Evaluation of [18F]FDG Synthesis and Quality Control: Experience in the Seoul National University Hospital (SNUH), Seoul, South Korea
Received: 05-Sep-2021 / Accepted Date: 20-Sep-2021 / Published Date: 27-Sep-2021 DOI: 10.4172/2167-7964.1000340
Abstract
Objective: The purpose of this study was to evaluate the radiochemical yield at the end of synthesis and the quality of synthesized [18F]FDG according to the acceptance criteria mentioned in different Pharmacopeia (USP, EP) and Korea Food and Drug Administration (KFDA) to reduce the hazards of the patients and obtain the best quality of image for proper diagnosis.
Method: Upon bombardment with a 16.5MeV cyclotron, Oxygen-18 enriched water is transformed into [18F]-fluoride ion. [18F]FDG was prepared by nucleophilic fluorination of Mannose triflate followed by basic hydrolysis. The entire synthesis was performed forty-six times using the chemistry module FASTlab2. The quality of produced [18F] FDG in the SNUH, South Korea was evaluated in a standard quality control laboratory. Radiochemical purity, radionuclide purity, chemical purity, pH, endotoxin tests were performed to ensure the quality of [18F] FDG before release. Sterility tests (FTM and TSB) and residual solvents (Acetonitrile and Ethanol) were analyzed after the release of the radiotracer.
Result: The average decay corrected yield was (81.52 ± 8.33) % more than the average non-decay corrected yield (71.20 % ± 8.02 %). The TLC result of [18F] FDG showed more than 95% radiochemical purity. Gamma emission spectrum shown more than 99.5% of the gamma emissions correspond to 511KeV photons. The results of the other quality control parameters were in the desired range. The total produced [18F] FDG in forty-six batches had been used for the treatment of around 2100 patients and found no immediate or afterward hazards to any patients.
Conclusion: The quality of synthesized [18F] FDG fulfilled all the requirements of USP, EP & KFDA and the radiochemical yield were in an acceptable range.
Keywords: Synthesis yield, Quality control
Introduction
Fludeoxyglucose (18F-FDG) is the most successful radiotracer in the expanding medical imaging technology of Positron Emission Tomography (PET) which is used for the diagnosis, staging, and restaging of several clinical conditions such as lung cancer, colorectal cancer, lymphoma, melanoma, head and neck cancer, brain and breast cancer . FDG-PET also has been used in neurology, cardiology and inflammation/infection [1-7].
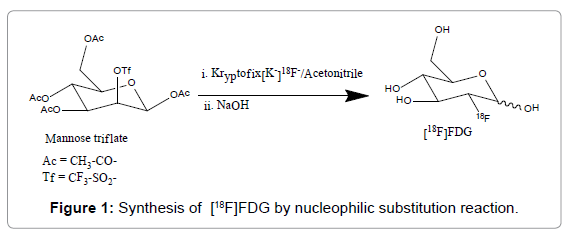
[18F]FDG synthesis initiates with the production of [18F]fluoride in a cyclotron, usually in a target chamber containing [18O]water, followed by a nucleophilic substitution reaction with 1,3,4,6-tetra-Oacetyl- 2-O-trifluoromethanesulfonyl-β-Dmannopyranose (mannose triflate) [8], and subsequent removing of protecting groups (acetyl) by acidic or basic hydrolysis, resulting in FDG formation (Figure 1). Following GMP regulations, the synthesis is performed in an automated synthesis module placed inside a hot cell. The mannose triflate (precursor molecule) is a sugar molecule containing a wellsuited leaving group (trifluoromethanesulfonyl) at carbon-2 for a facile nucleophilic substitution reaction, while the other four potential reaction sites are protected with four acetyl groups [1,5].
Installing a medical cyclotron (13 MeV, EBCO, Canada), [18F] FDG was first synthesized in the department of Radiopharmaceutical Production, Research and Development (RPRD), SNUH in 1995. After that a variety of radiopharmaceuticals such as [11C] PIB, [11C] MET, [18F] FDOPA, [18F] FLT, [18F] AV1451, [13N] NH3 etc. have been produced for clinical research, diagnosing and treating disease. In 2011, for radiopharmaceuticals production and their quality assurance, a state-of-the-art facility to comply with the GMP regulations was installed in Cancer Imaging Center of Cancer Hospital, SNUH. In addition, new 16.5 MeV medical cyclotrons (GEMS, Sweden) were installed and produced many radionuclides (11C, 13N, 15O, 18F) including metal radionuclide (64Cu, 89Zr, etc.). From the last few years (3.5-4.0) Ci [18F]FDG has been being synthesized routinely in two batches for the treatment of 40-50 patients per day [9].
