Research Article Open Access
Liquid Chromatography/Tandem Mass Spectrometry Method for Estimation of Cholic Acid in Rat Plasma, Urine and its Application
Surendra Yadav R1, 2* and Kilari Eswar Kumar31DMPK Laboratory (Biology Division), GVK BIO, Nacharam, Hyderabad, Andhra Pradesh, India
2Research and Development Cell, Jawaharlal Nehru Technological University, Kakinada, Andhra Pradesh, India
3Pharmacology Division, University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, Andhra Pradesh, India
- *Corresponding Author:
- Surendra Yadav R
DMPK Laboratory (Biology Division), GVK BIO, Nacharam
Hyderabad, Andhra Pradesh, India-500076
Tel: +919701532950
E-mail: suren.ravulapalli@gmail.com
Received date: July 13, 2014; Accepted date: August 11, 2014; Published date: August 14, 2014
Citation: Yadav SR, Kumar KE (2014) Liquid Chromatography/Tandem Mass Spectrometry Method for Estimation of Cholic Acid in Rat Plasma, Urine and its Application. J Anal Bioanal Tech 5: 200. doi: 10.4172/2155-9872.1000200
Copyright: © 2014 Yadav SR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Cholic acid is a primary bile acid synthesised from cholesterol in liver. Apart from being major catabolic products of cholesterol bile acids facilitates absorption of fats in intestine. A selective, sensitive MRM based liquid chromatography/ tandem mass spectrometry method has been developed and validated for the estimation of cholic acid in rat plasma and urine. Telmisartan was used as internal standard (IS). Electrospray ionization (ESI) probe with negative mode of operation was used for the ionisation of cholic acid and IS. Chromatographic separation was performed through X-bridge C18 column with the mobile phase of 0.1% formic acid in water (aqueous reservoir), 100% methanol (organic modifier). A short LC gradient of 3 minutes run time was used with flow rate of 0.7 mL/min. Charcoal stripped plasma and urine were used for the preparation of calibration standards and quality control samples. The analyte and IS were isolated from plasma and urine by a simple organic solvent based protein precipitation. The assay was linear in the concentration range of 31.26-10000 ng/mL. The method has been validated according to published FDA guidelines and showed excellent performance. The developed method was applied for the estimation of cholic acid in plasma and urine samples from liver toxicity experiment in rats. Liver toxicity was induced by intraperitoneal injection of carbon tetrachloride (CCl4).
Keywords
Bile acids; Cholic acid; Charcoal stripping; MRM; Fragment ion; Liver toxicity
Introduction
Bile acids are synthesised from Cholesterol in liver and plays important role in physiology [1]. Bile acids help the absorption of lipophilic nutrients in the intestine by emulsification and regulates cholesterol homeostasis. Bile acids control glucose, lipid and energy homeostasis [1-4]. Out of five bile acids two of them are synthesised directly from cholesterol in liver and called primary bile acids (Cholic acid Chenodeoxy cholic acid), remaining three bile acids are generated by intestinal bacteria from primary bile acids and called secondary bile acids (Ursodeoxy cholic acid, Deoxy cholic acid, Lithocholic acid) [1]. Cholic acid is formed from cholesterol by the activity of CYP7A1 enzyme (cholesterol 7α-hydroxylase.
A marked increase in plasma bile acids levels is observed in liver toxicity due to cholestasis [5-7]. Plasma and urine bile acid levels also serves as a marker for liver toxicity along with other markers for liver toxicity like plasma or serum alanine transaminase, total bilirubin. Cholic acid present in plasma and urine at significant levels as free form and in conjugation with Glycine and Taurine [8]. So many analytical techniques were reported for estimation of bile acids in plasma samples like high performance liquid chromatography (HPLC), gas-liquid chromatography (GLC), and radioimmuno assay (RIA) [8]. Mass spectrometric analysis is more specific than other analytical techniques and HPLC-MS/MS methods are more suitable for the estimation of bile acids in biological fluids and tissues [9]. Although some of the workers claimed that they used MRM mode of mass spectrometric methods, they used same parent and fragment ions which were not true MRM mode [1]. Current method describes the mass spectrometric method with MRM mode in which parent ion to specific fragment ion was monitored and this is more specific. Some of the methods used more than 10 minutes of run time with tedious procedures for bile acids extraction from biological fluids and tissues [10-18]. Current method was developed with a short run time of 3 minutes using MRM mode of detection. Cholic acid was extracted from rat plasma and urine samples using simple protein precipitation procedure using acetonitrile.
