Research Article Open Access
Evaluation of the Optimal Injection Solution in Hybrid Endoscopic Submucosal Dissection (ESD) for Various Organs in an Ex Vivo Porcine Model
Yuta Nakagawa, Kenshi Matsumoto*, Akihito Nagahara, Tsutomu Takeda, Kohei Matsumoto, Hiroya Ueyama, Yuji Shimada, Daisuke Asaoka, Mariko Hojo and Sumio Watanabe
Department of Gastroenterology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-Ku, Tokyo 113-8421, Japan
- *Corresponding Author:
- Kenshi Matsumoto
Department of Gastroenterology
Juntendo University School of Medicine
2-1-1 Hongo, Bunkyo-Ku
Tokyo 113-8421, Japan
Tel: 81-3-3813-3111
Fax: +81-3-3813-8862
E-mail: kmatumo@juntendo.ac.jp
Received date: December 06, 2015 Accepted date: December 21, 2015 Published date: December 28, 2015
Citation: Nakagawa Y, Matsumoto K, Nagahara A, Takeda T, Matsumoto K et al. (2015) Evaluation of the Optimal Injection Solution in Hybrid Endoscopic Submucosal Dissection (ESD) for Various Organs in an Ex Vivo Porcine Model. J Gastrointest Dig Syst 5:366. doi: 10.4172/2161-069X.1000366
Copyright: © 2015 Nakagawa Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: For flat and/or depressed lesions ranging from 11-20 mm, hybrid ESD, i.e., EMR with circumferential incision (CI), is now prevalent. However, there is no clear standard for choosing an injection solution. Sodium hyaluronate (SH) is often used, despite its disadvantages. This study aimed to clarify the most effective injection solution for safe hybrid ESD for trainees in various gastrointestinal tract organs.
Methods: CI was performed on 30 resected porcine esophagi, stomachs, duodena and colons. The following three solutions were injected into submucosa, and their ability to maintain mucosal elevation height (MEH) was evaluated: Solution A, normal saline (NS); Solution B, 1:1 mixture of NS and 0.4% SH; and Solution C, 0.4% SH. We measured the minimum snarable MEH and the average procedure time for snaring, and the optimal concentration of SH was identified both graphically and histopathologically.
Results: The lesion-lift ability was superior at higher SH concentrations except, for duodenum. No solution obtained sufficient MEH in duodenum. The minimum snarable MEH was 5.02 mm, 5.73 mm, and 6.05 mm, and the snaring procedure time was 58.8 s, 56.2 s, and 75.3 s in esophagus, stomach, and colon, respectively. Hybrid ESD could be performed successfully with Solution B for esophagus, Solution A for stomach and Solution C for colon, and these results were confirmed histopathologically.
Conclusions: The optimal solutions for hybrid ESD in an ex-vivo porcine model for trainees were Solution B for esophagus, Solution A for stomach, and Solution C for colon. Further study is needed for duodenum.
Keywords
Endoscopic mucosal resection (EMR); Endoscopic Submucosal dissection (ESD); Hybrid endoscopic submucosald Dissection (ESD); Sodium hyaluronate; Injection solution
Introduction
When performing endoscopic resection, en bloc resection plus R0 resection is desirable for a successful treatment outcome. Endoscopic mucosal resection (EMR) is widely used to remove nonpolypoid early stage neoplastic lesions of the gastrointestinal (GI) tract that are 10 mm or less in diameter because lesions greater than 11 mm may result in a piecemeal resection [1-7]. Endoscopic submucosal resection (ESD) allows en bloc resection, regardless of tumor size, but it is difficult to perform and can cause problematic complications [8,9]. A combination of circumferential incision (CI) followed by EMR is now prevalent for the removal of larger (11-20 mm) flat or depressed-type lesions [1,10-13]. This procedure has been named hybrid ESD [14,15].
Hybrid ESD is useful because it is a shorter and simpler procedure than ESD and because it fills the treatment gap for patients who require more treatment than EMR. In hybrid ESD, mucosal elevation height (MEH) after CI can vary significantly depending on the composition of the injection solution and the organ treated. This variability can lead to complications, such as the underlying muscle layer being gripped when snaring a lesion with a low MEH, which results in perforation; central portions of the lesion being left behind; or piecemeal resection. The most effective way to avoid these risks is to use submucosal injections to create an adequate submucosal fluid cushion between the lesion and the muscle layer.
