Epigenetics - Role as Biomarker in Cancer Diagnosis
Received: 11-Nov-2017 / Accepted Date: 29-Nov-2017 / Published Date: 05-Dec-2017
Abstract
Cancer is a disease either caused by genetic mutation or epigenetic modifications at the transcription level. Disease diagnosis at the early stage is a challenging step to be taken care for improving the outcome of patient survival. Latest advances has renewed interest and ended in the development of different potential biomarkers. Biomarkers of cancer include DNA, RNA, proteins, lipids, sugars, small metabolites, cytokinetic and cytogenetic parameters and the entire tumour cells found in the body fluid. Biomarkers should be thoroughly investigated for diagnosing the disease accurately, and to aid in the selective targeted therapies to improve the disease outcome. This review gives a brief description on various biomarkers for diagnosis, prognosis and therapeutic purposes, and how the epigenetic biomarkers act as cancer biomarker effectively.
Keywords: Hypomethylation; Microarrays; Malignant tumors; Chemotherapy
Introduction
Epigenetics alludes to change in the gene expression levels without bringing any alteration in DNA sequence. DNA methylation and Histone modifications are considered as significant epigenetic mechanisms that confer the heritable changes in cellular phenotype [1-5]. These play a vital role in DNA based processes like Replication, Transcription and DNA repair. Consequently, genomic alterations or abnormal expression in chromatic regulators can have profound effect leading to induction of Cancer [6,7].
DNA methylation
Hypermethylation of CpG islands located in Promoter regions of tumor suppressor genes is considered to be important mechanism for gene inactivation. Hypomethylation refers to the reduced levels of global DNA methylation which promotes the different types of malignancies leading to cancer [8-10].
Histone modifications
Histone acetylation involves the regulation of chromatin structure leading to the increased or decreased levels of gene transcription. HAT and HDAC are the enzymes involved in the addition and removal of acetyl groups from lysine residues on the histone N-terminal tails. Histone methylation is carried out by conserved proteins known as HMTs which facilitates the addition of methyl groups to the amino terminals of histone proteins and is related to different biological processes ranging from transcriptional regulation to epigenetic silencing [11-14].
DNA methylation is linked with many key processes like telomeres, centromeres, X-chromosome inactivation, and suppression of repetitive elements, genomic imprinting and carcinogenesis. There are two types of abnormal DNA methylation associated with human malignancies. Global hypomethylation is often associated with chromosomal instability and loss of imprinting whereas hypermethylation occurs at CpG islands located in Promoter regions and often associated with inactivation of tumor suppressor genes [15-20].
Epigenetic aberrations have an impact on the stages of tumorigenesis, eventually promoting the neoplastic cells with increase in pathogenicity. Identification of those alterations can be used as prognostic biomarkers for diagnosing Cancer at the early onset of the disease. These biomarkers will be helpful for characteristic patients whose malignancies are sensitive to particular cytotoxic chemotherapies that can hold guarantee for anticipating from which patients will be benefited from newer agents targeted at oncogenes [21-28].
These biomarkers will be useful for trademark patients whose malignancies are delicate to particular cytotoxic chemotherapies that can hold guarantee for anticipating from which patients will be profit by more current focused on operators coordinated at oncogenes.
Biomarkers
Discovery of novel biomarkers in cancer research is the future challenging step. A biomarker is defined as a biological entity found in tissues, blood, or other body fluids that indicates normal or abnormal process of a condition or disease [29-35]. Different types of cancer biomarkers are developed for screening the individuals at risk or for the detection of the presence of a specific type of cancer or for the prediction of the tumour’s outcome or help in recognizing the specific drug treatment for a patient [35-56].
Types of biomarkers
Biomarkers are mainly classified into three categories (Figure 1):
Diagnostic and prognostic biomarkers are often called as quantifiable traits that facilitate doctors to aid in the best treatment for the patients. These biomarkers exist in various forms; ancient biomarkers comprise assessing the patients with radiological techniques and using tumor specific antigens.
The Progress in human genome technologies like high-throughput DNA sequencing, mass spectrometry, and microarrays has led to the development of Cancer related biomarkers by comparing the sequence and expression levels of DNA, RNA and Protein. Genetic and genome based methodologies, for example, the investigation of gene expression pattern given by micro-array technology has significant role in diagnosis and prognosis of Cancer and many other diseases [57-63].