The quantitative determination of endogenous compounds in biological samples is more complicated both analytically and validation point of view. It is often difficult to obtain analyte-free samples of authentic biological matrix to construct calibration curve [19]. Cholic acid is present in rat plasma and urine as such as this is endogenous compound. For preparation of calibration curve and quality control samples plasma and urine were stripped with dextran coated charcoal [19]. The developed method is very sensitive and rapid compared to existing methods for bile acids analysis. Method was employed to estimate the cholic acid in plasma, urine samples of rats in Carbon tetrachloride induced liver toxicity experiment and the free cholic acid levels in plasma and urine were proved to be reliable markers for liver toxicity. This can be used to evaluate hepatoprotective activity of new chemical entities in preclinical models
Experiment
Materials
Cholic acid, telmisartan, dextran coated charcoal and olive oil were purchased from sigma-Aldrich Co. (St.Louis, MO, USA). Acetonitrile methanol, water (all HPLC grade), formic acid (90% pure), and carbon tetrachloride were purchased from Merck specialities pvt Ltd (Mumbai, India). Sprague dawley rats were procured from Bioneeds Ltd (Bangalore, India). Blood collection vacutainers (lithium heparin as anticoagulant) were purchased from BD (Franklin Lakes, USA). Alanine transaminase kit was purchased from Yerba.
Charcoal stripping of rat plasma and urine
Cholic acid is present in rat plasma and urine at significant levels and for the preparation of calibration standards and quality control samples blank matrix which is free of target analyte is needed. To remove endogenous basal levels of cholic acid, dextran coated charcoal was added to plasma and urine at concentration of 100 mg/mL and 50 mg/mL respectively followed by incubation for 4 hours with mixing at 4°C, after incubation samples were centrifuged for 30 minutes at 19650 g at 4°C and supernatant was separated leaving charcoal at bottom.
Preparation of calibration and quality control samples
Master stock solutions of cholic acid telmisartan were prepared in methanol at 1 mg/mL concentration. Working standard solutions of cholic acid were prepared by serial dilution from master stock in methanol: water (1:1 v/v). Working standard solutions were prepared at 25-fold higher concentrations to the calibration and quality control samples concentrations need to be achieved in plasma and urine. A total of nine calibration standards and four quality control samples were prepared. Calibration standards (31.26, 62.51, 208.37, 744.19, 2126.25, 4725.00, 7560.00, 9000.00, 10000.00 ng/mL) and quality control samples (32.05, 100.80, 5040.00, 8400.00 ng/mL) of cholic acid were prepared in plasma and urine by spiking 2 μL of the working standard solution in to 48 μL of blank plasma and urine. The working solution for IS (200 ng/mL) was prepared by dilution of an aliquot of master stock solution with acetonitrile. All cholic acid and telmisartan solutions were stored at 4°C in polypropylene tubes.
Sample preparation
A 50 μL aliquot of plasma and urine samples (blank control plasma, urine and samples from liver toxicity experiment) were transferred in to a 96 well polypropylene plate and cholic acid was extracted with acetonitrile containing internal standard. Samples were vortex mixed for 5 minutes at 1200 rpm and centrifuged at 4000 rpm for 5 minutes at 4°C. 100 μL of supernatant was separated and transferred in to a fresh plate for analysis and diluted with 200 μL of methanol: water (1:1, v/v), from this 10 μL was injected for LC-MS/MS analysis.
LC-MS/MS analysis
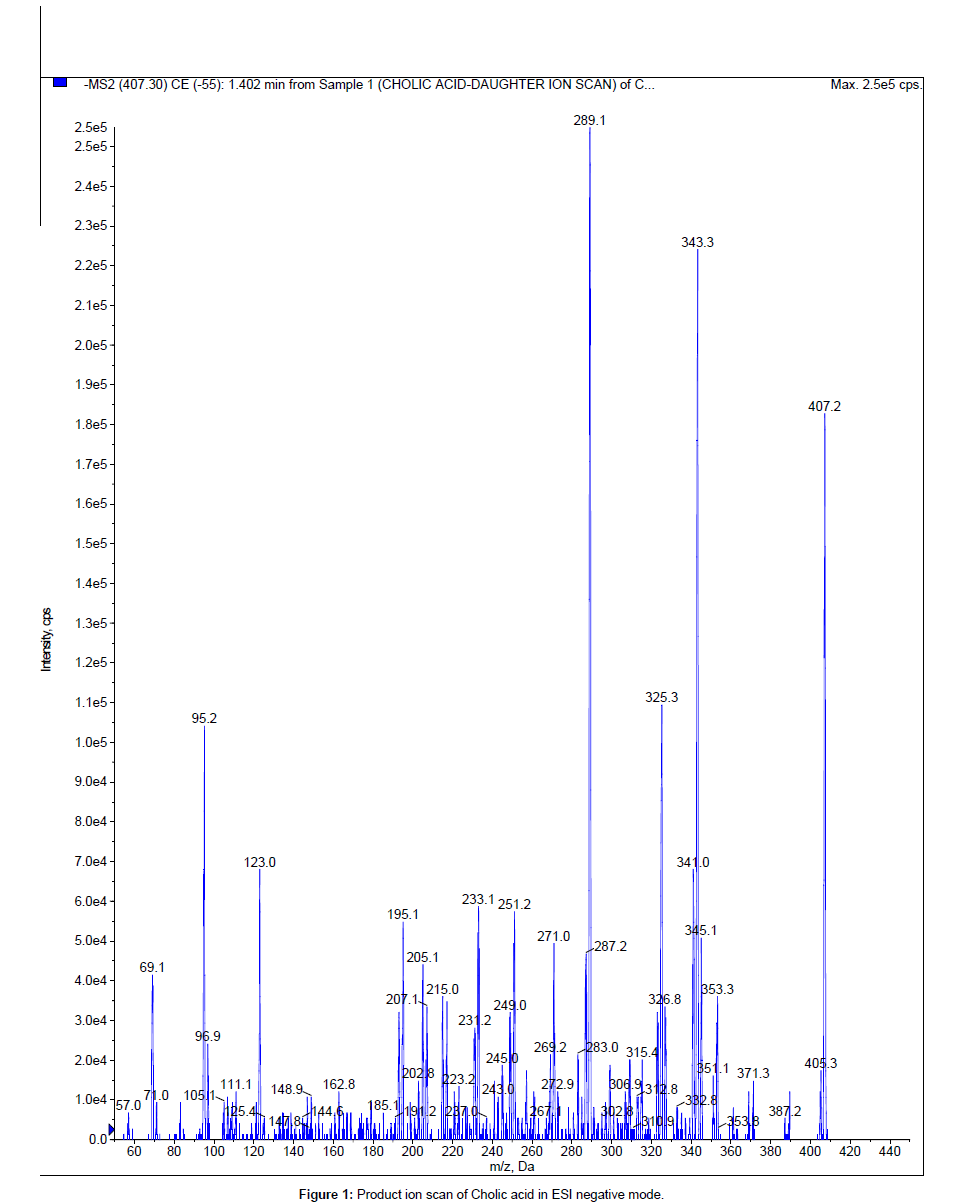