To meet the increasing demand for [18F] FDG requires an increase in production without a decrease in quality. The quality control criteria of [18F]FDG are listed in USP, EP, BP, Chemistry Manufacturing and Controls (CMC) section from United States Food and Drug Administration (US FDA) and KFDA [2,10]. The purpose of this study was to evaluate the radiochemical yield and the quality of synthesized [18F]FDG in some consecutive runs and compare with the acceptance criteria of different Pharmacopeia and KFDA to reduce the hazards of the patients and obtain the best quality of image for proper diagnosis. Several tests were performed to determine radionuclides purity, radiochemical purity, chemical purity, pH, residual solvent, sterility, and bacterial endotoxin level.
Materials and Methods
FDG Synthesis
[18F]FDG was synthesized in forty-six independent runs with standard method from mannose triflate with alkaline hydrolysis as initially proposed in Ref.[11]. Highly pure ≥ 98%) H218O was purchased from Taiyo Nippon Sanso Corporation (Tokyo, Japan). Cyclotron GE PETtrace10 with high yield niobium target (GEMS, Uppsala, Sweden) was the source of anionic fluorine. Produced activity in 18O (p, n) 18F reaction with 16.5 MeV proton beam current at 40-50μA, was 2.05 ± 0.55 Ci (55.50–96.20 GBq) after 30-50 min of irradiation. The activity (aqueous [18F]-fluoride) was transferred to the FASTlabTM2 unit (GE Healthcare, Belgium), where the synthesis and purification were performed within 24 min. In the synthesis path, the aqueous [18F]- fluoride ion was passed through an ion-exchange column to trap the [18F]-fluoride ions. [18F]-fluoride was then eluted to the reaction vessel with a mixture of potassium carbonate and Kryptofix 2.2.2, then water was removed by isotropic drying with acetonitrile and [18F]-fluoride reacted with mannose triflate. After alkaline hydrolysis, the solution was purified with a sequence of tC18 and alumina N-cartridges and eluted with distilled water (DW). The final product was diluted to 22 mL with DW and passed through a 0.2 μm SuporTM AEF filter (Pall medical, Switzerland) and collected in shielded sterile empty 20 mL Vials (ALK, USA) (Figure 1).
Quality control of 18F-FDG
Identification: tests were performed as described in Ref [12]. For γ-spectrometry, Canberra multichannel analyzer (MCA) with Highpurity Germanium (HPGe) detector (Canberra, USA) was used. Halflife was measured with CRC®-25PET Dose Calibrator (Capintec, USA). 100-300 μL (2.5–7.5mCi) sample was taken in 2mL vial (ALK USA). The initial and final activity of [18F]FDG sample were measured at 10 min intervals and an average value was calculated as an observed halflife of 18F by using the standard formula t1/2 = (0.693 × t) ÷ ln(A0/ At), where: t1/2 (half-life), t (time interval in minutes), A0 (initial activity), At (activity measured after 10 min) [5]. Radiochemical identity of synthesized [18F]FDG was also confirmed by comparison of retention factor (Rf) to the certified reference standard (CRS) of the main compound (ABX, Rydberg, Germany).