Cancer biomarkers
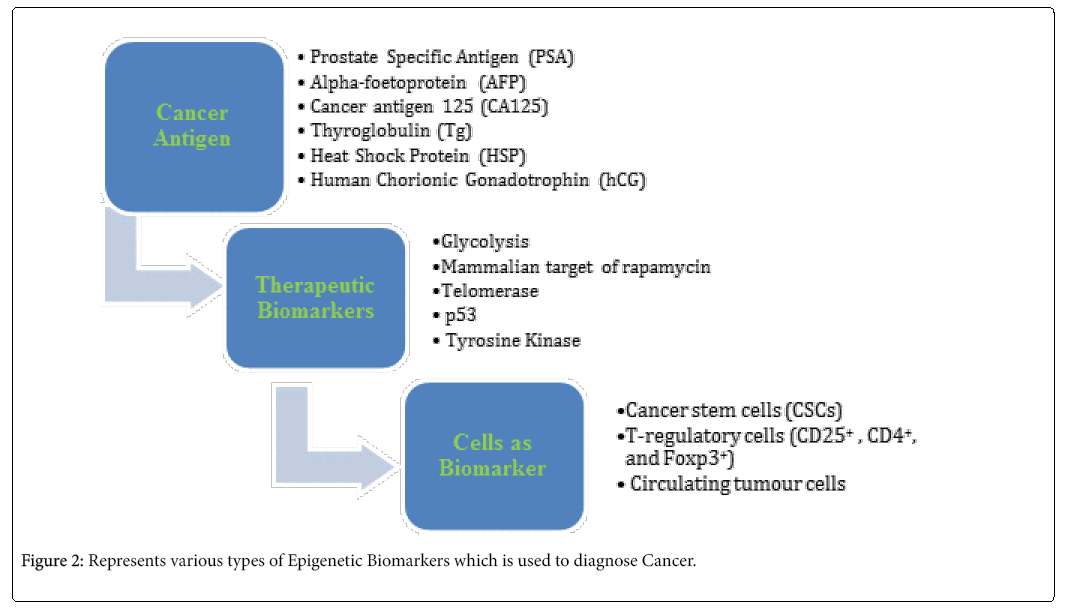
Cancer is defined as a group of diseases including genetic modifications that include point mutations, gene rearrangements and gene amplifications, bringing about changes in molecular pathways regulating cell growth, survival and metastasis [64-70]. When these types of changes appear among large number of patients suffering with specific type of tumor, then such progressions can be utilized as biomarkers to diagnose the targeted therapies (Figure 2) [71,72].
Cancer antigen based biomarkers
Tumor cells secrete few macromolecules into the extracellular fluid of which some proteins continue to persist in the blood stream which tends to act as potential serum biomarkers [73,74].
Prostate specific antigen (PSA)
Prostate specific antigen (PSA) is probably the most widely considered biomarker in prostate cancer. It belongs to the family of Kallikrein genes and is produced by both normal and neoplastic prostate epithelial cells. PSA is available in small quantities in the serum of normal men, and is frequently raised in the vicinity of prostate cancer [75-79].
Alpha-foetoprotein (AFP)
Alpha-foetoprotein (AFP) is a well-known diagnostic biomarker utilized in hepatocellular carcinomas (HCC). But it is not specific to detect in the early stages of hepatocellular carcinoma. It is the major serum foetal protein found in mammals, and is actively produced and secreted by the liver hepatocyte. Due to the fact that the levels of AFP may be elevated in serum from patients with other chronic liver diseases; AFP cannot be used for screening in patients suffering with hepatitis C or cirrhosis [80,81].
Cancer antigen 125 (CA125)
CA-125 antigen is known to be the primary serum tumour marker used in ovarian cancer for prognosis, response to chemotherapy and disease progression. The CA 125 antigen is found out to be a membrane glycoprotein delivered by tissues derived from coelomic epithelium expressed by most epithelial ovarian cancers. The Major drawback in using the CA125 test as a screening tool is its lack of sensitivity and its inability to detect early stage cancers. It may also be elevated in other malignant cancers, including those originating in the lungs, fallopian tubes, breast, endometrium, and gastrointestinal tract [82-87].
Thyroglobulin (Tg)
Thyroglobulin (Tg) is an organ-specific tumour marker; associated with patients possessing differentiated thyroid cancer that emerge from the follicle cells which increases the levels of thyroglobulin in the blood. It is considered to be a large glycoprotein stored in the follicular colloid of thyroid gland which acts as pro-hormone in the intrathyroid synthesis of thyroxine (T4) and triiodothyronine (T3) [88-90].
Heat shock protein (Hsp)
Hsp has gained importance due to its implication in tumour progression and response to therapy which has led to the development of targeted therapy by the usage of Hsps as immunological adjuvant in anticancer vaccines. Heat shock proteins (Hsps) are overexpressed in various human cancers and are involved in cell cycle related processes. It might be due to the stimulation of the Hsp induction by physiopathological features of the tumour microenvironment [91-95].
Human chorionic gonadotrophin (hCG)
Human Chorionic Gonadotrophin (hCG) is a hormone normally produced by placenta, which are found to be high in the blood of patients with certain types of ovarian cancers, testicular and choriocarcinoma. The elevation of serum levels of hCG and its metabolites cannot be considered for prognosis as it is estimated that βhCG might directly modify the growth of the cancer, leading to a worse outcome. Pregnant women are tested with the presence of hCG levels and may not be useful as a marker under this condition [96-100].
Therapeutic biomarkers
Cytotoxic chemotherapy and radiotherapy are considered to be the best medications available for malignancy; however, these may cause serious side effects causing damage to normal cells along with the tumor cells. Recent advancements in understanding the underlying mechanism Cancer has led to the development of targeted therapies which may inhibit the growth of the tumor cells interfering in their molecular pathways leading to apoptosis. For example, Imatinib and Erlotinib drugs can inhibit the activity of Protein tyrosine kinases targeting the Epidermal Growth Factor Receptor (EGFR). Other targeted therapies like antibody bevacizumab will act upon a growth factor that stimulates tumor blood vessel growth [101-105].
Glycolysis
Most of the malignant tumours depend on glucose for their development. When human tumour cell lines were studied with varying degrees of glycolysis, it has been observed that there is an inverse relationship between the rate of glycolysis and damage induced by chemotherapeutic drugs and radiation. Studies have shown that 2- DG selectively sensitizes tumour cells to ionizing radiation, without causing damage to normal cells. Thus, Clinical trials in brain tumour patients using a hypofractionted radiotherapy protocol combined with 2-DG proved to be successful. Combination therapy has resulted in minimal acute toxicity and late radiation effects and there is significant increase in survival rate and improvement in quality of life has been reported [106-110].