The LC system used was Shimadzu UFLC consisting of two isocratic pumps, a vacuum degasser, a temperature controlled micro well plate autosampler (further connected to rack changer to accommodate more plates) set at 4ºC and a thermostatic column oven set at 40°C (Shimadzu, Kyoto, Japan). The stationary phase used for the chromatography was X-bridge, C18 with 3.5 μm particle size and dimensions of 20×2.1 mm (Waters, Ireland). The mobile phase consisted of 0.1% formic acid in water (aqueous reservoir) and 100% methanol (organic modifier) was used at flow rate of 0.7 mL/minute. A common reverse phase gradient programme (time (min) / % B= 0.01/10, 0.70/95, 2.00/95, 2.10/10, 3.00/10) was used with a short run time of 3.0 minutes. All mass spectrometric detections were performed on API4000 triple quadrupole instrument (AB SCIEX, foster City, USA) with a turbo VTM ionisation source interface. Turbo V source with ESI probe was operated in negative ion mode for the LC-MS/MS (MRM) analysis. Data acquisition and processing for quantification were performed using Analyst software version 1.5 (AB SCIEX). The mass spectrometric conditions were optimized for the compounds by infusing a 500 ng/mL solution in methanol: water (1:1, v/v) at 10 μL/ minute flow rate using Harvard infusion pump (Harvard Apparatus, Holliston, USA) connected directly to the mass spectrometry. Flow dependent source parameters were optimised by flow injection analysis (FIA) with 0.7 mL/minute flow rate of mobile phase without column. The Turbo V source with ESI probe was operated with optimised settings as follows: polarity: negative, curtain gas: 20 psi, nebuliser gas (GS1): 50 psi, heater gas (GS2):55 psi, ion spray voltage: -4500 V, source temperature: 550°C. The mass spectrometry was operated in MRM mode in which both parent ion and fragment ion are fixed. The m/z value of cholic acid parent and fragment ions used were 407.3 and 343.10 (Figure 1) with optimum declustering potential (DP) and collision energy (CE) of -120 V and -44 V respectively. For telmisartan 513.20 and 287.00 were the m/z values used for parent and fragment ions with DP and CE of -60 V and -45 V respectively.
Method validation
The method was validated in SD rat plasma and urine separately. System suitability experiment was performed by injecting six consecutive injections using aqueous standard mixture of cholic acid and IS at the start of each batch during the method validation. The method was validated for selectivity, linearity, precision and accuracy of calibration standards and quality control (QC) samples, recovery, matrix effect, stability of cholic acid in different conditions. The percent deviation of the mean from nominal concentrations expressed as percent accuracy serves as measure of accuracy, coefficient of variation (% CV) serves as measure of precision. Selectivity was performed in six lots each of charcoal stripped plasma and urine and were checked for interference at the retention time of cholic acid.
Three precision and accuracy batches were analysed on different days (batch1 on day1, batch 2 and 3 on day 2) consisting of nine nonzero calibration standards and four QC samples (LLOQC, LQC, MQC, HQC). Six replicates of QC samples at each level were analysed in every batch to evaluate intra and interday precision and accuracy. The area ratio of analyte to IS was used for regression analysis. Each calibration curve was analysed individually by using least square weighted (1/x2) linear regression. The lowest standard on the calibration curve was accepted as lower limit of quantitation (LLOQ), if the analyte area in blank plasma or urine sample is Ë?20% of analyte area in lowest standard. The deviation of calibration standards (except LLOQ) and for QC samples (except LLOQC) from nominal concentrations should not be more than ± 15%, for LLOQ and LLOQC it should not be more than ± 20%. Recovery was calculated by comparing cholic acid area in extracted QC samples (LQC, MQC, HQC) against area in post extraction spiked QC samples. Matrix effect was evaluated in a quantitative manner by comparing cholic acid area in post extraction spiked QC samples against area in neat solutions at all QC levels and it was reported as matrix factor. To assess post preparative stability, six replicates of LQC, HQC were processed and kept in autosampler at 4°C and analysed against freshly prepared calibration standards. To evaluate freeze-thaw stability, six replicates of LQC, HQC were processed after three freeze-thaw cycles and analysed against freshly prepared calibration curve. Bench top stability of cholic acid in plasma and urine samples at room temperature was evaluated by keeping six replicates of LQC, HQC for 6.0 hours at room temperature, processed and analysed against freshly processed calibration curve standards. The samples were considered stable if the deviation of stability QC samples was within ± 15%.