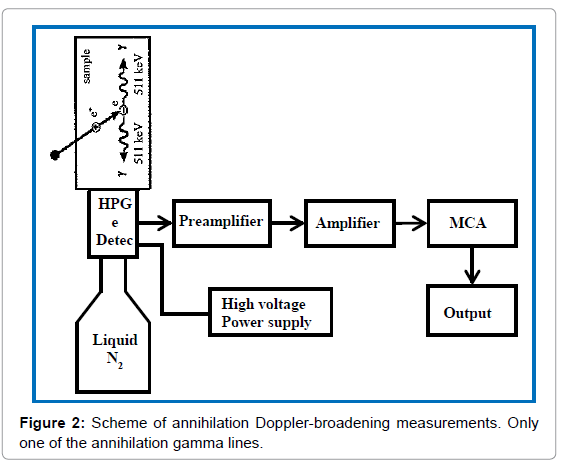
Radionuclides purity: is defined as the ratio between the radio activities of the desired radionuclide to the total activity of a radioactive compound, expressed as a percentage according to the Pharmacopoeia [12]. Radionuclide sample cannot be 100% pure and contains some contaminants which originate from the production process or the decay of the primary radioisotope. The radiation dose received by the patient is increased by radionuclide impurities and degrades the quality of the imaging procedure performed. The radionuclide purity (USP) of 18F radionuclide can be identified by the gamma spectrum detection and half-life measurement [6]. In the quality control laboratory, Canberra multichannel analyzer (MCA) with High-purity Germanium (HPGe) detector (Canberra, USA) is used to detect the gamma spectrum immediately after synthesis. An aliquot (<1 μL) of the [18F] FDG solution was mixed in a 20 mL DW containing vial and placed on the detector. Then the interaction of the emitted positron of 18F with the DW emitted 511 keV gamma photons. These Gamma photons were absorbed in the HPGe detector produced electrical pulse and the pulse amplitude was proportional to the energy deposited in the detector, which allowed for the measurement of gamma-ray energies (Figure 2).
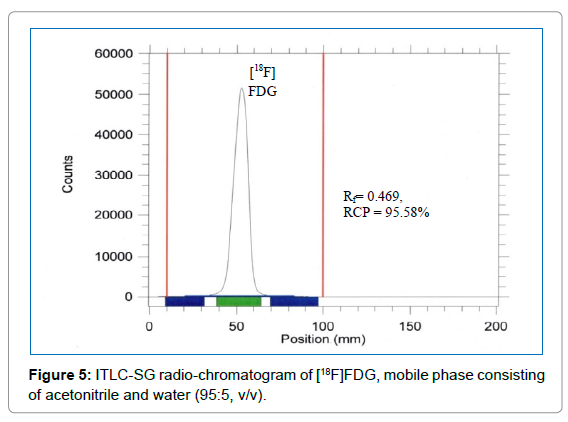
Radiochemical Purity (RCP): The radiochemical purity of [18F] FDG was determined by silica gel thin-layer chromatography (radio- TLC). Radio-TLC strips (1.5 × 9 cm) were marked at 1cm from one end as an origin, and an aliquot (≤ 2 μL) of the tracer solution was spotted onto the origin. The strips were placed in a solvent tank and developed in a 95:5 acetonitrile-water mixture until the solvent mixture reached the top of the strip. RCP was easily determined by scanning radio-TLC strips using a Bio-Scan AR-2000 system imaging scanner (Bios-can, WI).
Chemical purity: Ethanol, Acetonitrile and Kriptofix (K222) are the possible chemical contaminants in the synthesized [18F]FDG solution and the amount of these chemicals were measured to ensure the chemical purity of [18F]FDG.