Sodium hyaluronate (SH) is widely used clinically for endoscopic submucosal injection because of its high efficacy and low toxicity [16,17]. Nonetheless, the use of high-concentration SH has some disadvantages because it can cause defects in transpiration due to electrification, it can confuse gripping the muscle layer by increasing the grip force of snaring, and it has a very high cost. Therefore, the SH concentration should only be high enough to obtain sufficient MEH for a safe resection. In a past report, the ability to obtain MEH was examined only in an EMR model. No published studies have examined the effect of SH concentration on MEH after a CI for hybrid ESD, and variations in concentration have not been considered for different organs. Therefore, our first aim was to examine the change in MEH after injection in a CI model in various porcine organs. Furthermore, hybrid ESD can be performed in a short time compared with ESD because the process of dissection is omitted by snaring; as a result, if sufficient MEH is provided to resect a lesion safely, then a high concentration of SH is not necessary. Moreover, as mentioned above, because ESD is difficult, it is important that even trainees be able to perform hybrid ESD safely. Therefore, our second aim was to examine the correlation between the trainee’s procedure time for snaring and the minimum snarable MEH and to optimize the concentration of SH required for successful hybrid ESD by trainees in various porcine organs. The present study examines the snaring method, which is safe and has minimal disadvantages; therefore, we did not focus on the difficulties in circumferential incision and instead highlighted the process after the circumferential incision.
Materials
Circumferential incision models
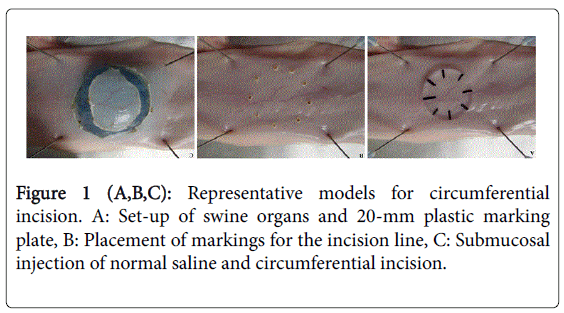
Fujishiro et al. reported that performing controlled, reproducible MEH and precise MEH measurements in live pigs is very difficult; therefore, they conducted their studies on resected specimens [18]. This study used porcine organ specimens (esophagus, stomach, duodenum, and colon), acquired from a company that provides no human animal models for research, within 2 hours of resection to best simulate in vivo conditions. This study was granted an exemption from requiring ethics approval by the Institutional Animal Care and Use Committee of The Juntendo University. Each specimen was stretched flat neutrally on a rubber board with pins, and the lesions were marked precisely with dots by tracing around the circumference of a 20-mm circular plastic plate (Figure 1A) using a Dual Knife (KD-650Q; Olympus Co., Tokyo, Japan) set at forced-coagulation mode (Effect3, 30 W) (ICC350; ERBE, Tübingen, Germany) (Figure 1B). To avoid the influence of solution, we used only NS for CI. A 23G needle was employed for the submucosal injection of indigocarmine and NS in the esophagus, stomach, and colon, and a 25G needle was used in the duodenum because of the thinner and more muscular mucosa. We used an ITknife 2 (KD-611 L; Olympus Co.) for CI of the stomach, and an ITknife nano (KD-612 L; Olympus Co.) (Figure 1C) set at the end-cut mode (Effect 4, 50 W) for the esophagus, duodenum, and colon. The CI models were made from 30 specimens of each organ. The CI models used in the following experiments were performed adequately by the same endoscopist (Y.N.) because the quality of CI can affect values such as MEH and procedure time.
Statistics
All data are expressed as the mean ± standard deviation. The results of MEH were analyzed using the Mann-Whitney U test. The odds ratio, absolute difference, 95% confidence interval and p values are reported. Statistical significance was defined as p<0.05. All statistical analyses were performed using the SPSS® Statistics 17.0 software (SPSS Inc., Chicago, IL, USA).
Methods
Method 1: Changes in mucosal elevation height after injection
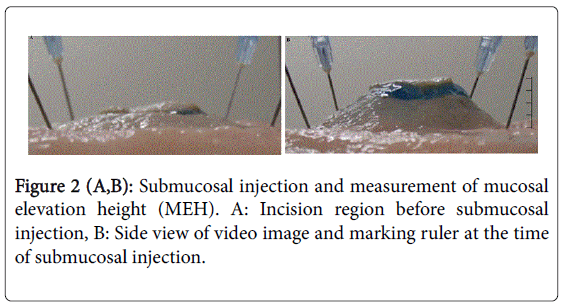
SH (0.4%, MucoUp, Johnson & Johnson Co., Tokyo, Japan) containing 80 mg of SH in 20 ml (SH concentration: 4 mg/ml) was developed and is used exclusively for endoscopic treatment in Japan. We applied three variations of this injection solution in our trials: Solution A, 0.9% NaCl (normal saline, NS); Solution B, 1:1 mixture of NS and 0.4% SH (final SH concentration: 2 mg/ml); and Solution C, 0.4% SH (final SH concentration: 4 mg/ml). We injected each solution into the submucosal layer of the CI models upon obtaining an initial maximum MEH, using 10 specimens for each organ. Avoiding the mucous membrane and muscle layers, as occurs in clinical practice, we injected the solution into the submucosal layer until the elevation was sufficient without fixing the amount of the injection solution or speed of injection. The MEH was recorded immediately from the lateral direction using a video camera (HC-W850 M, Panasonic Co., Osaka, Japan). The camera was fixed horizontally to the mucosal surface to perform this measurement precisely, and we set up a ruler to measure MEH in the screen view. We isolated still images from the recorded injection procedure at 0, 30, 60, 90, 120, 150, 180 and 300 seconds post-injection and measured MEH to 1/100 mm using Photoshop CC 14.1 (Adobe Systems Inc., San Jose, CA, USA) (Figure 2). We compared the mean MEH at each time point for each of the three solutions that were injected into each resected organ.