Dilution integrity experiment was conducted by diluting the stock solution prepared as spiked standard at 50000 ng/mL concentration. The precision and accuracy for dilution integrity QC sample at the dilution factor of 10 (5000 ng/mL) was determined by analysing the samples (6 replicates) against calibration curve standards. Carry over experiment was performed by running two injections of extracted blank sample from same well and higher calibration standard in between. The difference in analyte area between two blank samples was considered as carry over.
Application
The bioanalytical method was applied to the liver toxicity study in sprague-dawley (SD) rats. Two groups of SD rats (each group containing six rats) were used in the study. Group 1 was dosed 2 mL/kg of olive oil through intraperitoneal route and serves as control group. Group 2 was dosed with 2 mL/kg of olive oil: carbon tetrachloride (1:1, v/v) through intraperitoneal route, final dose of carbon tetrachloride is 1 mL/kg [20-22]. Serial blood samples were collected into vacutainers containing lithium heparin as anticoagulant. Blood samples were collected at 24, 48, 72 hrs after CCl4 dosing. At each time point 200 μL of blood was collected by retro orbital puncturing. Plasma was separated by centrifugation at 4000 rpm for 10 min at 4°C, from this 25 μL was used for alanine transaminase estimation and remaining volume was stored frozen at -80°C until cholic acid assay. Rats were maintained in metabolic cages during the experiment to collect urine samples of 0-24 Hr, 24-48 Hr, and 48-72 Hr time periods. Increase in plasma alanine transaminase levels was used to confirm liver toxicity. Free cholic acid levels were estimated in plasma and urine samples of both the groups.
Results and Discussion
LC-MS/MS analysis
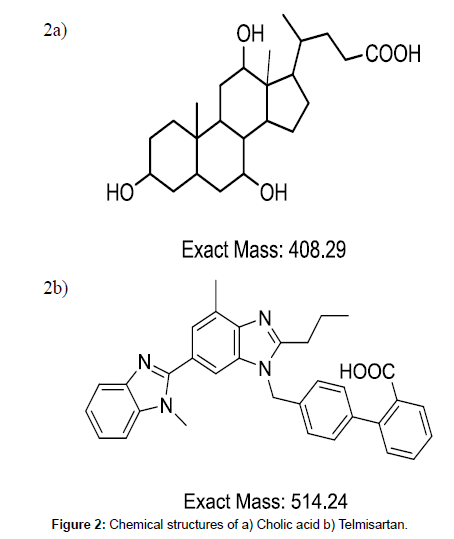
Presence of carboxylic group (Figure 2a) resulted favourable sensitivity for cholic acid in ESI negative mode. Structurally similar compounds or deuteriated derivatives are better to use as internal standard. Telmisartan was used as internal standard although it is not structurally similar to cholic acid. Deuteriated derivatives are very expensive to procure. Other bile acids which are very close to cholic acid in chemical structure cannot be used as they will interfere in quantitation of plasma and urine samples due to presence of their basal levels. Telmisartan will ionise in both positive and negative mode of ionisation with good sensitivity due to presence of terminal methyl groups and carboxylic group respectively (Figure 2b). Telmisartan chromatography was well studied in our lab and found to be suitable for any C18 column with general gradient of starting with low organic and increasing to high organic followed by coming back to high aqueous. Telmisartan can be used with any extraction technique with good recovery. With ESI interface both cholic acid and telmisartan formed single charged ions [M-H]- at m/z 407.30 and m/z 513.20 respectively. Cholic acid and IS both have fragment ions at m/z 343.10 and m/z 287.00 respectively.