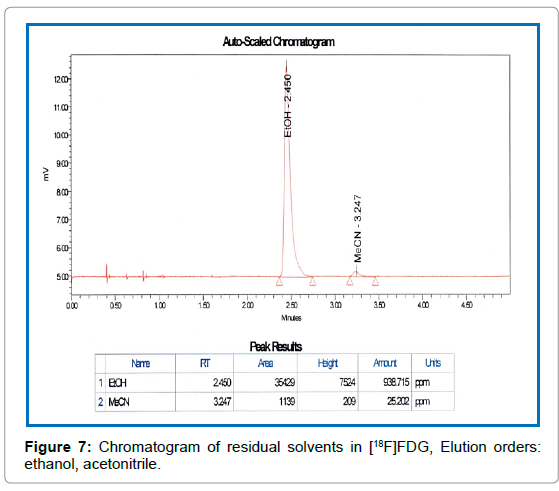
Solvents residues: The amount of residual solvent (Ethanol and Acetonitrile) was measured by Clarus 680 Gas Chromatograph (PerkinElmer, Singapore) [13]. 0.5 μL of [18F]FDG internal standard (solution of ethanol 5 mg/g and acetonitrile 0.41 mg/g in water) and synthesized QC sample solution were injected separately into a hot injector port of the Gas Chromatograph (GC). The temperature of the injector (250) was higher than the components boiling points. The gaseous components (evaporated sample at injector) of the [18F]FDG were pushed by carrier gas nitrogen on to the GC capillary column (Elite-624, dimension 30 m × 0.53 mm × 3.0 μm, PerkinElmer, USA). The separation of the organic compounds taken place between the carriers gas (pressure: 3.7psi, flow rate: 5 mL/min) nitrogen (mobile phase) and the high boiling liquid (stationary phase) within the GC capillary column (at constant temperature 50). Each component passed through an FID detector just before exits the GC instrument. The detector was heated at 250 by Hydrogen gas (flow rate: 40mL/min) / Air (flow rate: 450 mL/min) flame. The mixture of organic components reached the detector at varying times to oxidize and produced electrically charged particles (ions). The ions had been collected and produced an electrical signal. The signal was sent by detector to the recorder through an amplifier. The result with all peaks had been sent to the system computer which displayed, stored and analyzed the data
Kryptofix222: Two TLC silica gel 60 supports with chromogen substrate (Caltech, Italy) were taken and placed inside a shielded fume hood. 5 μL of the [18F]FDG injectable solution or kryptofix standard solution titled with 50 μg/mL (Caltech, Italy) was gently added at the center of O-ring of the TLC separately from the vertical position. After 4 minutes 10 μL of development solution (solution S, Caltech, Italy) was gently added at the center of each TLC from the vertical position and dried for 5 min.
pH measurement:Narrow range pH paper verified by the standard pH buffers displays a colour change for each 1.0 pH unit and gives an approximate value of pH. However, pH meter gives specific value of a substance [10]. In SNUH properly calibrated pH meter (Orion Star A221, Thermo Scientific) and PEHANON pH. (Germany) paper both have been used to check the pH of [18F]FDG. According to EP the physiological range of pH for [18F]FDG is 4.5-8.5 and it has been maintained from batch to batch.
Sterility and bacterial endotoxins (LAL test):Bacterial endotoxin (BET) and sterility tests are evaluated for the conformation of the microbiological purity of the final [18F]FDG product.
The sterility test is initiated within a reasonable period of time, which allows for the decay of radioactivity. A sterility test involves the incubation of a test sample for the cultivation of microorganisms with two different growth media, Tryptic Soy Broth (TSB) and Fluid Thioglycollate Medium (FTM) (10 mL fill, Hardy Diagnostics, Santa Maria, CA) at different temperature 22.5°C and 32.5°C respectably for 14 days. Three tubes were taken for each liquid culture media. [18F]FDG sample was added to two tubes (0.1mL sample to each tube) and other one was incubated without adding sample (negative control). The tubes were monitored for up to 14 days.
The chromogenic method quantifies bacterial endotoxins by means of a portable test system Endosafe®-PTS (Charles River Laboratory). This device includes a pumping system, a portable spectrophotometer and built-in software for sample data analysis. 200μL endotoxin-free water is added to 5 μL [18F]FDG sample for 41 times dilution and then added the diluted sample (25μL) into each sample reservoirs of limulus gametocyte lysate (LAL) reagent containing cartridge (Endosafe®-PTS, Charleston, SC) without creating bubbles. It took around 10 min to get the printed report.
Results And Discussion
[18F]FDG synthesis yield, radionuclide purity, radiochemical purity, chemical purity, microbiological purity and pH of synthesized [18F]FDG had been evaluated (Table-2) at the Seoul National University Hospital laboratory.