Result 1: Changes in mucosal elevation height after injection
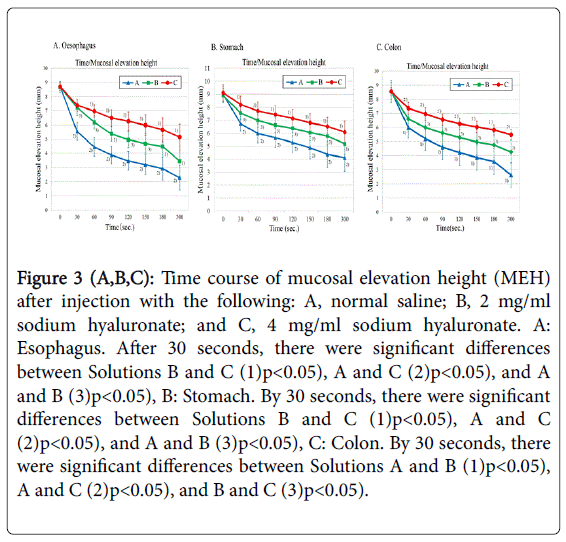
A time course of the change in MEH after submucosal injection in the different resected organs is shown in Figure 3. There were no significant differences in the initial MEH for each solution in the esophagus, stomach and colon. Analysis of the duodenum was prevented by the immediate spread of the solution after injection, which precluded any lasting increase in MEH. The lift potential of Solutions A and B in the esophagus was similar up to 30 seconds, and Solution C was most able to maintain the MEH after 30 seconds (Solutions C > B > A) (Figure 3A). A similar rating was observed in the stomach (Solutions C > B > A), with Solution C being most able to maintain the MEH at 30 seconds post-injection (Figure 3B). There was a significant difference in the colon between Solutions B and C at 30 seconds, and both solutions were superior to Solution A. However, Solution C had the ability to maintain the MEH at 60 seconds (Solutions C > B > A) (Figure 3C).
Figure 3 (A,B,C): Time course of mucosal elevation height (MEH) after injection with the following: A, normal saline; B, 2 mg/ml sodium hyaluronate; and C, 4 mg/ml sodium hyaluronate. A: Esophagus. After 30 seconds, there were significant differences between Solutions B and C (1)p<0.05), A and C (2)p<0.05), and A and B (3)p<0.05), B: Stomach. By 30 seconds, there were significant differences between Solutions B and C (1)p<0.05), A and C (2)p<0.05), and A and B (3)p<0.05), C: Colon. By 30 seconds, there were significant differences between Solutions A and B (1)p<0.05), A and C (2)p<0.05), and B and C (3)p<0.05).
Consequently, it was found that even after the CI, the ability of the solution with high SH concentration to maintain MEH was superior. Therefore, to compare the ability of lesion-lift in each organ with the same solution, we calculated reduction rates using MEH at 0-300 seconds and compared them in each organ. The ability of Solution C to maintain MEH in each organ was compared by evaluating MEH at 300 seconds relative to the initial MEH, which showed that the stomach was able to maintain MEH significantly better than the esophagus (p=0.041) but that there was no significant difference between the esophagus and colon, or the stomach and colon. The ability of Solution B to maintain MEH in each organ was compared by evaluating MEH at 300 seconds relative to the initial MEH, which showed that the stomach was able to maintain MEH significantly better than the esophagus (p=0.025) and the colon (p=0.048), and the colon was able to maintain MEH significantly better than the esophagus (p=0.013). These results were same in the case of Solution A.
As a result, we found that the ability to maintain MEH could vary in each organ, even when the same solution was used. These results led us to further explore the optimal solution for hybrid ESD in consideration of these variations.
Method 2: Minimum snarable mucosal elevation height
We examined the minimum MEH with which snaring can be performed successfully. However, measuring MEH directly during snaring is difficult because the form of the lifted mucosal membrane is changed by the influence of the snaring itself. Therefore, we used a 2-step procedure to measure the minimum snarable MEH (i.e., the minimum mucosal height at which snaring is possible). First, we measured the “snarable time” that is, the number of seconds during which snaring is possible after the injection (duration of time for possible snaring). We injected Solution A, which showed the lowest ability to maintain MEH in Result 1 (Figure 3), into the submucosa of each CI model, and we snared the lesion with a 25-mm snare (Snare Master; SD-210U-25, Olympus Co, Tokyo, Japan) at 0, 30, 60, 90, 120, 150, 180, and 300 seconds. Because the MEH can change if snaring is attempted in the same CI model, we performed the snaring for each time point with a different model (one model for 0 seconds, another model for 30 seconds, another model for 60 seconds, and so on). We used an endoscope and performed it in the same way as assumed for actual endoscopic technique. The duration of time for possible snaring was measured by three endoscopists (K.M, Y.N, and Y.A) individually on each of the ten resected organs. The mean duration of time for possible snaring was calculated and rounded to the nearest 30 seconds to yield a value for “snarable time”. The “snarable time” obtained from the measurement was converted to MEH based on Result 1 (Figure 3). Ultimately, the “minimum snarable MEH” for each organ was defined as the mean MEH at the “snarable time” plus one standard deviation (1SD) of time as a safety margin.
