert the chemical energy of a chemical reaction between two solid reactants (active mass) into electrical energy by consuming the active mass. Once one of the active mass is fully consumed, the current-producing reaction ceases, thus no current flow through the system. Thus, batteries have limited supply of fuel and has to be recharged or replaced once all the chemical energy stored in the active mass is consumed. Meanwhile, in glucose biofuel cells, the chemical reaction takes place between liquid and/or gaseous reactants and electrical power is generated as long as there is a continuous supply of glucose and the enzymes remain active. Thus, making enzymatic glucose biofuel cells ‘green’ alternative to convention fuel cells for generating power with high conversion efficiency and at ambient temperatures. |
| Enzymatic Glucose Biofuel Cells |
| Enzymatic glucose biofuel cells employ electrodes modified with naturally occurring glucose and oxygen selective enzymes derived from micro-organisms to oxidize and reduce glucose and oxygen, respectively. These purified redox enzymes are renewable and less expensive compared to the precious metal catalysts used in the development of conventional fuel cells. However, in order to successfully generate electrical energy using these enzyme modified electrodes, an important factor: the enzyme’s ability for bioelectrocatalysis to the electrode must be considered. Previously researched biofuel cells employ Direct Electron Transfer (DET), as well as employ Mediator Electron Transfer (MET) [2,3,6-8]. DET is promoted by ensuring that the immobilized enzyme is directly wired to the electroactive surface of the nanostructured electrode and that the enzyme is capable of transferring electrons directly from its active center to the current collector. This mechanism of bioelectrocatalysis is limited to Pyrroloquinoline Quinone (PQQ)-dependent, Flavin Adenine Dinuclucleotide (FAD)-dependent, and heme-containing enzymes. To improve the performance of the biofuel cell it is important to achieve DET between the enzymes and the electrode. |
| When the enzymes cannot efficiently transfer electrons to the electrode, MET is employed as the mechanism of bioelectrocatalysis. Naturally occurring oxidoreductase enzymes and coenzymes, such as Nicotinamide Adenine Dinucleotide (NAD+) dependent (i.e., Glucose Dehydrogenase (GDH)) are employed in glucose biofuel cell anodes. Although MET offer higher current-density over DET based biofuel cells, the major disadvantage of MET is that it requires the immobilization of additional components such as redox-mediated or redox polymers along with the enzymes, thereby introducing a layer of complexity, which further destabilizes the system. For this reason, biofuel cells based on DET are preferred to those based on MET. |
| Aston et al. [9] studied the immobilization of a biocatalyst and a mediator at both the anode and cathode of an enzymatic biofuel cell and showed that a maximum power density of 4 μW/ cm2 at a cell potential of 40 mV can be obtained by operating in 1 mM glucose (pH 7). Demonstrated anodic oxidation of glucose in a compartmentless glucose-oxygen fuel cell using glucose dehydrogenase, and a power density of 58 μW/cm2 was achieved at a pH of 7. The development of stable and continuous enzymatic biofuel cells designed to catalyze the oxidation of fuel has been limited by low power densities [10,11] employed Single-Walled Carbon Nanotube (SWCNT) anodic substrate modified with Pyroloquinoline Quinone Glucose Dehydrogenase (PQQ-GDH) in the implementation of glucose biofuel cell. The use of SWCNT as the bioanode substrate material in half-cell electrochemistry, for the first time, demonstrated DET between the active sites of PQQ-GDH and SWNT as characterized by cyclic voltammetry. |