[18F]FDG synthesis yield
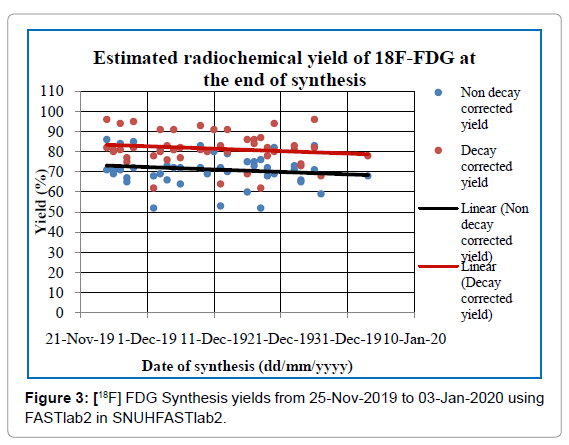
The radioactivity concentration has been considered as an important parameter in the [18F]FDG stability. Free radicals are formed due to auto radiolysis of water with ionizing radiation [14]. [18F]FDG samples with radioactivity concentration (20-130)mCi/mL and with trace amount (0.1% -0.4%) ethanol remained stable over 16h [15]. That’s why radiochemical yield is very important to synthesized FDG with proper radioactive concentration. [18F]FDG is the most widely produced radiopharmaceutical in SNUH and, as evaluated, the nondecay corrected synthesis yield (mean ± SD) was (71.20 ± 8.02) % and decay corrected yield was (81.52 ± 8.33) % as shown in Figure 3. The radioactive concentration of [18F]FDG was kept 24 mCi/mL to 116 mCi/mL (Figure 3).
Identification
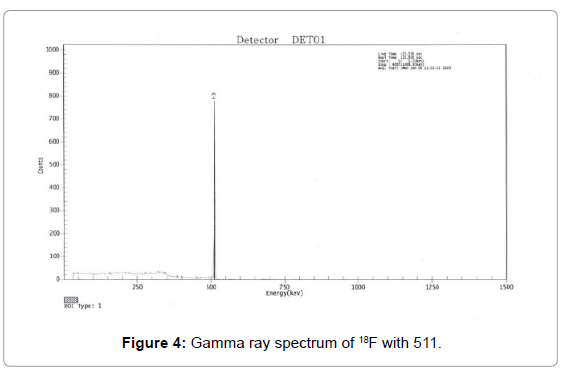
18F has been identified by recording the main gamma peak at 511 keV(Figure 4) and determination of half-life (108.56 ± 2.45). Radiochemical identity of [18F]FDG was confirmed, comparing the Rf of reference standards (cold FDG) with the observed value of Rf in the radio-TLC chromatograph of the [18F]FDG. The observed value of RF was within 0.4-0.6.
Radionuclide Purity
Gamma-ray spectrometry: Gamma spectrum was recorded for identification and determination of radionuclides purity [10]. All the [18F]FDG samples synthesized in forty-six batches, showed no other peak except the peak with energy 511 keV as shown in Figure 4. From the spectrum it was confirmed that radionuclides purity was more than 99.5% in all batches (Figure 4).
Half-life test:The 18F half-life (109.7 min) was almost the same as the mean of the measured half-life (108.56 min) of [18F]FDG shown in Table 1. For all batches, the measured mean half-life (106.5 - 110.5 min) found in good agreement with the expected range (105-115 min) mentioned in Pharmacopeia [10] (Table 1)
| No. | Time | Initial activity (mCi) | Final activity (mCi) | Half-life (Min) |
Average Half-life (Mean ± SD) |
|---|---|---|---|---|---|
| 1 | 11:51-12:01 | 5.51 | 5.17 | 108.80 | (108.56 ± 2.45)min |
| 2 | 12:01-12:11 | 5.17 | 4.84 | 105.09 | |
| 3 | 12:11-12:21 | 4.84 | 4.54 | 108.32 | |
| 4 | 12:21-12:31 | 4.54 | 4.23 | 112.02 |
Table 1: Radio activity measured by CRC®-25PET Dose Calibrator and calculated average half-life for one [18F] FDG sample.
Radiochemical Purity
The TLC method has been used in the quality control laboratory to test the RCP of the radiotracer [18F]FDG. Forty-six consecutive batches were analyzed. The free 18F- (Rf = 0.0), [18F]FDG (Rf = 0.4-0.6) and partially hydrolyzed tetra acetyl impurities (Rf = ~0.5-1.0) were determined quantitatively [10,16]. A typical radio-chromatogram is shown in Figure 5. Average RCP of all samples of [18F]FDG was (96.70 ± 0.38)% with the lowest result 95.58%. For the TLC method, the radioactivity of [18F]FDG is not less than 95% at Rf ~0.45 mentioned in EP [10,16] (Figure 5).