Mean procedure time of snaring
Based on the trials described above, we measured the procedure time of snaring (tsnare) and evaluated the optimal injection solution. Our definition of tsnare was that the time measurement started at 0 seconds when the injection needle was removed after the injection; the snare was then put through the endoscope and out from the tip of the endoscope, and the time measurement ended when the lesion was held firmly with the snare. The time measurement ended right before the excision, and the time for the excision was not included. Trainees must perform the procedure safely, and tsnare depends on the practitioner’s skill. Therefore, we measured tsnare for one expert who had attended more than 1000 cases of EMR and 500 cases of ESD (K. M) as a reference, and for two trainees who had attended up to 30 cases of EMR (Y. N and Y. A). We used an upper endoscope for the esophagus, stomach, and duodenum and a lower endoscope for the colon. We calculated the average of ten tsnare values for each practitioner.
In this study, we fastened the organ on a rubber board placed on an even surface and set the lesion in the horizontal direction almost the same as with endoscopic technique, which is the 6 o’clock direction. Based on Result 1, which indicated that NS showed the lowest ability to maintain MEH, we evaluated the minimum MEH for possible snaring using only NS.
Optimal injection solution
From these results, the minimum snarable MEH can be derived. If the MEH is lower than the minimum snarable MEH, snaring cannot be performed. In other words, if the MEH after the injection is higher than the minimum snarable MEH, snaring can succeed. On the graph that shows the transition of MEH, we indicated the minimum snarable MEH, the length of snarable time for each injection solution, and the time required for the trainees to snare. Then, it was considered that snaring can be successfully performed with an injection solution with MEH that is higher than the minimum snarable MEH at the time required for trainees to snare. Among these solutions, the one with the lowest SH concentration was considered optimal.
Histopathological investigation
To evaluate the optimal solution for each organ in practice, the removed specimens and remaining organ samples using each solution were examined histopathologically. The specimens were fixed in formalin, cut into 2-mm slices, embedded in paraffin, and stained using hematoxylin-eosin and azan to determine whether the muscle layer of the remaining organ was perforated and/or whether residual lesions remained.
Results 2: Minimum snarable mucosal elevation height
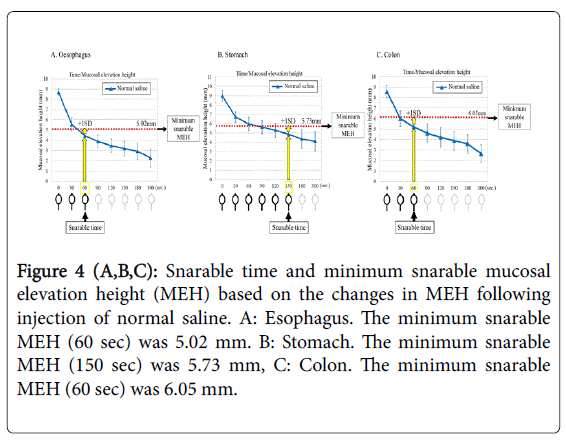
The snarable times are shown in Table 1, and the minimum snarable MEH is shown in Figure 4. The mean time of possible snaring in the esophagus was scored as 58 seconds, and the snarable time was thus determined to be 60 seconds. The addition of +1 SD of height to the MEH was achieved using Solution A at 60 seconds, which led to a minimum snarable MEH of 5.02 mm (Figure 4A). The mean time of possible snaring was 145 seconds in the stomach, and the snarable time was determined to be 150 seconds. The addition of + 1 SD of height to the MEH was achieved with Solution A at 150 seconds as a margin of safety, which led to a minimum snarable MEH of 5.73 mm (Figure 4B). No snaring was achievable in the duodenum because the lesion did not remain elevated after the injection of Solution A. The mean time of possible snaring was 56 seconds in the colon, and the snarable time was thus determined to be 60 seconds. The addition of + 1 SD margin of safety produced a minimum MEH for the colon of 6.05 mm (Figure 4C).
Figure 4 (A,B,C): Snarable time and minimum snarable mucosal elevation height (MEH) based on the changes in MEH following injection of normal saline. A: Esophagus. The minimum snarable MEH (60 sec) was 5.02 mm. B: Stomach. The minimum snarable MEH (150 sec) was 5.73 mm, C: Colon. The minimum snarable MEH (60 sec) was 6.05 mm.
Mean snaring procedure time
The mean procedure times for snaring are shown in Table 2. The average tsnare in the esophagus was 40.2 seconds for the expert and 58.8 seconds for the trainees.
There was a significant difference in the average tsnare between the expert and the trainees for the stomach (39.6 versus 56.2 seconds, respectively). The mean tsnare in the colon was approximately 26 seconds faster for the expert than for the trainees (48.7 versus 75.3 seconds, respectively).