| Other studies have been focused on miniaturized membraneless enzymatic biofuel cells operating on glucose and oxygen [8,12-17]. A biofuel cell device based on glucose oxidase and laccase both mediated by osmium-based redox hydrogels 7 μm diameter carbon fibers have been shown to achieve power density of 137 μW/ cm2 operating in 15 mM glucose at a temperature of 37°C and a pH of 5 [18] employed laccase mediated by 2, 2’-azinobis-(3-ethylbenzothiazoline-6- sulphonate) (ABTS) to enhance the bioelectrocatalytic capability of cathode using a three dimensional porous electrodes instead of a planar electrode. Beden et al. [19] fabricated a membraneless biofuel cell comprising of anodic and cathodic enzymes mediated by a redox polymer, which generated a power density of 50 μW/cm2 at 0.50 V (37 C). The power density, however, dropped from 50 μW/cm2 to 30 μW/cm2 over a period of 48 hours. Most recently, [20-26] demonstrated one year stability of their MWCNT-based glucose biofuel cell under continuous operation using 5 mM glucose (pH 7, 37°C). The power output rapidly decrease by 25% after 1 h of operation due to the deactivation of laccase at neutral pH. Using an intermittent reactivation/ discharge cycle, wherein the biocathode is reactivated in phosphate buffer (pH 5), the maximum power density was restored to 22% of its initial value. Stability of biofuel cells is a major concern that still remains to be addressed. Consequently, a major advancement in biofuel cells is the achievement of the greatest lifetime of immobilized enzymes (> 1 year) due to the encapsulation of the enzyme in micellar polymers [26-31]. |
| Although these studies focused on anodes and cathodes composed of different enzyme systems under a common pH, temperature and electrolyte simultaneously, the low operating pH (pH 5), which is optimal for the laccase cathode but not optimal for the currentlimiting glucose oxidase anode have significant impact on the power densities of the biofuel cell devices. Due to the low operating pH of laccase, [32-38] immobilized Bilirubin Oxidase (BOD) and its functional analogue, 2, 5-dimethyl-1-phenyl-1H-pyrrole-3- carbaldehyde, onto Multi-Walled Carbon Nanotube (MWCNT) via 1- Pyrenebutanoic Acid, Succinimidyl Ester (PBSE) crosslinker. This cathodic modification resulted in an increased current density, which was found to be 20 times higher than that of unmodified BOD cathode. Moreover, the performance of the enzyme is optimized in pH neutral buffers, thereby making them more attractive for powering ultra-low power consuming bio-implantable devices. |
| In context, glucose-based biofuel cell circuit is completed with an external load to allow the conduction of electrons from the bioanode to the biocathode. Thus, the choice of the anodic and cathodic material depends on several factors including but not limited to the biocatalyst to catalyze the electrode reactions; the integration of the biocatalyst with the appropriate physicochemical transduction element for generating energy from the various concentration of glucose; effective transduction element surface area in order to increase the number of adsorption sites for the biocatalyst; ease of fabrication; and enhanced durability to ensure extended functional lifetimes of biofuel cell devices for implantation [39-46]. Currently, high performance glucose biofuel cells mostly employ Carbon Nanotubes (CNTs) as the electrode materials due to their high surface area and their excellent electrochemical properties for mediating electron transfer [47-50]. |
| In vivo Implantations of Enzymatic Biofuel Cell |
| For in vivo energy conversion, Mano et al. [51] implanted a glucose biofuel cell composed of a GOx and laccase electrically connected to the bioanode and biocathode, respectively via osmium-based redox hydrogel in a grape and it delivered a stable but very low power of 0.47 μW/cm2. Once the glucose biofuel cell was relocated to be in close proximity to the skin of the grape, the biofuel cell delivered a power of 2.4 μW/cm2 at a cell voltage of 0.54 V. This increase in power density is attributed to the higher oxygen concentration in the sap closer to the skin of the fruit, thereby suggesting that the power density produced by cell is oxygen-transport dependent. Researchers [51-55] implanted a biofuel cell based on buckypaper modified with PQQ-GDH and FAD dependent fructose dehydrogenase at the anode and laccase at the cathode inside an orange. Such assembly produced an open