Chemical purity
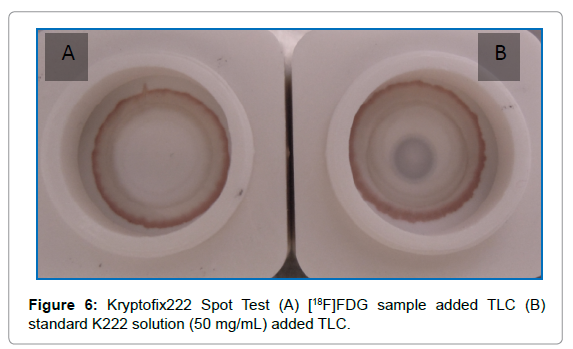
Kryptofix spot test: K222 has toxicity, that’s why USP and FDA recommend maximum contents of kryptofix equivalent to 50μg/mL in the injectable solution. As its toxicity is more than acetonitrile, according to Good Laboratory Practice (GLP), it is better to keep Kryptofix concentration in an [18F]FDG injectable solution as low as possible. In the case of kryptofix standard solution added TLC silica gel substrate, a bluish-colored ring was formed (Figure 6B) but no ring was formed at [18F]FDG injectable solution added TLC silica gel substrate (Figure 6A). This confirmed that the amount of K222 in the FDG was negligible (Figure 6).
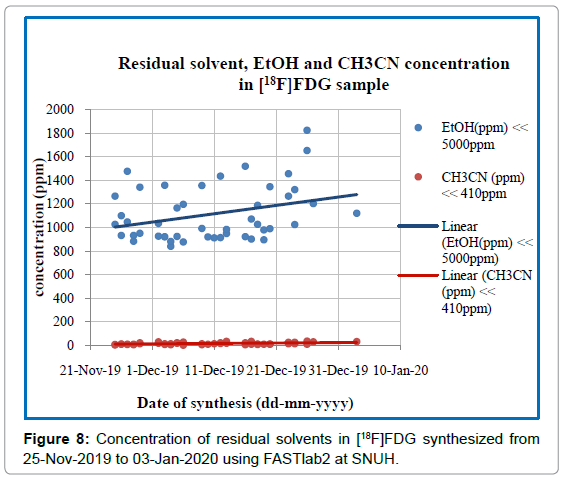
Residual solvent test:The concentration of acetonitrile and ethanol in all samples was quantitatively determined by the GC equipped with FID detector. The separation of acetonitrile and ethanol was completed at 3.5 min (Figure 7) with a total analysis time of 5 min. Detailed results of all [18F]FDG samples during the whole period of production are shown in Figure 8, where the average content of ethanol is (1113.92 ± 231.5) ppm and (CH3CN) is (16.59 ± 9.03) ppm. The maximum concentration of ethanol and acetonitrile in the forty-six FDG samples was found to be 1824.235ppm and 37.425ppm respectively, which was much lower than their acceptance limit (Figure 8).
Sterility and bacterial endotoxins test
The test tubes were visually examined. No turbidity was found during the [18F]FDG incubation period (14 days) of the [18F]FDG test sample with both TSB and FTM Medium. [18F]FDG samples successfully passed this test. During the whole FDG synthesis period no unsterile product was detected. The results of the sterility test of each FDG synthesis were the same as in Figure 9 (n=2). Also the sensitive apyrogenity test showed that the average bacterial endotoxins level was always deep below the acceptance limit (<175/V EU) mentioned in pharmacopoeia for intravenous applications (Figure 9 and Table 2).
| Quality parameter | Specification | Results | Comment |
|---|---|---|---|
| 1. Appearance | Colorless/Clear /No particle | þ Colorless þ Clear þ No Particle |
 |
| 2. Bacterial Endotoxin Test | < 175 EU/V |  |
|
| 3. Kryptofix2.2.2 Test | < 50µg/mL |  |
|
| 4. PH | 4.5-8.5 | 6.5 ± 0.05 |  |
| 5. Radiochemical Purity | > 95% | 99.95 % |  |
| 6. Half Life | 105-115min | 109.5 ± 0.21 min |  |
| 7. Gamma-ray Energy | 511/1022KeV | 511 KeV |  |
| 8. Residual ACN | < 410 ppm | 30.2 ± 18.32 ppm |  |
| 9. Residual Ethanol | < 5000 ppm | 980.3 ± 25.36 ppm |  |
| 10. Sterility test (FTM & TSB) | No microbial growth | No colony was found |  |
Table 2: QC results summer.
