Estimation of Anti-Tubercular Drug Resistance in Relapse TB Patients in Rims Hospital, Srikakulam
Received: 18-Mar-2019 / Accepted Date: 10-May-2019 / Published Date: 21-May-2019 DOI: 10.4172/2332-0877.1000404
Abstract
Relapse TB means the patient had a TB he was previously been treated for TB. Most of such patients had Multi-Drug resistance for first line TB treatment. Method and Materials: These studies were conducted based on observational study in this study we utilized quantitative method to find an effected population. Result: we gathered total 150 population in the period of six months in that 63 individuals were sent for CBNAT test (Rifampicin Resistant test) out of that 40 samples were got a positive results to Rifampicin Resistant, in that male 36 (57.1%) were higher than females 4(6.3%) and adults 31(49.2%) were more than the other age groups, based on the location Srikakulam zone were higher than the other zones. Conclusion: Relapse TB and Multidrug resistant caused due to failure of TB treatment, infected with drug resistant mycobacteria.
Keywords: Relapse Tb; Cartridge Based Nucleic Acid AmplificationTest (Cbnaat); Drug Resistance; Dot; Treatment; Acid Fast Bacilli; Dst;Drt; Patient
Abbrevations
AFB: Acid Fast Bacilli; CBNAAT: Cartridge Based Nucleic Acid Amplification Test; DOT: Directly Observed Treatment; DST: Drug Susceptibility Testing; DRT: Drug Resistance Testing; EMB/E: Ethambutol; HIV: Human Immunodeficiency Virus; INH/H: Isoniazide; MTB: Mycobacterium Tuberculosis; MDR: Multi Drug Resistance; PZA/Z: Pyrazinamide; PM: Proportion Method; PCR: Polymerase Chain Reaction; RMP/R: Rifampicin; RR: Rifampicin Resistance; RNTCP: Revised National Tuberculosis Control Program; S: Streptomycin; TB: Tuberculosis; WHO: World Health Organization; XDR: Extensive Drug Resistance.
Introduction
Drug resistance in Mycobacterium tuberculosis was defined as a decrease in the sensitivity of sufficient degree to be reasonably strain concerned is different from wild-type strains, which are isolated that never come into contact with the drug. The vast majority of drug resistance in the Mycobacterium tuberculosis complex is caused by single-nucleotide polymorphisms, although insertions or deletions are also possible [1]. Drug resistance Tuberculosis is characterized by both the types of drugs to which the bacteria lack susceptibility and the manner in which resistance was acquired. Resistance to single agents is the most common type resistance to multiple agents is less frequent but of greater concern [2].
TB is an airborne bacterial infection caused by mycobacterium tuberculosis which affects any part of the body and most commonly the lungs (Pulmonary TB). Live released tuberculin bacilli spread via the blood stream or lymphatic channels to any part of the body tissues or organs such as larynx, lymph nodes, spine, bones or kidneys (Extra Pulmonary TB) [3].
Patients who have previously been treated for TB, where declared cured or treatment completed at the end of their most recent course of treatment and are now diagnosed a recurrent episode of TB known as Relapse TB [4]. Recurrence of TB can be due to re-growth of same strain of mycobacterium tuberculosis (MTB) [5]. Recurrent TB poses significant threats, especially emergence of drug resistance which can pose challenges to TB control programs [5].
India is the country with the highest burden of TB. The WHO statistics for 2015 give an estimated incidence figure of 2.2 million cases of TB for India out of a global incidence of 9.6 million. It is estimated that about 40% of Indian population is infected with TB bacteria, the vast majority of whom have latent rather than active TB. The global data show that 32% of Relapse cases are actually Multidrug resistant TB [6]. Tuberculosis (TB) has been classically associated with poverty, overcrowding and malnutrition. Important social inequalities, HIV infections and drug or alcohol abuse may co-exit, all factors strongly associated with TB. The lack of adequate TB treatment, poor adherence, low treatment completion rates and absence of effective TB prevention and control programmers, lead to the development of drug resistance. HIV infections, extremes of age, diabetes, alcohol, severe malnutrition and anti-tumor necrosis alpha factor (TNF-α) these factors lead to TB. Socio-economic factors such as poor living conditions, homelessness, incarceration, poverty, tobacco use and alcohol abuse, place people who use drugs at higher risk for developing TB [7].