circuit voltage and a short circuit current of 0.6 V and 0.33 mA/cm2, respectively and a peak power of 670 μW. To decrease the complexity of biofuel implantation, [56] partially implanted a two-compartment biofuel cell (bienzyme anode consisting of trehalose and GOx and BOx cathode) in the abdomen section of a cockroach. In this work, only the bioanode was implanted in the cockroach and the BOD cathode was used as an open-air cathode and maintained outside the cockroach in order to avoid low oxygen levels in the body. A maximum power and current densities of 55 μW/cm2 at 0.2 V and 460 ± 70 μA/cm2 were obtained. Thus, [57-61] demonstrated that bioelectricity can be produced on a micro-scale from trehalose within an insect and the oxygen from air. Another research group implemented a trehalose/O2 biofuel cell in Blaberus discoidalis (tropical cockroach) species, where the chemical energy stored in haemolymph was used to generate power [51,62,63]. The maximum power output was observed to be 0.12 μW at 0.1 V. |
| Invertebrates such as cockroaches and mollusks have open circulatory blood system meaning, their blood flow is not confined to distinct blood vessels but flows freely throughout the animal’s body thus, supplying nutrients to different parts of their body. Similarly, snail has an open circulatory system in which haemocyanin is the main blood (haemolymph) component that carries oxygen. A biofuel cell comprising of buckypaper electrodes modified with PQQ-GDH at anode and laccase at cathode was implanted between the body wall and internal organ of the snail. Such assembly produced an open circuit voltage of 0.53 V and a maximum current of 42.5 μA. The peak power obtained was 7.45 μW at an optimum resistance of 20 kΩ [64-67]. The open circuit potential observed in this work is much higher than those previously reported [68-70]. Using the same buckypaper electrode platform, [71-73] placed their biofuel cell in direct contact with the exposed tissue of a rat without having to implant the electrodes. The active surface area of each of the electrode was 2 cm2. The tissue exposed biofuel cell produced a current density of 5 μA/cm2 and exhibited an open circuit voltage and short circuit current of 140 ± 30 mV and 10 ± 3 μA, respectively. Although the electrical parameters were low, it provided a promising path for the in vivo implantations. |
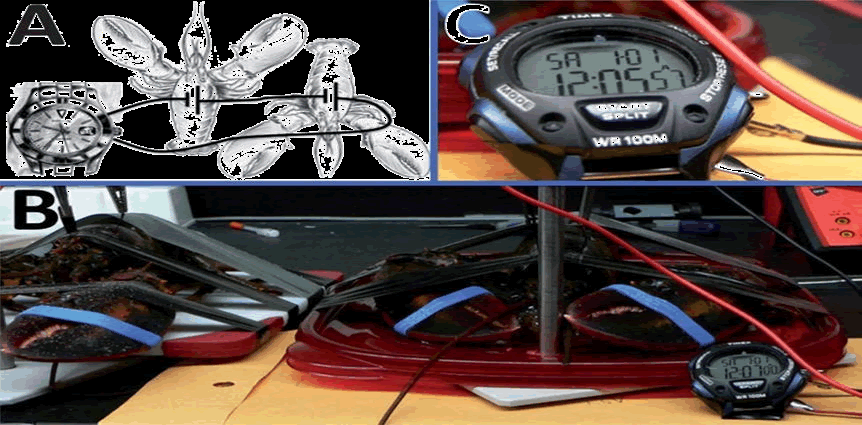
| To achieve higher voltages in vivo, Katz et al. connected glucose biofuel cells in series using two lobsters each implanted with a biofuel cell (Figure 3). This arrangement resulted in an enhanced potential of 1.2 V as opposed to 0.54 V for a single cell [74-76] implanted a similar biofuel cell based on buckypaper modified with PQQ-GDH and laccase and achieved an open circuit voltage, short circuit current and maximum power of 0.8 V, 25 μA and 5.2 μW, respectively for series combination and 0.36 V, 300 μA and 37 μW for parallel combination. Thus, series or parallel combination of the enzymatic biofuel cells can be implemented based on the desired electrical parameter. Nevertheless, the DET enabled by the use of nanomaterials such as CNTs has paved the way for lower overpotentials of the oxidation and reduction of glucose and oxygen, respectively. It is now possible to achieve an open circuit potential of 1 V using GOx and laccase, which can be used to power ultra-low power electronic devices via a charge pump circuit for power management. |
| In order to generate enough energy to power a cardiac pacemaker circuit, it is important that the size of the biofuel cell remains as small as possible, thus using multiple biofuel cells connected in series will increase the bulkiness and the complexity of the biofuel cell circuit. Researchers [77-85] implemented a pacemaker circuit using a single glucose biofuel cell and boost convertors consisting of a charge pump and a DC-DC convertor to generate the 3 V to drive the pacemaker circuit. In this work, the charge pump assembly was used to excite the input voltage (0.3 – 0.5 V) to 2 V and the DC-DC convertor was used to amplify the 2 V supply to result in a stable 3V power supply used to drive the pacemaker circuit. Thus, a single implanted biofuel cell inside a lobster exhibited an open circuit voltage of 0.47 V and a short circuit current density of 0.83 mA/cm2 and delivered sufficient energy to power the cardiac pacemaker circuit. |