Optimal injection solution
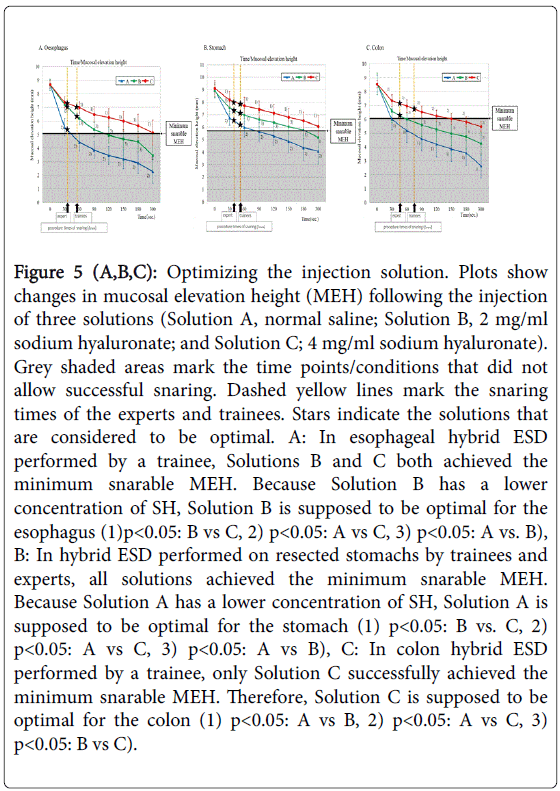
For the esophagus (Figure 5A), the minimum snarable height was 5.02 mm. If the MEH is lower than 5.02 mm, snaring cannot be performed (the gray shaded area in Figure 5A). In other words, when the MEH after the injection is higher than the minimum snarable MEH of 5.02 mm for the esophagus, snaring can succeed. The graph indicates that with normal saline, snaring can be performed for approximately 30 seconds but that snaring cannot be performed at 60 seconds because MEH decreases below 5.02 mm. For B, snaring can be performed for approximately 120 seconds, but at 150 seconds, MEH has decreased below 5.02 mm. For C, snaring can be performed for 300 seconds. For the trainees, the mean procedure time for snaring, Tsnare, was 58.8 seconds. The graph indicates that at 58.8 seconds, the MEH for Solution A is below 5.02 mm but that the MEH for Solutions B and C is above 5.02 mm. Consequently, it was found that trainees can perform snaring with Solutions B and C. Considering the disadvantages of SH, it is better to use a lower concentration of SH; therefore, Solution B can be the optimal injection solution for the esophagus.
Figure 5 (A,B,C): Optimizing the injection solution. Plots show changes in mucosal elevation height (MEH) following the injection of three solutions (Solution A, normal saline; Solution B, 2 mg/ml sodium hyaluronate; and Solution C; 4 mg/ml sodium hyaluronate). Grey shaded areas mark the time points/conditions that did not allow successful snaring. Dashed yellow lines mark the snaring times of the experts and trainees. Stars indicate the solutions that are considered to be optimal. A: In esophageal hybrid ESD performed by a trainee, Solutions B and C both achieved the minimum snarable MEH. Because Solution B has a lower concentration of SH, Solution B is supposed to be optimal for the esophagus (1)p<0.05: B vs C, 2) p<0.05: A vs C, 3) p<0.05: A vs. B), B: In hybrid ESD performed on resected stomachs by trainees and experts, all solutions achieved the minimum snarable MEH. Because Solution A has a lower concentration of SH, Solution A is supposed to be optimal for the stomach (1) p<0.05: B vs. C, 2) p<0.05: A vs C, 3) p<0.05: A vs B), C: In colon hybrid ESD performed by a trainee, only Solution C successfully achieved the minimum snarable MEH. Therefore, Solution C is supposed to be optimal for the colon (1) p<0.05: A vs B, 2) p<0.05: A vs C, 3) p<0.05: B vs C).
For the stomach (Figure 5B), the minimum snarable height was 5.73 mm. If the MEH is lower than 5.73 mm, snaring cannot be performed (the gray shaded area in Figure 5B). In other words, when the MEH after the injection is higher than the minimum snarable MEH of 5.73 mm for the stomach, snaring can succeed. The graph indicates that with normal saline, snaring can be performed for approximately 60 seconds but that snaring cannot be performed at 90 seconds because MEH has decreased below 5.73 mm. For B, snaring can be performed for approximately 180 seconds, but at 300 seconds, MEH has decreased below 5.73 mm. For C, snaring can be performed for 300 seconds. For the trainees, the mean procedure time for snaring, Tsnare, was 56.2 seconds. The graph indicates that at 56.2 seconds, the MEH for Solutions A, B and C is above 5.73 mm, which suggests that the trainees can perform snaring with any of the three injection solutions. Considering the disadvantages of SH, it is better to use a lower concentration of SH; therefore, Solution A can be the optimal injection solution for the stomach.