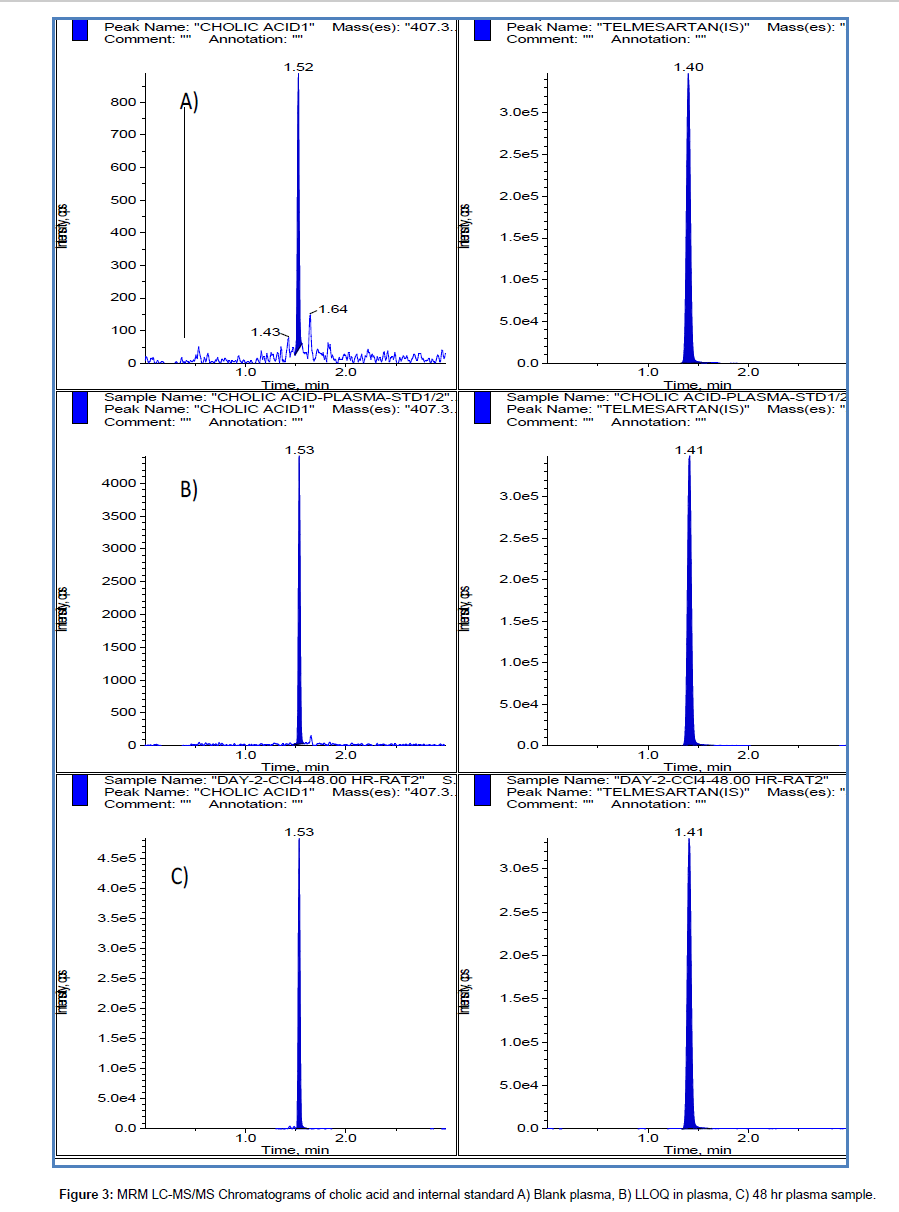
A waters X-bridge C18 column using gradient elution has been used successfully for chromatography. A short run time of 3 minutes was used without any ion suppression and interference peaks from plasma and urine samples. With ammonium acetate as aqueous component of mobile phase low sensitivity was observed for cholic acid. With acetonitrile as organic modifier very sharp peak with good sensitivity was observed at LLOQ level but the response was not linear with concentration at higher concentrations of calibration curve. Finally 0.1% formic acid in water and 100% methanol were used as mobile phase components. Sample chromatograms for blank, LLOQ, application study samples were shown in Figures 3 and 4 for plasma and urine respectively.
Optimization of sample preparation
Solid phase extraction and liquid-liquid extraction are very common techniques employed for extraction of endogenous compounds from biological matrices to avoid interference from other endogenous compounds of same group. Matrix effect will be minimised with SPE and LLE, but performing SPE is expensive and LLE is tedious procedure and involved many of the steps where there is a high chance of manual errors. A simple protein precipitation with acetonitrile containing IS was developed and no matrix effect was observed. Four times of acetonitrile to sample volume was used for effective precipitation for both plasma and urine samples. High recoveries were achieved from both the matrices.
Method validation
The developed method was validated in both plasma and urine to meet the acceptance criteria of industrial guidance for bioanalytical method validation [23]. Throughout the method validation, the % CV of system suitability (area ratio) was observed below 4%. Calibration curves were linear from 31.26–10000 ng/mL. Linear regression analysis with 1/(x × x) weighing resulted optimum accuracy of corresponding calculated concentrations at each level (Tables 1 and 2). Intra and inter assay performance for QC values were mentioned in Tables 3 and 4 for plasma and urine respectively. Stability studies results were summarised in Table 5 for plasma and urine. Recovery of cholic acid was found to be 73.87%, 90.20% among 3 QC levels from plasma and urine with precision of 3.6, 0.5% CV values respectively. Matrix factor for cholic acid from plasma and urine samples were 0.92 and 0.95 among 3 QC levels with % CV values of 3.7, 0.1. Dilution integrity was performed with a dilution factor of 10, accuracy and precision values for dilution QC samples were 100.59%, 4.5% for plasma and for urine these were 99.56%, 4.60%.