Types of drug resistance
• Resistance to one first-line Anti-TB drug only.
• Resistance to more than one first-line Anti-TB drug, other than both Isoniazide and Rifampicin.
• Resistance to at least both Isoniazide and Rifampicin.
• Resistance to any Fluoroquinolone and at least one of three secondline inject able drugs (Capreomycin,Kanamycin and Amikacin) in addition to multi drug resistance.
• Resistance to Rifampicin detected using Phenotypic or Genotypic methods, with or without resistance to other Anti-TB drugs. It includes any resistance to Rifampicin, in the form of Mono- Resistance, Poly-Resistance, MDR or XDR [8].
The World Health Organization has recently recommended the following terminology changes for the different types of resistance to Anti-TB drugs. Isolation of drug resistant Mycobacterium tuberculosis from patients without a history of previous treatment should be referred to as “ Drug Resistance Among New Cases ” (Primary Resistance).
Isolation of a drug-resistant strain from patients who have been treated for Tuberculosis for at least 1 month should be referred to as “ Drug Resistance Among Previously treated Patients ” (Acquired Resistance).
The more common of these occurrences is “Drug Resistance Among Previously Treated Patients” in which inadequate treatment or lack of adherence by the patient results in the selection of naturally occurring resistant mutants [8].Identifying the trends in the drug resistance is one of the important aspects in the assessment of TB epidemiologic trends and TB control planning.
The study included a review of the records for the period of 6 Months and the collection of information on personal characteristics, sputum mycobacteriologic studies and Anti-TB drug resistance detected by Drug Susceptibility Testing (DST) for first line agents. Retreatment patients were defined as patients who has previously received Anti-TB treatment for at least 1 month, including those with treatment failures and Relapse [9].
Globally accepted standard methods of Drug Susceptibility Testing (DST) are the Proportion Method (PM), the absolute concentration method, and the resistance ratio method. These methods are based on visual detection of slow growing colonies of Mycobacterium tuberculosis and can take up to 6 weeks that may be crucial for early initiation of intensive phase therapy and reduction of bacterial load in smear-positive cases [10].
Drug resistance in relapse TB
Most drug resistance in Relapse TB the Rifampicin resistants due to mutations in the rpoB gene that codes for the beta Sub-Unit of the RNA Polymerase. Isoniazid resistance due to mutations in the inh A and kat G causing an over expression of inh A, which decrease the affinity of isoniazid. Ethambutol resistance majorly caused by mutations in emb B gene, which codes for arabinosyl transferase, which is involved in the synthesis of arabinogalactan. Pyrazinamide resistance caused due to mutation in pnc A and ribosomal protein-1. Streptomycin resistance is by mutation in rps L and rrS genes [11].
Major characteristic of mycobacterium is that they are acid fast bacilli; that is once stained with aniline dyes, they are difficult to decolorize even when treated with a mixture of acid and phenol [12]. Gram positive, acid fast bacteria do not take the crystal violet into the cell wall, appearing very light purple rather than the deep purple of normal gram-positive bacteria [13]. These bacteria are gram positive, aerobic, non-sporulating, non-motile and often Pleomorphic [14].
Cartridge-based nucleic acid amplification test (CBNAAT) is a recently introduced polymerase chain reaction (PCR) based method for detection of TB. It also detects Rifampicin resistance as it targets the rpo-B gene of Mycobacteria. CBNAAT is a mycobacterium tuberculosis-specific automated, cartridge-based nucleic acid amplification assay, having full integrated and automated amplification and detection using real-time PCR, providing results within 100 minutes. It is a highly specific test as it uses 3 specific primers and 5 unique molecular probes to target the rpo B gene of Mycobacterium tuberculosis, which is the critical gene associated with Rifampicin resistance [15].Transmission occurs through exposure to tuberculin bacilli in air born droplet nuclei produced by people with pulmonary TB during expiratory efforts such as coughing or sneezing [16].