| Researchers [86-99] implanted a glucose biofuel cell based on CNTs inside the abdominal cavity of the rat. The in vivo biofuel cell delivered a stable power of 38.7 μW, which corresponds to a peak power density of 193.5 μW/cm2 at 0.57 V. This implanted biofuel cell assembly was capable of powering a digital thermometer for several minutes. A summary of in vivo implantations successfully achieved in mammals, crustaceans and mollusks are depicted in (Table 1). |
| Application of in vivo Glucose Biofuel Cells |
| The energy converted from glucose in the body using glucose biofuel cells finds potential application in powering bioelectronic devices and portable electronic devices, such as continuous glucose monitors (CGMs) and cardiac pacemaker. Continuous monitoring of blood glucose levels in people suffering from diabetes is essential since diabetes is the 7th leading cause of death in the United States [100-118]. Current CGM technologies require the patient to periodically prick his/ her finger using a test-strip in order to maintain tight control over their blood glucose level. In addition, CGM uses potentiostatic circuit to acquire blood glucose concentration and this technique requires the use of an external power source such as a battery. The current approach for monitoring blood glucose is very cumbersome and is associated with a degree of discomfort to the patient especially when one has to prick multiple times to maintain normal blood glucose level. Thus, using self-powered glucose biosensors implanted in vivo will help patients monitor blood glucose levels without using any invasive technique |
| Furthermore, cardiac pacemakers are currently powered by lithium batteries and is used to treat arrhythmias or abnormal rhythm of the heart -beat. Usually these batteries last anywhere between 5-8 years and needs to be replaced once worn out [119-122]. The battery consumption depends on a number of factors including but not limited to the age of the pacemaker, the pacemaker work load and power settings. Typically the pacemaker consumes 10 – 30 μW [122-127] and lithium battery replacement requires an open heart surgery, which results in significant surgical cost and risk to the patient. Thus, miniaturized glucose biofuel cells implanted inside the heart will utilize the glucose present in the blood stream to produce bioelectricity to power the pacemaker circuit. Since, glucose is abundant in the body, the glucose biofuel cell will continuously generate electricity as long as there is a continuous supply of glucose with the assumption that the enzymes does not loss its activity over time. Thus, numerous efforts have been made to developed lightweight glucose biofuel cells that can power ultra-low power devices over a long period of time without the need for surgical replacements. |
| Conclusion |
| One of the major application of enzymatic biofuel cells is to power implantable devices thus decreasing our reliance on batteries. Although there has been a considerable amount of research in biofuel cells development, improving the stability of immobilized enzymes on transducer surfaces still present a great challenge in the quest of developing biofuel cells with a lifetime greater than 3 months. Hydrogels and polymers have been incorporated to improve the stability and longevity of the enzymatic biofuel cells. Future advancement in energy conversion approaches using glucose biofuel cells will continue to be driven by the ever increasing demand of renewable source of energy that are cost-effective, environment friendly and readily available. |
| Acknowledgment |
| This work was supported by National Science Foundation (Award ECCS�# #1349603). |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22090
- [From(publication date):
December-2015 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 16737
- PDF downloads : 5353