Mammalian target of rapamycin (mTOR)
Mammalian target of rapamycin (mTOR) is a serine-threonine protein kinase which fits in with the PIKK [phosphoinositide 3-kinase (PI3K)-related kinase] family, assumes a noteworthy part in regulating cell development and proliferation. When mTOR is activated, the phosphorylation levels of its downstream targets p70S6K and 4EBP1 are promoted, which leads to increased levels of ribosome biogenesis, translation, inhibition of autophagy and reorganization of the actin cytoskeleton. Studies have shown that the (PI(3)K)–PTEN–mTOR signaling pathway is aberrantly activated in many tumours, resulting in dysregulation of cell growth and proliferation. Loss of PTEN mRNA or protein production in tumour tissue can be considered as Biomarkers to evaluate the activation of the pathway. Proliferative marker Ki-67 is used to evaluate the Inhibition of mTOR by rapamycin, which can measure the presence of phosphorylated form of the ribosomal protein S6, and its therapeutic effects on tumour cells [110-114].
Telomerase
Telomerase is an enzyme known as reverse transcriptases, which uses RNA as a template for producing DNA and it contains both RNA and protein components. The enzyme is sole responsible for protecting the cell from degradation and death by the maintenance of telomere. Thus it can be treated as one of the best diagnostic markers for human cancer, related to malignant tumours, thus making it an ideal target for chemotherapy [115-119].
p53
The p53 gene is known to be a tumour suppressor gene which prevents the uncontrolled multiplication of abnormal cells. Radiation and many other anticancer drugs cause damage to the DNA of cancer cells, which activates the p53 gene leading to apoptosis. During treatment, an intact wild type p53 gene is essentially required to stimulate programmed cell death of a cancer cell. Thus p53 gene is a well-studied potential biomarker for predicting prognosis and patient’s response to therapy [120,121].
Tyrosine kinase
Tyrosine kinases belong to group of enzymes that regulate various cellular processes like cell growth, differentiation, migration, and apoptosis that aids in tumour development and progression. Thus targeting protein Tyrosine kinases as a therapeutic biomarker is an attractive approach to inhibit the tumour growth. For example, tyrosine kinase inhibitor Gefitinib and Trastuzumab have proved to be anti-cancer agents [122-124].
Cells as Biomarker
Circulating tumour cells (CTCs)
Circulating tumour cells can be considered as a powerful biomarker to predict the disease progression and response to therapy. Increase in CTCs at any time during therapy is an indication of progression, whereas decreased number of CTCs shows the effectiveness of the therapy. Studies have shown that they can be considered as standard tumour markers (e.g., Ca27-29) in predicting prognosis [124].
T-regulatory cells (CD4+, CD25+ and Foxp3+)
T regulatory cells (T-regs) are considered to be important in inducing and maintaining peripheral self-tolerance, consequently preventing immune pathologies. They are assumed to control both natural and acquired immune responses. T-regs is well known as a surrogate immune marker of cancer progression; also acts as a predictor of response to targeted therapies. The presence of FoxP3+ cells within tumours has proven to predict the prognosis, metastatic ability and invasiveness of some tumours by modulating the ability of the immune system to target tumour cells [125-127].
Cancer stem cells (CSCs)
Cancer stem cells are subpopulation of cells that have the capacity to self-renew and to generate the more differentiated progeny which make up the bulk of a tumour. Studies have shown cancer stem cells (CSCs), tumourigenic cancer cells, or tumour-initiating cells, can give rise to new tumours when transplanted into immuno-deficient animals. Therefore, it is utmost importance to identify CSCs for every possible tumour which may lead to new therapeutic avenues [128-131].
Epigenetic biomarkers
It is well known that, in cancer cells, genes are either altered by mutations, or through epigenetic modifications to chromosomes that change gene-expression patterns. Epigenetic modifications are supposed to occur either through DNA methylation of genes or by acetylation, methylation, or phosphorylation of histones and other proteins around which DNA is wound to form chromatin [132-134]. Recent advancement in the field has led to the awareness of the epigenetic changes driving neoplasia to be used as significant cancer biomarkers. Studies have shown that the activity of DNA methyltransferases (DNMTs), are altered in tumour cells and are known to be associated with several developmental abnormalities [135-140]. Epigenetic changes can be either through Hypermethylation or Hypomethylation of genes. Hypermethylation markers can be utilized for detecting both primary and metastatic cancers. For example, hypermethylation of p16 promoter in the circulating serum DNA is found in recurrent colorectal cancer. These biomarkers have been proved to be useful for identifying patients sensitive to specific cytotoxic chemotherapies and may help in predicting which patients benefit from newer targeted agents directed at oncogenes.
Conclusion
Epigenetic alterations have been associated with the development and progression of human cancer. Studies have shown that epigenetic modifications are reversible, in contrast to genetic mutations. This has led the researchers to focus on developing epigenetic drugs in treatment of cancer patients. Although, advancements in epigenetics research has led to improved disease outcome of patients with certain forms of lymphoma and leukemia’s by using the drugs that alter DNA methylation and histone acetylation, more attention should be paid for optimizing and validating the methylation markers in clinical trials. Therapeutics designed to reverse the epigenetic alterations in cancer cells, along with diagnostic and prognostic assays based on genemethylation patterns, are promising new avenues for future progress in patient care.
References
- Souza-Pardo APD (2015) Side-by-Side Epigenetics and Genetics Share Importance in Cancer Development. Human Genet Embryol
- Gaal Z, Balint BL, Rejto L, Olah E (2015) Decreased Expression Levels of Tumor Suppressor MicroRNAs in Hairy Cell Leukemia . J Leuk (Los Angel) 3: 169.