Materials and Methods
It is prospective and observational study design is to be conducted after taking ethical committee approval. Approaching to TB centers and collecting the Primary data from them. Then the collected data by CBNAAT test for the identification of Anti-Tubercular drug resistance in Relapse TB patients.
Study location
This study will be carried out in RIMS Hospital, it is a 12 multispecialty and 700 bedded capacity tertiary care Govt. District Hospital in Srikakulam.
Study approval
This study was approved by the Rajiv Gandhi Institute of Medical Sciences and RIMS Government General Hospital. Institutional Ethics Committee (EC registration no: ECR/492/Inst/AP/2013) Srikakulam.
Study setting
To approach the DOT Centers in RIMS Hospital and collect the Relapse TB patients all types of data in that CBNAAT test Rifampicin resistant result was using in this study.
Study criteria
a. Inclusion criteria: This study includes Relapsed Pulmonary Tuberculosis (R. PTB), Extra Pulmonary Tuberculosis (R.ETB), HIV and Relapse TB.
b. Exclusion criteria: Pregnancy and Lactation, Patients those who are unconscious, Patients those who have not signed in consent form.
Source of data
In this study we used primary data of the Relapsed Tuberculosis patient lab investigation CBNAAT results for the Anti-Tubercular drug resistance were collected.
Study procedure
We approached to the ethical committee by preparing protocol with proper study design and objectives of the study and got approval from the Institutional Ethical Committee RIMS Hospital, Srikakulam for conducting a study. Then we approached to the TB centers and TB wards to collect the Relapsed TB patient’s data from them. After collecting the data, then approach to the patient and follow-up them. Then the collected data by CBNAAT test for the identification of Anti- Tubercular drug resistance in Relapse TB patients. Those data were gathered for observing quantitative data for the Rifampicin resistant patients. For this study we are using data collecting annexure like patient consent form, standard questionnaires form. Based on this study requirement we can include or exclude the annexure and documented.
Collection and documentation of data
These study data were collected from primary sources like patients prescribed Anti-Tubercular Drug Resistant data from Relapse TB patients by using CBNAAT report annexure and these collected data is analyzed and documented.
Forms used in study
In this study we are using data collecting annexure like patient profile form, patient consent form, CBNAAT lab Report form. Based on this study requirement we can include or exclude the annexure.
Results
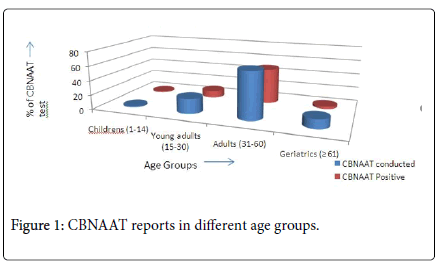
This study was conducted in 150 population of Relapse TB patients in that 63 were conducted CBNAAT test, from that 40 patients (63.5%) got a positive report towards Rifampicin resistance. As per the age criteria in our study, only 1 (1.6%) child (1-14) was send to CBNAAT test and got positive results, in these study this is the lower value. The highest value of this study Adults (31-60) patients 41 (65%) were send for CBNAAT test in that 31 (49.2%) Adults were got positive results from the total CBNAAT conducted, this data was summarized in Table 1 and Figure 1.
| Age | CBNAAT conducted |
Percentage | CBNAAT Positive |
Percentage |
|---|---|---|---|---|
| Children’s (1-14) | 1 | 1.6% | 1 | 1.6% |
| Young adults (15-30) | 13 | 20.6% | 6 | 9.5% |
| Adults (31-60) | 41 | 65% | 31 | 49.2% |
| Geriatrics (= 61) | 8 | 12.7% | 2 | 3.2% |
Table 1: CBNAAT reports in different age groups.