As described in Result 1, it was difficult to examine the duodenum. For the colon (Figure 5C), the minimum snarable height was 6.05 mm. If the MEH is lower than 6.05 mm, snaring cannot be performed (the gray shaded area in Figure 5C). In other words, when the MEH after the injection is higher than the minimum snarable MEH of 6.05 mm for the colon, snaring can succeed. The graph indicates that with normal saline, snaring cannot be performed at 30 seconds because MEH has decreased below 6.05 mm. For B, snaring can be performed for approximately 60 seconds, but at 90 seconds, MEH has decreased below 6.05 mm. For C, snaring can be performed for 150 seconds. For the trainees, the mean procedure time for snaring, Tsnare, was 75.3 seconds. The graph indicates that at 75.3 seconds, the only MEH above 6.05 mm is that of Solution C, which suggests that trainees can perform snaring only with Solution C. Therefore, Solution C can be the optimal injection solution for the colon.
Histopathological examination
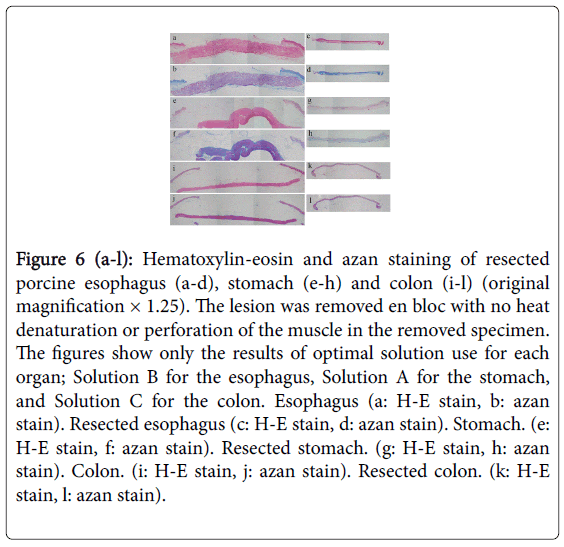
We performed hybrid ESD on resected porcine organs using each solution. We used histopathological methods to verify that, when using Solutions B and C for the esophagus, Solutions A, B and C for the stomach, and Solution C for the colon (Figure 6), all lesions were removed en bloc, and we observed no denaturation by heat or perforation in the muscle layers in either the removed specimen or the remaining organ.
Figure 6 (a-l): Hematoxylin-eosin and azan staining of resected porcine esophagus (a-d), stomach (e-h) and colon (i-l) (original magnification × 1.25). The lesion was removed en bloc with no heat denaturation or perforation of the muscle in the removed specimen. The figures show only the results of optimal solution use for each organ; Solution B for the esophagus, Solution A for the stomach, and Solution C for the colon. Esophagus (a: H-E stain, b: azan stain). Resected esophagus (c: H-E stain, d: azan stain). Stomach. (e: H-E stain, f: azan stain). Resected stomach. (g: H-E stain, h: azan stain). Colon. (i: H-E stain, j: azan stain). Resected colon. (k: H-E stain, l: azan stain).
These results indicated that for the esophagus hybrid ESD could be performed successfully with Solution B or C, but Solution B with a lower concentration of SH can be optimal. Similarly, for the stomach, Solution A can be better than Solution B or C, and for the colon, only Solution C can be used.
Discussion
Various solutions have been used as a submucosal injection solution for endoscopic resection, but there are no clear standards regarding its choice, which depends on the individual practitioner. Therefore, injection solutions such as hypertonic saline [19], dextrose water [20], glycerin solution [21], and SH [22] have been examined. Fujishiro et al. injected a fixed quantity of various solutions into the submucosa of the porcine stomach and evaluated the ability of each solution to maintain MEH. Their results showed that SH was superior at maintaining MEH over other solutions [18]. However, unusually, CI was not performed in their study. Procedures involving CI such as hybrid ESD and ESD are performed widely, and SH is widely used clinically, although the concentration of SH depends on the individual practitioner. Furthermore, in hybrid ESD, MEH after CI can vary significantly depending on the composition of the injection solution and the organ treated, but no study has examined the optimal injection solution for hybrid ESD in each organ.
Given the above, we evaluated the MEH after CI according to the concentration of SH for the first time in this study using a commercial solution of 4 mg/dl SH. In stomach models without CI, Fujishiro et al. reported that a 30% decrease in MEH required at least 1200 seconds using the same 4 mg/dl SH solution [18]. As shown in Figure 3, a high concentration of SH was superior at maintaining the MEH even after CI. However, the MEH for Solution C decreased by 30% in 300 seconds in our study (line C in Figure 3B), possibly because the injection solution immediately flows into the surrounding regions from part of the CI. Thus, one should be aware that the injected solution might flow into areas surrounding the lesion when performing a procedure based on CI. From this, it is clear that CI makes it difficult to sustain MEH.