| Concentration (ng/mL) | Statistical parameters | ||||||
| Actual conc. | Calculated con. | Mean | SD | % CV | Accuracy | ||
| set-1 | set-2 | set-3 | |||||
| 31.26 | 31.48 | 31.19 | 31.13 | 31.27 | 0.187 | 0.6 | 100.03 |
| 62.51 | 62.33 | 64.05 | 62.38 | 62.92 | 0.979 | 1.6 | 100.65 |
| 208.37 | 196.10 | 191.59 | 213.18 | 200.29 | 11.389 | 5.7 | 96.12 |
| 744.19 | 792.70 | 771.43 | 771.45 | 778.53 | 12.274 | 1.6 | 104.61 |
| 2126.25 | 2178.08 | 2165.30 | 2185.57 | 2176.32 | 10.249 | 0.5 | 102.35 |
| 4725.00 | 4953.16 | 5031.85 | 4869.14 | 4951.38 | 81.370 | 1.6 | 104.79 |
| 7560.00 | 7736.82 | 7657.28 | 7808.41 | 7734.17 | 75.600 | 1.0 | 102.30 |
| 9000.00 | 8261.12 | 8364.61 | 7866.51 | 8164.08 | 262.847 | 3.2 | 90.71 |
| 10000.00 | 9755.35 | 9960.23 | 9813.10 | 9842.89 | 105.639 | 1.1 | 98.43 |
Table 1: Calculated concentrations of cholic acid calibration standards prepared in rat plasma (n=3).
| Concentration (ng/mL) | Statistical parameters | ||||||
| Actual conc. | Calculated con. | Mean | SD | % CV | Accuracy | ||
| set-1 | set-2 | set-3 | |||||
| 31.26 | 31.68 | 32.74 | 31.49 | 31.97 | 0.676 | 2.1 | 102.29 |
| 62.51 | 59.98 | 56.61 | 62.33 | 59.64 | 2.877 | 4.8 | 95.40 |
| 208.37 | 212.56 | 200.77 | 194.78 | 202.71 | 9.045 | 4.5 | 97.28 |
| 744.19 | 826.14 | 846.87 | 825.25 | 832.75 | 12.232 | 1.5 | 111.90 |
| 2126.25 | 2076.24 | 2052.71 | 1998.78 | 2042.58 | 39.713 | 1.9 | 96.06 |
| 4725.00 | 4828.67 | 4877.41 | 4865.82 | 4857.30 | 25.461 | 0.5 | 102.80 |
| 7560.00 | 6668.24 | 6819.50 | 7186.92 | 6891.55 | 266.737 | 3.9 | 91.16 |
| 9000.00 | 8994.92 | 8879.41 | 8623.43 | 8832.59 | 190.123 | 2.2 | 98.14 |
| 10000.00 | 10171.52 | 10591.60 | 10730.62 | 10497.91 | 291.083 | 2.8 | 104.98 |
Table 2: Calculated concentrations of cholic acid calibration standards prepared in rat urine (n=3).
| Type | Statistical parameter | Concentration (ng/mL) | |||
| LLOQC (32.05) |
LQC (100.80) |
MQC (5040.00) |
HQC (8400.00) |
||
| Intraday, set-1, N=6 |
Mean | 30.06 | 101.98 | 4879.78 | 8258.0 |
| SD | 2.326 | 3.777 | 245.857 | 241.88 | |
| % CV | 7.7 | 3.7 | 5.0 | 2.9 | |
| Accuracy | 93.80 | 101.17 | 96.82 | 98.31 | |
| Intraday, set-2, N=6 |
Mean | 32.77 | 104.35 | 4887.30 | 8262.61 |
| SD | 1.242 | 4.094 | 262.359 | 260.361 | |
| % CV | 3.8 | 3.9 | 5.37 | 3.15 | |
| Accuracy | 102.26 | 103.52 | 96.97 | 98.36 | |
| Intraday, set-3, N=6 |
Mean | 29.54 | 101.49 | 4802.93 | 8046.87 |
| SD | 1.706 | 6.969 | 233.780 | 280.376 | |
| % CV | 5.77 | 6.87 | 4.87 | 3.48 | |
| Accuracy | 92.17 | 100.68 | 95.30 | 95.80 | |
| Inter day, N=18 |
Mean | 30.79 | 102.60 | 4856.67 | 8189.15 |
| SD | 2.242 | 5.006 | 235.873 | 266.434 | |
| % CV | 7.28 | 4.88 | 4.86 | 3.25 | |
| Accuracy | 96.08 | 101.79 | 96.36 | 97.49 | |
Table 3: Precision and accuracy of cholic acid in plasma quality control samples.