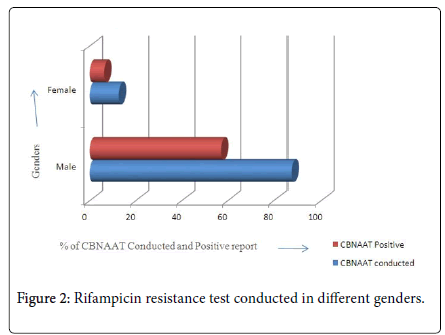
In our study, the total population were 150 Relapse TB patients in RIMS Hospital. In that 55 (87.3%) males patients samples were send to CBNAAT test for Rifampicin resistance in which 36 (57.1%) patients were got positive results in CBNAAT test. Same in the case of females, total 8 (12.7%) patients samples were send for CBNAAT test for Rifampicin resistance in that 4 (6.3%) which means half of the females patients got positive results in CBNAAT test. Based on gender difference, Males are more prone than Females to Rifampicin resistance done by CBNAAT test. The data was summarized in Table 2 and Figure 2. The study was conducted on 150 Relapse TB patients of 12 zones who were admitted in RIMS Hospital Srikakulam of 6 months duration. 63 (42%) patient samples from all 12 zones were send for CBNAAT test for the detection of Rifampicin resistance. In that zones, Sompeta region does not have any Relapse TB samples. Srikakulam register the maximum results as 13 (20.6%) patient samples were send and 11 (17.5%) patient samples were detected positive. In Ranasthalam, 2 (3.2%) patient samples were send and both of those are reported as positive of CBNAAT test. In the case of Palakonda and Naransannapeta, 3 (4.8%) patient samples were sending to CBNAAT test in that only 1 (1.6%) patient was reported as positive for Rifampicin resistance. In spite of all the zones, maximum cases detected in Srikakulam, minimum cases were detected in Ranasthalam and no cases detected in Sompeta.
| Gender | CBNAAT Conducted | Percentage | CBNAAT positive | Percentage |
|---|---|---|---|---|
| Male | 55 | 87.3% | 36 | 57.1% |
| Female | 8 | 12.7% | 4 | 6.3% |
Table 2: Rifampicin resistance test conducted in different genders.
All this data was summarized in Table 3, Figure 3. The total study population were 150 Relapse TB patients from 12 zones were admitted in RIMS Hospital Srikakulam in duration of 6 months, in that 63 (42%) patients were send for CBNAAT test for Rifampicin resistance from that 40 (26.7%) Relapse TB patients were finalized as CBNAAT positive. These samples were completely resistance towards Rifampicin were observed in our study, this data was summarized in Table 4, Figure 4.
| Location | CBNAAT Performed | Percentage | CBNAAT Positive | Percentage |
|---|---|---|---|---|
| Ponduru | 9 | 14.3% | 3 | 4.8% |
| Srikakulam | 13 | 20.6% | 11 | 17.5% |
| Tekkali | 6 | 9.5% | 4 | 6.3% |
| Rajam | 7 | 11.1% | 6 | 9.5% |
| Narasannapeta | 3 | 4.8% | 1 | 1.6% |
| Pathapatnam | 4 | 6.3% | 4 | 6.3% |
| Palasa | 6 | 9.5% | 3 | 4.8% |
| Amadalavalasa | 5 | 7.9% | 2 | 3.2% |
| Kotturu | 5 | 7.9% | 3 | 4.8% |
| Ranasthalam | 2 | 3.2% | 2 | 3.2% |
| Sompeta | 0 | 0% | 0 | 0% |
| Palakonda | 3 | 4.8% | 1 | 1.6% |
Table 3: Rifampicin resistance report of different zones.
| Name of the test | CBNAAT Conducted (63) | Percentage |
|---|---|---|
| CBNAAT Positive | 40 | 63.4% |
| CBNAAT Negative | 23 | 36.5% |
Table 4: CBNAAT resistance test was conducted in total population.