- Dawson MA, Kouzarides T (2012) Cancer Epigenetics: From Mechanism to Therapy. Cell 150: 12-27.
- Zhang Q, Wu H, Zheng H (2015) Aberrantly Methylated CpG Island Detection in Colon Cancer. J Proteomics Bioinform S9: 007.
- Fragale A, Marsili G, Battistini A, (2013) Genetic and Epigenetic Regulation of Interferon Regulatory Factor Expression: Implications in Human Malignancies. J Genet Syndr Gene Ther 4: 205.
- Zhang Q, Wu H, Zheng H (2015) Aberrantly Methylated CpG Island Detection in Colon Cancer. J Proteomics Bioinform S9: 007
- Boulding T, Wu F, Zafar A, Rao S (2015) Multi-Layered Epigenetic Regulatory Mechanisms Mediate Epithelial to Mesenchymal Transition in Cancer. J Integr Oncol 4: 127.
- Gaal Z, Balint BL, Rejto L, Olah E (2015) Decreased Expression Levels of Tumor Suppressor MicroRNAs in Hairy Cell Leukemia. J Leuk 3: 169
- Jeon J, Lee YH (2014) The Rise of Epigenetics in Microbial Eukaryotes. Fungal Genom Biol 4: 112.
- Jordaan G, Liao W, Coriaty N, Sharma S (2014) Identification of Histone Epigenetic Modifications with Chromatin Immunoprecipitation PCR Array in Chronic Lymphocytic Leukemia Specimens. J Cancer Sci Ther 6: 325-332.
- Ohta T (2013) Epigenetics and Evolutionary Mechanisms. Human Genet Embryol 3: 113.
- Lee J, R SH (2013) Cancer Epigenetics: Mechanisms and Crosstalk of a HDAC Inhibitor, Vorinostat. Chemotherapy 2: 111.
- Roy S, Majumdar APN (2013) Cancer Stem Cells in Colorectal Cancer: Genetic and Epigenetic Changes. J Stem Cell Res Ther S7-006
- Vinci MC (2012) Sensing the Environment: Epigenetic Regulation of Gene Expression. J Phys Chem Biophys S3: 001
- Wang Y, Moore BT, Peng X, Xiao P (2012) Epigenetics and Hematopoietic Stem or Progenitor Cells. Human Genet Embryol S2: 004
- Archer T, Oscar-Berman M, Blum K (2011) Epigenetics in Developmental Disorder: ADHD and Endophenotypes. J Genet Syndr Gene Ther 2: 104.
- Shewale SJ, Huebinger RM, Allen MS, Barber RC (2013) The Potential Role of Epigenetics in Alzheimer’s Disease Etiology. Biol Syst Open Access 2: 114.
- Azad GK,Tomar RS (2015) Epigenetics of Curcumin: A Gifted Dietary Therapeutics Compound. J Carcinog Mutagen 6: 206
- Mohammed RHA (2014) Epigenetics: Understanding Molecular Roots of Autoimmunity. Rheumatology (Sunnyvale) S5-e001
- Weaver ICG (2014) Epigenetics: Integrating Genetic Programs, Brain Development and Emergent Phenotypes. Cell Dev Biol 3: 132.
- Archer T, Oscar-Berman M, Blum K, Gold M (2012) Neurogenetics and Epigenetics in Impulsive Behaviour: Impact on Reward Circuitry. J Genet Syndr Gene Ther 3: 115
- Hait NC (2013) Epigenetics and Novel Therapeutic Approaches. J Mol Genet Med 7: 74
- Qin T (2012) Cancer Epigenetics: It is Time to Move Forward to Therapy. J Bioanal Biomed S5 e001
- Maiti A (2012) Mechanism of Active DNA Demethylation: Recent Progress in Epigenetics. J Biomol Res Ther 1: e106
- Falasca M (2012) Biomarkers, Epigenetics and Pancreatic Cancer. J Mol Biomark Diagn 3: e113
- Arlen M, Arlen P, Wang X, Saric O, Martin DA, et al. (2013) The Clinical Detection of Pancreatic Carcinoma: A Comparison of the Standard Biomarkers to that of a Newer Class of Biomarkers used for both Diagnosis and Therapy. Pancreat Disorders Ther S4: 001
- Yarsan E, Yipe M (2013) The Important Terms of Marine Pollution "Biomarkers and Biomonitoring,Bioaccumulation, Bioconcentration, Biomagnification". J Mol Biomark Diagn S1-003
- Paap BK, Hecker M, Koczan D, Zettl UK (2013) Molecular Biomarkers in Multiple Sclerosis. J Clin Cell Immunol S10: 009
- Matsuzaki H, Lee S, TakeiKumagai N, Hayashi H, Miura Y, et al. (2012) Exploration of Biomarkers for Asbestos Exposure and Occurrence of Malignant Mesothelioma Based on the Immunological Effects of Asbestos. J Data Mining in Genom Proteomics S2-001
- Engelborghs S (2013) CSF Biomarkers for Alzheimer Disease Diagnosis: Recent and Future Perspectives. J Neurol Disord 1: e102
- Coccini T, Signorini C, Roda E (2012) Biomarkers for Pulmonary Effects Induced by In vivo Exposure to Cadmium-Doped Silica Nanoparticles. J Mol Biomark Diagn S1-001
- Konstantinidis TG, Parasidis TA (2013) Immune Biomarkers in Predictive and Personalized Medicine. J Anc Dis Prev Rem 1: e102
- Tan Y, Miele L, Sarkar FH, Wang Z (2012) Identifying Biomarkers and Drug Targets Using Systems Biology Approaches for Pancreatic Cancer. Pancreat Disorders Ther 2: e128