| Type | Statistical parameter | Concentration (ng/mL) | |||
| LLOQC (32.05) |
LQC (100.80) |
MQC (5040.00) |
HQC (8400.00) |
||
| Intra day, set-1, N=6 |
Mean | 34.00 | 108.06 | 4980.72 | 8230.2 |
| SD | 1.475 | 3.744 | 199.151 | 285.62 | |
| % CV | 4.3 | 3.5 | 4.0 | 98.0 | |
| Accuracy | 106.09 | 112.61 | 98.82 | 97.98 | |
| Intra day, set-2, N=6 |
Mean | 31.87 | 4.12 | 4998.89 | 8209.88 |
| SD | 2.046 | 3.657 | 111.851 | 320.507 | |
| % CV | 6.4 | 3.9 | 2.24 | 3.90 | |
| Accuracy | 99.44 | 111.71 | 99.18 | 97.74 | |
| Intra day, set-3, N=6 |
Mean | 31.84 | 106.58 | 5096.46 | 8126.53 |
| SD | 1.441 | 4.013 | 249.840 | 148.745 | |
| % CV | 4.53 | 3.76 | 4.90 | 1.83 | |
| Accuracy | 99.34 | 105.74 | 101.12 | 96.74 | |
| Interday, N=18 |
Mean | 32.57 | 109.08 | 5025.36 | 8188.88 |
| SD | 1.889 | 4.561 | 190.888 | 250.692 | |
| % CV | 5.80 | 4.18 | 3.80 | 3.06 | |
| Accuracy | 101.62 | 108.22 | 99.71 | 97.49 | |
Table 4: Precision and accuracy of cholic acid in urine quality control samples.
Application study
The validated method was successfully applied to CCl4 induced liver toxicity study in order to establish free cholic acid plasma and urine levels as markers for liver damage. Liver toxicity was confirmed by increased plasma levels of Alanine transaminase in CCl4 induced group compared to control group. Alanine transaminase was estimated by kit which involves IFCC method, kinetic.
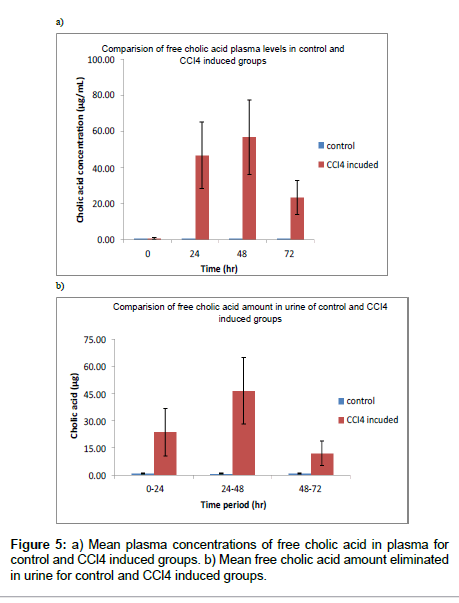
Plasma free cholic acid levels were increased very high in 24, 48 hr samples (Table 6 and Figure 5a) and recovery was observed in 72 hr samples. For urine samples amount of free cholic acid was calculated and found huge increase in CCl4 induced group compared to control group (Table 7 and Figure 5b). The method is useful to confirm liver damage by measuring free cholic acid level in urine as urine collection is non-invasive technique.
| Storage condition | Analyte concn. (ng/mL) |
Calculated con.(ng/mL) | % CV | Accuracy (%) | |||
| Plasma | Urine | Plasma | Urine | Plasma | Urine | ||
| Three freeze-thaw cycles | 100.80 | 105.96 ± 3.091 | 94.66 ± 4.737 | 2.9 | 5.0 | 105.1 | 93.9 |
| 8400.00 | 7902.81 ± 34.742 | 8059.43 ± 180.754 | 0.4 | 2.2 | 94.1 | 95.9 | |
| Autosampler (24 hr) |
100.80 | 106.28 ± 4.775 | 107.68 ± 2.734 | 4.5 | 2.5 | 105.4 | 106.8 |
| 8400.00 | 7878.79 ± 168.324 | 7851.06 ± 285.283 | 2.1 | 3.6 | 93.8 | 93.5 | |
| Bench top (6 hr) |
100.80 | 104.81 ± 2.272 | 100.65 ± 5.470 | 2.2 | 5.4 | 104.0 | 99.9 |
| 8400.00 | 8037.10 ± 133.286 | 8057.51 ± 259.936 | 1.7 | 3.2 | 95.7 | 95.9 | |
Table 5: Summary of stability studies for cholic acid in plasma and urine.
| Time(hr) | Free cholic acid concentration (µg/mL) | |||||
| Control group | CCL4 induced group | |||||
| Mean | SD | % CV | Mean | SD | % CV | |
| 0 | 0.54 | 0.313 | 58.1 | 0.73 | 0.552 | 76.0 |
| 24 | 0.64 | 0.284 | 44.7 | 46.81 | 18.425 | 39.4 |
| 48 | 0.69 | 0.185 | 27.0 | 56.83 | 20.805 | 36.6 |
| 72 | 0.66 | 0.250 | 37.8 | 23.33 | 9.594 | 41.1 |
Table 6: Free cholic acid plasma levels in control and CCl4 induced rats, N=6.
| Time(hr) | Free cholic acid amount eliminated in urine (µg ) | |||||
| Control group | CCL4 induced group | |||||
| Mean | SD | % CV | Mean | SD | % CV | |
| 0-24 | 1.12 | 0.764 | 68.3 | 23.88 | 13.047 | 54.6 |
| 24-48 | 0.77 | 0.434 | 56.4 | 46.55 | 18.352 | 39.4 |
| 48-72 | 1.09 | 0.761 | 69.6 | 12.19 | 6.621 | 54.3 |
Table 7: Free cholic acid amount eliminated in to urine in control and CCl4 induced rats, N=6.