Discussion
The present study gives information about the prevalence of drug resistance among Relapse cases who receives DOTS category I, Treatment. In this study we are conducting CBNAAT test (Rifampicin resistance test) in 150 population were taken and 63 samples were send for the test, in that 40 (63.4%) samples were detected as Rifampicin Resistance but 23(36.5%) samples were not detected for Rifampicin resistance in CBNAAT test.
Our study had a higher population and detect a more Rifampicin resistance positive cases than Malhotra B.et.al; total population 44 Relapse TB patients in that 3(6.8%) Rifampicin resistance patients were found [17]. In our present study, Adults 31-60 age group 31(49.25%) were more affected and declared as Rifampicin Resistance than all other age groups. Anamika Gupta et.al; study says that Adults (91.7%) were more affected to Rifampicin resistance than other age groups [18] as like our study but it has higher affected subjects than our study. Nair et al. study highlights Adults (3.1%) was higher than other age groups [19] compare to our study this is lesser Rifampicin resistant individuals. Rajib Saha et.al; study declared Young Adults (53.9%) and Adolescents (53.9%) both age groups are same but these are compared to our study young adults 15-30 age group 6 (9.5%) was higher [20]. In our study we are categorized Rifampicin resistance based on Gender out of 150 population, 40 patients were Rifampicin resistance affected TB Relapsed patients, in these affected patients Males 36 (57%) were higher than the Female 4 (6.3%) patients. In our study Male 36 (57%) individuals were higher than the Male (13.7%) individuals of A.Thomas et.al; out of 62 Rifampicin resistance population [21]. Praveen.B.Gautham et.al; study says 38(30.6%) Male patients out of 168 population in that 44 (26.1%) Rifampicin resistance Relapse TB patients (22). By comparing this study with our study Male patients were near to lesser out of 150 population in that 40 Rifampicin resistance patients. Relapse TB patients admitted in RIMS Hospital Srikakulam was finalized Rifampicin resistance patients were 40 members out of 150 population in 6 months duration, we found in different zones in Srikakulam District in that Srikakulam zone 11 (17.5%) is higher than the Palakonda and Narasannapeta 1(1.6%) but in Sompeta had nil Rifampicin resistance Relapse TB patients. By comparing our study with Vishal Goyal et.al; study of Hyderabad region 14(13.7%) Rifampicin resistance Relapse TB patients out of 102 population [22] this is very lesser than our study but in Jaipur Malhotra B.et.al; were conducted a study by saying out of 44 population of Relapse TB patients in that Rifampicin resistance Relapse TB patients 3(6.8%) (17) were very few found than our study.
Conclusion
Our data demonstrated that Relapse TB is a major mechanism leading to TB re-occurrence in patients who are admitted in RIMS Hospital Srikakulam. Moreover Males patients had a higher risk of Rifampicin resistance than Females due to there occupational effects like over-crowding, travelling and unhygienic working areas. The reason behind the unfavorable outcomes of retreatment cases are irregular DOTS therapy, quality of drugs and health care. Adults in Srikakulam are more affected in our study due to immigration of more people from different areas in aspect of livestock. To control the reoccurrence of TB, RNTCP should implement still more awareness programs along with rural educational instituions for eradication of TB and conduct health screening camps in rural areas. This study better to continue in rural areas by the health care eduactional institutions as a student research studies.
Conflict of Interest
These study was conducted on the intension of observing the Rifampicin resistant Relapse TB patients for the benefit of avoiding prolong hospital stay and higher economic cost of health.
References
- Schon T, Miotto P, Köser CU, Viveiros M, Böttger E, et al. (2017) Mycobacterium Tuberculosis drug resistance testing: challenges, recent developments and perspectives. Clin Microbiol Infect 23:154-160.
- Nachega JB, Chaisson RE (2003) Tuberculosis drug resistance. A global threat. Clin Infect Dis 36:24-30.
- Akosua AA, Ofori-Asenso R (2017) Tuberculosis: An overview. J Public Health Emerg 1:7.
- Gadoev J, Asadov D, Harries AD, Parpieva N, Tayler-Smith K, et al. (2017) Recurrent tuberculosis and associated factors: A five-year countrywide study in Uzbekistan. PLOS ONE 12: e0176473.