- Ahmad MI, Sharma N (2012) Biomarkers in Acute Myocardial Infarction. J Clin Exp Cardiolog 3: 222
- Pehlivan S (2012) Circulating Cell-Free Nucleic Acids as Potential Biomarkers for Noninvasive Diagnosis of Diseases in the Future. Biochem Physiol 1: e109
- Hwang-Verslues WW, Lee W-H, Lee EY-HP (2013) Biomarkers to Target Heterogeneous Breast Cancer Stem Cells. J Mol Biomark Diagn S8-006
- Galizia G, Gemei M, Pinto M, Zamboli A, Mabilia A, et al. (2012) Different Biomarkers Address Different Colorectal Cancer Stem Cell Populations: Who’s the Killer?. J Mol Biomark Diagn 2012, S8-004
- Yang X (2012) Use Circulating microRNAs as Biomarkers of Drug-Induced Liver Injury. J Vaccines Vaccin 3: e105
- McGeough CM, Bjourson AJ (2013) Diagnostic, Prognostic and Theranostic Biomarkers for Rheumatoid Arthritis. J Clin Cell Immunol S6: 002
- Jin D, Xie S, Mo Z, Liang Y, Guo B, et al. (2012) Circulating DNA-Important Biomarker of Cancer. J Mol Biomark Diagn S2-009
- LeBeau AL, Johnson GT, McCluskey JD, Harbison RD (2013) Evaluation of Urinary Pesticide Biomarkers among a Sample of the Population in the United States. J Clin Toxicol S5: 003
- Kamal-Eldin A (2012) Dietary Biomarkers and the Unresolved Challenges. JBB 4: 7-8
- Hubaux R, Becker-Santos DD, Enfield KSS, Lam S, Lam WL, et al. (2012) MicroRNAs as Biomarkers for Clinical Features of Lung Cancer. Metabolomics 2: 108
- Mimeault M (2012) Potential Biomarkers and Therapeutic Targets in Cancer Stem Cells. J Mol Biomark Diagn 3: e108
- Carasevici E (2012) Stromal Biomarkers as Putative Targets in Cancer Chemotherapy. Biochem Pharmacol 1: e108
- Palaghia M, Prelipcean CC, Cotea E, Vlad N, Leneschi L, et al. (2015) Metastatic Colorectal Cancer: Review of Diagnosis and Treatment Options. Surgery 10: 4
- Stephen Suh K (2012) Discovery of Novel Biomarkers for the Development of Personalized Medicine. Transl Med S1-e001
- Romick-Rosendale LE, Schibler KR, Kennedy MA (2012) A Potential Biomarker for Acute Kidney Injury in Preterm Infants from Metabolic Profiling. J Mol Biomark Diagn S3-001
- Lakkis M, Kai J, Santiago N, Puntambekar A, Moore V, et al. (2012) Novel Biomarkers Detection and Identification by Microfluidic- Based MicroELISA. Transl Med S1-006
- Katrina M Crader, Jonathan JD Repine, John E Repine (2012) Breath Biomarkers and the Acute Respiratory Distress Syndrome. J Pulm Respir Med 2: 111
- Cooper SJ, Tun HW, Roper SM, Kim Y, Kislinger T, et al. (2012) Current Status of Biomarker Discovery in Human clear Cell Renal Cell Carcinoma. J Mol Biomark Diagn S2-005
- Anastasi E, Granato T, Longo F, Viggiani V, Frati L (2012) HE4 and CA72.4 are Useful Biomarkers in the Follow-up of Epithelial Ovarian Cancer. J Mol Biomark Diagn 3: 122
- Shukla Y (2011) Concept of Toxicoproteomics in Identifying Biomarkers of Toxicant Action. JPB 4: 6-7
- Soendergaard M, Newton-Northup JR, Palmier MO, Deutscher SL (2012) Peptide Phage Display for Discovery of Novel Biomarkers for Imaging and Therapy of Cell Subpopulations in Ovarian Cancer. J Mol Biomark Diagn S2-004
- Gottlieb H, Klausen TW, Boegsted M, Olsen BS, Lausten GS, et al. (2011) A Clinical Study of Circulating Cellular and Humoral Biomarkers Involved in Bone Regeneration Following Traumatic Lesions. J Stem Cell Res Ther 1: 108
- Bousman CA, Everall IP (2011) Formidable Challenges in the Search for Biomarkers of Psychiatric Disorders. J Postgenom Drug Biomark Develop 1: 105e
- Karley D, Gupta D, Tiwari A (2011) Biomarkers: The Future of Medical Science to Detect Cancer. J Mol Biomark Diagn 2: 118
- Sai YRKM, Dattatreya A, Anand SY, Mahalakshmi D (2011) Biomarkers and their Role in Premonition, Interpretation and Treatment of Cancer. J Cancer Sci Ther S17-002
- Dizdaroglu M, Jaruga P (2012) Oxidatively Induced DNA Damage and Cancer. J Mol Biomark Diagn S2-002
- Huixiao H, Candee T, Laura PJ, Weida T, Jack AH, et al. (2008) SELDI Based Proteomic Determination of Hepatic Biomarkers in Mouse Serum Following Acetaminophen Administration. J Proteomics Bioinform 1: 424-436
- Imtiyaz Ahmad AB, Mir R, Zuberi M, Javid J, Yadav P, et al. (2013) Inactivation of RIZ1 Gene by Promoter Hypermethylation is Associated with Disease Progression and Resistance to Imatinib in Indian Chronic Myelogenous Leukemia Patients, First Study from India. J Cancer Sci Ther 5: 045-051