We also examined changes in MEH in the esophagus and colon for the first time, and we showed the superiority of a high SH concentration at maintaining MEH, similar to the results in the stomach. Moreover, we compared the reduction of MEH in each organ using the same solution. The ability of 4 mg/dl SH to maintain MEH in each organ was compared by evaluating the MEH at 300 seconds relative to the initial MEH, which showed that the stomach was able to maintain MEH significantly better than the esophagus (p=0.041); however, there was no significant difference between the esophagus and colon or the stomach and colon. By contrast, the ability of 2 mg/dl SH to maintain MEH in each organ was compared by evaluating the MEH at 300 seconds relative to the initial MEH, which showed that the stomach was able to maintain MEH significantly better than the esophagus (p=0.025) and the colon were (p=0.048); furthermore, the colon was able to maintain MEH significantly better than the esophagus (p=0.013). These results were the same in the case of NS. That is, SH was superior at maintaining MEH in each organ equally in the case of using 4 mg/dl SH, but the ability to maintain MEH was organ-specific in the case of using 2 mg/dl SH and normal saline (stomach>colon>esophagus). In terms of the initial MEH, a previous report [18] that determined the volume of injection solution showed that SH was superior to NS. In this study, we injected solution until the maximum MEH was obtained without determining the injection solution volume, and there was no significant difference in the initial MEH between the solutions in the esophagus, stomach, or colon (esophagus vs. stomach: p=0.211, esophagus vs. colon: p=0.241, stomach vs. colon: p=0.058). As a result, there was no association with organ specificity for the initial MEH. That is, it has been suggested that the ability to maintain MEH is associated with organ specificity and viscosity [20], but the same may not be true for the initial MEH. The initial MEH may be associated with the capacity of the submucosal layer into which the solution is injected. In terms of the differences among organs in maintaining MEH, there are gross differences in the mucosal and submucosal layers, and there are certainly pathological and molecular biological differences among these tissues that may relate to their ability to maintain mucosal elevation. Esophageal mucosae are generally thin and composed of stratified squamous cells, and mucosae in the stomach and colon are composed of simple columnar epithelia, with a thicker layer in the stomach than the colon. Differences in the thickness and molecular composition of submucosae among these organs have not been reported, and it has thus been difficult to analyze the differences between human and porcine submucosal tissue molecularly.
This study showed that a higher concentration of SH has a greater ability to maintain MEH. As mentioned in the Introduction, however, the SH concentration should be as low as possible to avoid the disadvantages associated with its use. Hybrid ESD has a shorter procedure time than ESD because the dissection procedure can be omitted. For this reason, a high concentration of SH is not necessary if a sufficient MEH for safe resection can be obtained during the snaring time. Accordingly, we measured both the snaring procedure time and the snarable MEH, and we evaluated the optimal concentration of SH.
In this study, the optimal solutions were 2 mg/dl SH in the esophagus, NS in the stomach, and 4 mg/dl SH in the colon. There are two factors that prescribe the optimal concentration of SH on the graph of changes in MEH: the minimum snarable MEH and the snaring procedure time (tsnare). Regarding the minimum snarable MEH, the snarable time was up to 60 seconds in the esophagus, up to 150 seconds in the stomach, and up to 60 seconds in the colon. The MEH without 1SD was 4.448 mm at 60 seconds in the esophagus and 5.201 mm at 60 seconds in the colon; thus, there were significant differences between the esophagus and colon (p<0.05). This finding suggests that snaring may be more difficult to perform in the esophagus than in the colon, possibly because of histological differences in the mucosa (e.g., esophagus, stratified squamous cells; colon, simple columnar epithelia) and/or the density of the connective tissue of the submucosa. However, no studies have evaluated these mechanisms, and research on this subject may be expected in the future. By contrast, the mean tsnare by trainees was 58.8 seconds in the esophagus, 56.2 seconds in the stomach, and 75.3 seconds in the colon (Table 2). Thus, tsnare was longer in the colon than in the esophagus or stomach. This may be because the length and thickness of the colonoscope affect the time taken both to replace a snare catheter and for the snaring operation: a colonoscope is longer and thicker and more difficult to operate than an upper gastrointestinal endoscope. It is generally considered that the procedure time is affected by the number of years of experience [23-25]; thus, we measured tsnare for both trainees and an expert. Similar to a past study, tsnare was faster for the expert than for the trainees. These findings suggest that practitioners might perform hybrid ESD by using more conventional solutions according to their level of learning and expertise.
According to the present results, SH at a concentration of 4 mg/dl provided sufficient MEH in each organ equally, but the optimal solution varied for each organ due to organ specificity, the ease of snaring, and because tsnare was also different for each organ. This study has therefore shown that we should consider not only the characteristics of the organ but also differences in scope, procedure time, and the proficiency of the practitioner when selecting the optimal solution in hybrid ESD.