- Sadikot R, Mirsaeidi M (2018) Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol 7: 1-6.
- Gupta A (2016) Study to determine Relapse and mortality rates in tuberculosis cases and their epidemiological correlations: A historical cohort study. Int J Med Clin Res 4: 14781-14786.
- Millet JP, Moreno A, Fina L, Baño LD, Orcau A, et al. (2013) Factors that influence current tuberculosis epidemiology. Eur Spine J 22: 539-548.
- Somoskovi A, Parsons LM, Salfinger M (2001) The molecular basic of resistance to Isoniazide, Rifampin and pyrazinamide in Mycobacterium Tuberculosis. Respir Res 2: 164-168.
- Shamaei M, Marjani M, Chitsaz E, Kazempour, Esmaeili M, et al. (2009) First-line anti-tuberculosis drug resistance pattern and trends at the national TB referral center in Iran: Eight years of surveillance. Int J Infect Dis 13: e236-e240.
- Kohli A, Bashir G, Fatima A, Jan A, Wani N, et al. (2016) Rapid drug susceptibility testing of Mycobacterium tuberculosis clinical isolates to first line anti-tuberculosis drugs by nitrate reductase assay: A comparison with proportion method. Departement of Microbiology. Int J Mycobacteriol 5: 469-474.
- Palomino J, Martin A (2014) Drug resistance mechanisms in mycobacterium tuberculosis. Antibiotics 3: 317-340.
- Reynolds J, Moyes RB, Breakwell DP (2009) Differential staining of bacteria: acid fast stain. Curr Protoc Microbiol.
- Dixit P, Kotra LP (2007) Disease caused by acid fast Bacteria. Pharmacology: 1-5.
- Coleman JP, Smith CJ (2007) Microbial Classification. The Comprehensive Pharmacology: 1-4.
- Walker R, Edwards C (2012) Clinical pharmacy and Therapeutics. 5th edition, Churchill Livingstone, London.
- Malhotra B, Pathak S, Vyas L, Katoch VM, Srivastava K, et al. (2002) Drug susceptibility profiles of Mycobacterium tuberculosis isolates at Jaipur. Indian J Med Microbiol 20: 140-146.
- Gupta A, Mathuria JP, Singh SK, Gulati AK, Anupurba S, et al (2011) Anti-Tubercular drug resistance in four health care facilities in North India. J Health Popul Nutr 29: 583-592.
- Nair SA, Raizada N, Sachdeva KS, Denkinger C, Schumacher S, et al. (2016) Factors associated with Tuberculosis and Rifampicin resistance Tuberculosis amongst symptomatic patients in India: A retrospective analysis. PLOS ONE 11: e0150054.
- Rajib Saha (2016) Predictors of treatment outcome for retreatment pulmonary tuberculosis among tribal people of Eastern India District: A prospective cohort study. Tuberc Res Treat: 1-8.
- Thomas A, Gopi PG, Santha T, Chandrasekaran V, Subramani R, et al. (2005) Predictors of relapse among pulmonary Tuberculosis patients treated in a DOTS program in South India. Int J Tuberc Lung Dis 9: 556-561.
- Gautam PB, Mishra A, Kumar S (2018) Prevalence of Rifampicin resistance Mycobacterium Tuberculosis and associated factors among presumptive Tuberculosis patients in Eastern Uttar Pradesh: A cross-sectional study. Int J Community Med Public Health 5: 2271-2276.
Citation: Allu H, Mudakala S, Singupuram M, Kaviti US (2019) Estimation of Anti-Tubercular Drug Resistance in Relapse TB Patients in Rims Hospital, Srikakulam. J Infect Dis Ther 7: 404. DOI: 10.4172/2332-0877.1000404
Copyright: © 2019 Allu H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3171
- [From(publication date): 0-2019 - Oct 05, 2025]
- Breakdown by view type
- HTML page views: 2355
- PDF downloads: 816