- Falasca M (2012) Biomarkers, Epigenetics and Pancreatic Cancer. J Mol Biomark Diagn 3: e113
- Chan TA, Baylin SB (2012) Epigenetic biomarkers. Curr Top Microbiol Immunol 355: 189-216
- Grayson SE, Ponce de Leon FA, Muscoplat CC (2014) Epigenetics: Understanding How our Choices Lead to our Diseases. J Clin Case Rep 4: 447
- Bhatt AN, Mathur R, Farooque A, Verma A, Dwarakanath BS (2010) Cancer biomarkers - Current perspectives. Indian J Med Res 132: 129-149
- Stephen SK, Sarojini S, Milinovikj N (2013) Ovarian Cancer Biomarkers: Current Trends in Translational Research for Early Detection. Transl Med 3: e115
- Padmanabhan A (2013) The Search of New Cancer Biomarkers: Moving Forward. J Clinic Trials 3: e114
- Mathivanan S (2012) Quest for Cancer Biomarkers: Assaying Mutant Proteins and RNA that Provides the Much Needed Specificity. J Proteomics Bioinform 5: 13-17
- Nichols EM, Cohen RJ, Cheston SB, Feigenberg SJ (2015) Radiation Therapy in the Elderly with Early Stage Breast Cancer: Review and Role of New Technology. J Nucl Med Radiat Ther 6: 204
- Yashiro M (2014) Gastric Cancer Stem Cells and Resistance to Cancer Therapy. Chemotherapy (Los Angel) 3: 135
- Vargas AN (2014) Natural History of Ovarian Cancer. J Cancer Sci Ther 6: 247-252
- Feng Y, Wang N, Hong M (2014) Cancer Chemotherapy: Time for New Solution. Chemotherapy (Los Angel) 3: 130
- Zhao G, Guo Y, Chen Z, Yang Y, Yang C, et al. (2015) miR-203 Functions as a Tumor Suppressor by Inhibiting Epithelial to Mesenchymal Transition in Ovarian Cancer. J Cancer Sci Ther 7: 034-043
- Svensson MA, Menon R, Carlsson J, Vogel W, Andrén O, et al. (2015) Combination of Multiple Markers Predicts Prostate Cancer Outcome. J Mol Biomark Diagn 6: 213
- Ayari H (2015) FABP4 Expression as Biomarker of Atheroma Development: A Mini-Review. J Mol Biomark Diagn 6: 218
- Mahajan K (2015) Microparticles in Atherosclerosis: Biomarkers of Disease. J Clin Exp Cardiolog 5: 356
- Aureli A, Del Beato T, Sebastiani P, Di Rocco M, Marimpietri AE, et al. (2014) Potential Biomarkers for Intellectual Disability: A Gipsy Family Study. Hereditary Genet 3: 138
- Bernstein HS (2014) Future Prospects for Biomarkers in the Management and Development of Novel Therapies for Pediatric Heart Disease. Pediat Therapeut 4: e126
- Heck TG, Ludwig MS, Montagner GFFDS, Frizzo MN (2015) Subclinical Processes in the Development of Type Two Diabetes. J Nov Physiother 5: 246
- Fernandes FF, Rossetti RAM, Coelho-Castelo A, Panunto-Castelo A (2015) Molecular Modeling of Heat Shock Protein of 60-Kda from Paracoccidioides Brasiliensis: The First in silico Structural Model of a Fungal Hsp60. J Comput Sci Syst Biol 8: 241-244
- Ilangovan G (2014) High Level of Heat Shock Protein 25 (Hsp25) Expression in Atherosclerotic Lesions. J Cytol Histol 5: i106
- Venkatesan C, Sahul Hameed AS, Sundarraj N, Rajkumar T , Balasubramanian G (2014) Analysis of Immune Genes and Heat Shock Protein Genes under Exposure to White Spot Syndrome Virus (WSSV) and Herbal Immune Stimulant in Litopenaeus vannamei. J Bacteriol Parasitol 5: 205
- Ajili F, Nedri A, Kourda N, Maaloul A, Karay S, et al. (2014) Prognostic Significance of Heat Shock Protein 90 in Non Muscle Invasive Bladder Cancer Treated by BCG Immunotherapy. J Cytol Histol 5: 230
- Aziz N, Butch AW, Quint JJ, Detels R (2014) Association of Blood Biomarkers of Bone Turnover in HIV-1 Infected Individuals Receiving Anti-Retroviral Therapy (ART). J AIDS Clin Res 5: 360
- Rocha A, Bravo F, Beirao I, Vizcaiacuteno J, Oliveira JC, Lobato L, et al. (2014) Urinary Biomarkers for Kidney Disease in ATTR Amyloidosis. J Nephrol Ther 4: 181
- Dhawan V, Sharma I, Mahajan N, Sangwan SM, Jain S (2014) Implication of Endothelin-2 and Oxidative Stress Biomarkers in Essential Hypertension. J Hypertens 3: 170
- Tangutoori S (2014) The Cytoskeleton as Biomarker: Angiosarcoma- Cytoskeleton. J Mol Biomark Diagn 5: I101
- Yang J, Bai W, Xiao Z, Chen Y, Chen L, et al. (2014) Aberrant Hypomethylated KRAS and RASGRF2 as a Candidate Biomarker of Low Level Benzene Hematotoxicity. J Mol Biomark Diagn 5: 195