Conclusion
The results of this evaluation shown high radiochemical yield of [18F]FDG as expected. The radionuclides purity was more than 99.5% and radiochemical purity was more than 95%. The quality of final product fulfilled all the requirements set by relevant regulations. No immediate or delayed side effects have been reported after injecting the patients. In SNUH, [18F]FDG production has been adapted well to cGMP for routine clinical use.
Acknowledgement
We gratefully acknowledge the support of this work by the personnel of PET/ CT Unit, Department of Nuclear Medicine, Seoul National University Hospital, and Seoul, South Korea.
References
- Cyclotron Produced Radionuclides: Guidance on Facility Design and Production of [ 18F] Fluorodeoxy glucose (FDG) 172.
- Khwaj A (2014) 18F-FDG Synthesis and Quality Control and cost effectiveness in nuclear medicine centre in KHMC. Zagazig Univ Med J 20:1-8.
- Kilian K. (2014) Synthesis, quality control and determination of metallic impurities in 18F-fludeoxyglucose production process. Rep Pract Oncol Ther 19:S22-S31.
- Noh D.Y (1999) Detection of cancer in augmented breasts by positron emission tomography. Eur J of Surgery 165:847-851.
- Rahmani S (2017) Synthesis Quality Control and Stability Studies of 2-[18F] Fluoro-2-Deoxy-D-Glucose (18F-FDG) at Different Conditions of Temperature by Physicochemical and Microbiological Assays. Iran J Pharm Sci 16:602.
- Yu S (2006) Review of 18F-FDG synthesis and quality control. Biomed. Imaging Interv J 2.
- Zhang X (2016) Automated synthesis of [18F] (2S, 4R)-4-fluoroglutamine on a GE TRACER labâ„¢ FX-N Pro module. Appl Radiat Isot 112:110-114.
- Hamacher K HH, Coenen, G.Stöcklin (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using amino polyether supported nucleophile substitution. J Nucl Med 27:235-238.
- The department of Radiopharmaceutical Production, Research and Development (RPRD), Seoul National University Hospital, Seoul, South Korea
- Hung J C (2002) Comparison of various requirements of the quality assurance procedures for 18F-FDG injection. J Nucl Med 43:1495-1506.
- Füchtner F (1996) Basic hydrolysis of 2-[18F] fluoro-1, 3, 4, 6-tetra-O-acetyl-D-glucose in the preparation of 2-[18F] fluoro-2-deoxy-D-glucose. Applied Radiation and Isotopes 47:61-66.
- Walters L R (2012) Stability evaluation of 18F-FDG at high radioactive concentrations. J. Nucl Med Technol 40:52-56.
- Búriová E (2005) Auto radiolysis of the 2-deoxy-2-[18F] fluoro-D-glucose radiopharmaceutical. J Radioanal Nucl Chem 264:595-602.
- Dantas N M (2013) Radiolysis of 2-[18F] fluoro-2-deoxy-d-glucose ([18F] FDG) and the role of ethanol, radioactive concentration and temperature of storage, Appl Radiat Isot 72:158-162.
- Knust E J, Machulla H J, Dutschka K (1982) Radiopharmaceuticals 4: 18F-labelling with water target produced 18F Synthesis and quality control of 18F-3-deoxy-3-fluoro-D-glucose. Radiochemical and Radio analytical Letters 55:21-28.
- Fludeoxyglucose (2002) (18F) injection, in European Pharmacopeia, 4th Edition, European Directorate for the Quality of the Medicines: Strasbourg, France 2316-2319.
Citation: Uddin J, Rahman MM, Cho YH, Yoon CS, In WJ, et al. (2021) Evaluation of [18F]FDG Synthesis and Quality Control: Experience in the Seoul National University Hospital (SNUH), Seoul, South Korea. OMICS J Radiol 10: 340. DOI: 10.4172/2167-7964.1000340
Copyright: © 2021 Uddin J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 6038
- [From(publication date): 0-2021 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 5170
- PDF downloads: 868