Conclusion
A rapid, sensitive MRM based LC-MS/MS method for the determination of cholic acid in rat plasma and urine has been successfully developed and validated using protein precipitation as sample preparation procedure. The assay method demonstrated acceptable sensitivity (LLOQ: 31.26 ng/mL), precision, accuracy, recovery and stability. The validated method was successfully employed to analyse rat plasma and urine samples and represented the plasma, urine levels of free cholic acid in CCl4 induced liver toxicity experiment. Free cholic acid plasma and urine levels were proved to be good markers for liver damage in rat model. This approach can be used successfully to evaluate hepatoprotective activity of new chemical entities and herbal formulations in preclinical models.
Acknowledgements
Authors are thankful to Pratima Srivastava and Satish kumar for their guidance and cooperation during the work. Our special thanks to the management of GVK Biosciences, Hyderabad, India for allowing us to carrying out this work.
References
- Suzuki Y, Kaneko R, Nomura M, Naito H, Kitamori K, et al. (2013) Simple and rapid quantitation of 21 bile acids in rat serum and liver by UPLC-MS-MS: effect of high fat diet on glycine conjugates of rat bile acids. Nagoya J Med Sci 75: 57-71.
- Li T, Chiang JY (2012) Bile Acid signaling in liver metabolism and diseases. J Lipids 2012: 754067.
- Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J (2009) Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol 15: 804-816.
- Hofmann AF (2009) The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci (Landmark Ed) 14: 2584-2598.
- Chang CW, Beland FA, Hines WM, Fuscoe JC, Han T, et al. (2011) Identification and categorization of liver toxicity markers induced by a related pair of drugs. Int J Mol Sci 12: 4609-4624.
- Singh A, Bhat TK, Sharma OP (2011) Clinical Biochemistry of Hepatotoxicity. J Clinic Toxicol S4:001.
- Neale G, Lewis B, Weaver V, Panveliwalla D (1971) Serum bile acids in liver disease. Gut 12: 145-152.
- Street JM, Trafford DJ, Makin HL (1986) Capillary gas-liquid chromatography of glycine-conjugated bile acids without prior hydrolysis. J Lipid Res 27: 208-214.
- Griffiths WJ, Sjvall J (2010) Bile acids: analysis in biological fluids and tissues. J Lipid Res 51: 23-41.
- Perwaiz S, Tuchweber B, Mignault D, Gilat T, Yousef IM (2001) Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J Lipid Res 42: 114-119.
- Burkard I, von Eckardstein A, Rentsch KM (2005) Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 826: 147-159.
- Ando M, Kaneko T, Watanabe R, Kikuchi S, Goto T, et al. (2006) High sensitive analysis of rat serum bile acids by liquid chromatography/electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal 40: 1179-1186.
- Alnouti Y, Csanaky IL, Klaassen CD (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873: 209-217.
- Yang L, Xiong A, He Y, Wang Z, Wang C, et al. (2008) Bile acids metabonomic study on the CCl4- and alpha-naphthylisothiocyanate-induced animal models: quantitative analysis of 22 bile acids by ultraperformance liquid chromatography-mass spectrometry. Chem Res Toxicol 21: 2280-2288.
- Bobeldijk I, Hekman M, de Vries-van der Weij J, Coulier L, Ramaker R, et al. (2008) Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: compound class targeting in a metabolomics workflow. J Chromatogr B Analyt Technol Biomed Life Sci 871: 306-313.
- Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ, et al. (2010) High performance liquid chromatography-tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878: 51-60.
- Want EJ, Coen M, Masson P, Keun HC, Pearce JT, et al. (2010) Ultra performance liquid chromatography-mass spectrometry profiling of bile acid metabolites in biofluids: application to experimental toxicology studies. Anal Chem 82: 5282-5289.
- Huang J, Bathena SP, Csanaky IL, Alnouti Y (2011) Simultaneous characterization of bile acids and their sulfate metabolites in mouse liver, plasma, bile, and urine using LC-MS/MS. J Pharm Biomed Anal 55: 1111-1119.
- van de Merbel NC (2008) Quantitative determination of endogenous compounds in biological samples using chromatographic techniques. Trends in Analytical Chemistry 27: 924-933.
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A (2008) Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 73: 399-409.
- Kumar PV, Sivaraj A, Elumalai EK, Kumar BS (2009) Carbon tetrachloride-induced hepatotoxicity in rats - protective role of aqueous leaf extracts of Coccinia grandis. International Journal of PharmTech Research 1: 1612-1615.
- Eidi A, Mortazavi P, Bazargan M, Zaringhalam J (2012) Hepatoprotective Activity of Cinnamon Ethanolic Extract against CCl4-Induced Liver Injury in Rats. EXCLI Journal 11: 495-507.
- US Department of Health and Human Services (2001) Guidance for Industry-Bioanalytical Method Validation. Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15372
- [From(publication date):
September-2014 - Aug 25, 2025] - Breakdown by view type
- HTML page views : 10695
- PDF downloads : 4677