- Győrffy A, Kormos M, Bartha L, Szabó A, Győrffy B, et al. (2014) Validation of Biomarkers in Gene Expression Datasets of Inflammatory Bowel Disease: IL13RA2, PTGS2 and WNT5A as Predictors of Responsiveness to Infliximab Therapy. J Proteomics Bioinform 7: 272-277
- Cui Z, Lin D (2014) Circulating miRNAs: Potential Biomarkers for Diagnosis and Prognosis Prediction of Hematological Malignancies. J Leuk 2: 140
- Xu X, Strimpakos AS, Saif MW (2011) Biomarkers and Pharmacogenetics in Pancreatic Cancer. JOP. J Pancreas 12
- Hoimes CJ, Moyer MT, Saif MW (2009) Biomarkers for Early Detection and Screening in Pancreatic Cancer. JOP. J Pancreas 10
- Wang M, Brown DPG, You J, Bemis KG (2014) Putative Surrogate Biomarkers to Predict Patients with Acquired Platinum Resistance in Ovarian Cancer. J Mol Biomark Diagn 5: 184
- Troiani T, Napolitano S, Morgillo F, Ciardiello F, Belli G et al. (2014) Predictive Biomarkers to Anti-EGFR Inhibitors Treatment in the Management of Metastatic Colorectal Cancer. J Carcinogene Mutagene S10 – 001
- Eid HA (2014) The Use of Systemic Lupus Erythematosis (SLE) Biomarkers in Forensic Investigation: A Suggested Approach. J Forensic Res S12: 003
- Ciccone MM, Scicchitano P, Gesualdo M, Quistelli G, Cortese F et al. (2014) The Role of Biomarkers in the Emergency Cardiovascular Setting. Emergency Med 4: 192
- Pasini E, Aquilani R, Corsetti G, Dioguardi FS (2014) Biomarkers to Identify Protein Metabolism Impairment in Chronic/Acute Diseases. J Mol Biomark Diagn 5: 177
- Ghosh P, Lammel JAL, Lee TH, Wang X, Lee DY (2012) Controlled Molecular Targeting of Inducible Heat Shock Proteins. J Mol Imag Dynamic 2: 106
- Sok AJ, Gizak A, Mamczur P, Piotrowska A, Knapik A, et al. (2014) Demethylation with 5-Aza-2′-deoxycytidine Affects Oxidative Metabolism in Human and Mouse Non-small Cell Lung Cancer Cells. J Cancer Sci Ther 6: 036-044
- Serfin J, Carragher J, Groman A, Dexter EU, Yendamuri S (2011) Outcome Prediction Using Markers of Aerobic Glycolysis (the Warburg effect) Varies Between Tumor Regions in Stage I Non-Small Cell Lung Cancer. J Mol Biomark Diagn 2: 116
- Ponizovskiy MR (2012) The Detailed Description Mechanisms of the Herbs Extracts Operations in the New Method Cancer Disease Treatment via the Rearrangement of Metabolism from Pathological Development into Normal Development. J Clinic Trials 2: 124
- Mohamad RH, El-Said MGA, Zekry ZK, Al-Bastawesy AM, Farag RM, et al. (2015) New Aspects of Therapy of Hepatocellular Carcinoma Egyptian Patients. Biochem Physiol 4: 150
- Soejima H, Ogawa H, Yasud O, Kim-Mitsuyam S, Matsui K, et al. (2014) The Changes of Biomarkers by Telmisartan and their Significance in Cardiovascular Outcomes: Design of a Trial of Telmisartan Prevention of Cardiovascular Diseases (ATTEMPT-CVD). J Clin Trials 4: 162
- Huang HL, Huang IY, Lin CY, Huang MC (2013) Effective Strategies for Identifying Novel Genetic Markers Based on DNA Polymorphisms. J Mol Biomark Diagn 5: 156
- Jansen E, Viezeliene D, Beekhof P, Gremmer E, Rodovicius H, et al. (2013) Biomarkers of Selenium Toxicity after Sub-Acute Exposure in Mice. J Mol Biomark Diagn 4: 150
- Shukla HD, Vaietiecunas P (2013) Proteomic Analysis of Oxidatively Stressed Pancreatic Adenocarcinoma BxPC-3 Cells: Identification of Potential Biomarkers and Therapeutic Targets. J Mol Biomark Diagn 4: 149
- Fadri-Moskwik M, Zhou Q, Chai W (2013) Beyond Telomerase: Telomere Instability as a Novel Target for Cancer Therapy. J Mol Genet Med 7: 91
- Hartwig FP (2013) Up-Regulating Telomerase and Tumor Suppression: A Two-Step Strategy to Boost Hematopoietic Stem Cell Transplantation. J Stem Cell Res Ther S3: 003
- Ibrahim AM, Sabet SF, El-Shinawi M (2012) Investigation of Telomerase Activity in Inflammatory and Non Inflammatory Breast Cancer. J Cancer Sci Ther 4: 360-367
- Tan EY, Cheok CF (2014) Bringing p53 into the Clinic. J Cancer Sci Ther 6: 363-369
- Tyagi AK, Prasad S (2015) Targeting P53 Pathway by Curcumin for Cancer Prevention and Treatment. Cell Dev Biol 4: e131
- Leitch C, Andresen V, Gjertsen BT (2013) Impaired Ribosome Biogenesis and P53 Activation in Haematological Disease: Novel Therapeutic Strategies. J Bone Marrow Res 1: 121
- Naga Deepthi CH, VVL Pavan Kumar A, Rameshbabu, Indirapriyadarshini U (2011) Role of Tumor Suppressor Protein p53 in Apoptosis and Cancer Therapy. J Cancer Sci Ther S17-001
- Kuroda J, Taniwaki M (2013) Principles and Current Topics Concerning Management of Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia. Transl Med S2-001
- Chen MS, Liu CY, Wang WT, Hsu CT, Cheng CM (2011) Probing Real-Time Response to Multitargeted Tyrosine Kinase Inhibitor 4-N-(3′-Bromo-Phenyl) Amino-6, 7-Dimethoxyquinazoline in Single Living Cells Using Biofuntionalized Quantum Dots. J Nanomed Nanotechnol 2: 117
- Ahn JM, Cho JY (2013) Current Serum Lung Cancer Biomarkers. J Mol Biomark Diagn S4-001
- Falasca M (2012) Cancer Biomarkers: The Future Challenge of Cancer. J Mol Biomark Diagn S2-e001
- Trugenberger CA, Peregrim D (2013) Discovery of Novel Biomarkers by Text Mining: A New Avenue for Drug Research? J Mol Biomark Diagn S3-004
- Sharma M, Mohanty S (2013) Molecular Biomarkers in Cytogenetically Normal –Acute Myeloid Leukemia: harnessing the targets. J Mol Biomark Diagn S8-009
- ElDeeb WM (2013) A Novel Biomarkers for Pneumonia in Calves. J Veterinar Sci Technol 4: e111
- Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, et al. (2013) Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol Obstetric S4-003
- Valiyaveedan SG, Ramachandran B, Iliaraja J, Ravindra DR , James BL, et al. (2015) Acquisition of Cancer Stem Cell Behaviour Plays a Role in Drug Resistance to Combination Chemotherapy and Prognosis in Head and Neck Cancer. J Stem Cell Res Ther 5: 261
- Milner BL, Penny CB, Gibbon VE, Kay P, Ruff P (2015) CD133/EpCAM Cancer Stem Cell Markers of Tumour Stage in Colorectal Cancer Cells. J Tissue Sci Eng 6: 143
- Yashiro M (2014) Gastric Cancer Stem Cells and Resistance to Cancer Therapy. Chemotherapy (Los Angel) 3: 135
- Oliveira LR, Castilho-Fernandes A, Ribeiro-Silva A (2014) The Prognostic Influence of CD44 in Oral Squamous Cell Carcinoma. J Clin Exp Pathol 4: 175
- Clausell-Tormos J, Azevedo MM, Lorenzo IM, Vieira CR, Sanchez-Ripoll Y, et al. (2014) Nano-Volume Well Array Chip for Large-Scale Propagation and High-Resolution Analysis of Individual Cancer Stem Cells. J Nanomed Nanotechnol 5: 191
- Chen Y, Li P, Li J (2014) Metabolic Enzymes: The Novel Targets for Cancer Stem Cells. J Stem Cell Res Ther 4: 197
- Muqbil I, Bao GW, El-Kharraj R, Shah M, Mohammad RM, et al. (2013) Systems and Network Pharmacology Approaches to Cancer Stem Cells Research and Therapy. J Stem Cell Res Ther S7-005
- Salama R, Tang J, Gadgeel SM, Ahmad A, Sarkar FH (2013) Lung Cancer Stem Cells: Current Progress and Future Perspectives. J Stem Cell Res Ther S7-007
- Ahmed A, Ali S, Philip PA, Sarkar FH (2013) The Role of Cancer Stem Cells and MicroRNAs in the Development and Progression of Pancreatic Cancer. J Stem Cell Res Ther S7-004
- Chang CL, Chen YL, Chen YT, Leu S, Chang LT, et al. (2014) Lipocalin 2 Level Predicts Prognostic Outcome in Patients with Colorectal Cancer Undergoing Surgical Intervention. J Mol Biomark Diagn 5: 162
- Lam L, Czerniecki BJ, Fitzpatrick E, Xu S, Schuchter L, et al. (2013) Interference-Free HER2 ECD as a Serum Biomarker in Breast Cancer. J Mol Biomark Diagn 4: 151
- Zia A, Khan S, Bey A, Gupta ND, MukhtarUnNisar S, et al. (2011) Oral biomarkers in the diagnosis and progression of periodontal diseases. Biol med
- van Bogaert LJ (2013) Immunostaining by Human Herpes Virus 8 Latent Nuclear Antigen-1 of Kaposi’s sarcoma: A Potential Biomarker of Severity of Disease?. J Mol Biomark Diagn S5-002
- Prima V, Cao M, Svetlov SI (2013) ASS and SULT2A1 are Novel and Sensitive Biomarkers of Acute Hepatic Injury-A Comparative Study in Animal Models. J Liver 2: 115
- Mas VR, Maluf DG (2013) Biomarker Discovery and Validation in Kidney Transplantation. J Mol Biomark Diagn 4: e115
- Nagananda MS, Sengupta A, Rehman SMK, Santhosh J, Anand S (2012) Identifying Prospective Biomarkers for Cognitive Impairments during Pregnancy – Review of Current Status and Some Preliminary Results. Gynecol Obstet (Sunnyvale) S: 8
- Cheekurthy AJP, Rambabu C, Kumar A (2015) Biochemical Biomarkers-Independent Predictors of Type 2 Diabetes Mellitus. J Bioanal Biomed
Citation: Manasa (2017) Epigenetics - Role as Biomarker in Cancer Diagnosis. J Oncol Res Treat 2: 114.
Copyright: © 2016 Manasa P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 9373
- [From(publication date): 0-2017 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 8099
- PDF downloads: 1274