In this study, we obtained the optimal concentration of SH in hybrid ESD: 2 mg SH in the esophagus, normal saline in the stomach, and 4 mg/dl SH in the colon, and it is considered that EMR, hybrid ESD, and ESD in the duodenum are difficult. Matsumoto et al. reported that the incidence of complications in the duodenum was 35.7% for ESD and 3.4% for EMR [26]. Conversely, Kim et al. reported an incidence of 75% for ESD and 6% for EMR [27]. Accordingly, the complication rate varies by study, and a standard technique in the duodenum has not been established. These reports also suggested that EMR is safer than ESD in the duodenum. In this study, duodenal MEH could not be obtained due to the immediate spreading of the injection solution because of the thin mucosa and submucosa and the underlying Brunner gland in the duodenum. EMR is considered safer because it is performed without CI; however, hybrid ESD is not suitable in the duodenum because it is difficult to obtain sufficient MEH after performing CI. For this reason, in the duodenum, we should probably perform partial incision instead of CI at the beginning of ESD. Few reports mention the complications of hybrid ESD, and further consideration of this topic will be needed.
There are some limitations to this study. This study was conducted in resected porcine specimens, and thus, we did not assess the influence of blood flow, body temperature, peristalsis, or absorption from tissue in a live animal. Therefore, similar results may not be applicable to humans, and the safety of these methods in human subjects must be uniquely considered. Furthermore, there are anatomical differences between human and porcine versions of the same organ; however, we performed the examination in a part of the organ that is considered to be similar to human. MucoUp is sold only in Japan as SH for gastrointestinal endoscope treatment, while SH such as SUPARTZ (SH concentration: 10 mg/dl, Kaken Pharmaceutical Co., Tokyo, Japan) is also sold in other countries. Therefore, the solution recommended in the present study can be made by diluting these solutions with saline. Also for this reason, we did not use GLYCEOL (Chugai Pharmaceutical Co., Tokyo, Japan) for the investigation. Nonetheless, the results of this study provide important evidence demonstrating the optimal solution and technique for trainees performing hybrid ESD, and the results may lead to the improved performance of hybrid ESD in the future.
References
- Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, et al. (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33: 221-226.
- Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, et al. (1994) Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy 26: 352-358.
- Ichikawa J, Tanabe S, Koizumi W, Kida Y, Imaizumi H, et al. (2003) Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy 35: 203-206.
- Kiesslich R, Neurath MF (2004) Endoscopic mucosal resection: an evolving therapeutic strategy for non-polypoid colorectal neoplasia. Gut 53: 1222-1224.
- Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, et al. (2009) Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol 15: 4273-4277.
- Su MY, Hsu CM, Ho YP (2005) Endoscopic mucosal resection for colonic non-polypoid neoplasms. Am J Gastroenterol 100: 2174-2179.
- Huang J, Lu ZS, Yang YS, Yuan J, Wang XD, et al. (2014) Endoscopic mucosal resection with circumferential incision for treatment of rectal carcinoid tumours. World J SurgOncol 12: 23.
- Gotoda T (2006) Endoscopic resection of early gastric cancer: the Japanese perspective. CurrOpinGastroenterol 22: 561-569.
- Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41: 929-942.
- Watanabe K, Ogata S, Kawazoe S (2006) Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. GastrointestEndosc 63: 776-782.
- Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, et al. (2006) Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. GastrointestEndosc 64: 877-883.
- Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, et al. (2007) Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J GastroenterolHepatol 22: 821-826.
- Min BH, Lee JH, Kim JJ (2009) Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis 41: 201-209
- Toyonaga T, Man-I M, Morita Y, Azuma T2 (2014) Endoscopic submucosal dissection (ESD) versus simplified/hybrid ESD. GastrointestEndoscClin N Am 24: 191-199.
- Basford PJ, George R, Nixon E (2014) Endoscopic resection of sporadic duodenal adenomas: comparison of endoscopic mucosal resection (EMR) with hybrid endoscopic submucosal dissection (ESD) techniques and the risks of late delayed bleeding. SurgEndosc 28: 1594-1600
- Yamamoto H, Yahagi N, Oyama T (2008) Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. GastrointestEndosc 67: 830-839
- Fujishiro M, Yahagi N, Kashimura K (2005) Tissue damage of different submucosal injection solutions for EMR. GastrointestEndosc 62: 933-942
- Fujishiro M, Yahagi N, Kashimura K (2004) Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 36: 579-583
- Hirao M, Masuda K, Asanuma T (1988) Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. GastrointestEndosc 34: 264-269
- Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, et al. (2004) Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy 36: 584-589.
- Torii A, Sakai M, Kajiyama T (1995) Endoscopic aspiration mucosectomy as curative endoscopic surgery; analysis of 24 cases of early gastric cancer. GastrointestEndosc 42: 475-479
- Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, et al. (1999) A novel method of endoscopic mucosal resection using sodium hyaluronate. GastrointestEndosc 50: 251-256.
- Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, et al. (2005) The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. GastrointestEndosc 62: 860-865.
- Herreros de Tejada A (2014) ESD training: A challenging path to excellence. World J GastrointestEndosc 6: 112-120.
- Gotoda T, Friedland S, Hamanaka H, Soetikno R (2005) A learning curve for advanced endoscopic resection. GastrointestEndosc 62: 866-867.
- Matsumoto S, Yoshida Y1 (2014) Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol 20: 8624-8630.
- Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, et al. (2014) Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J GastroenterolHepatol 29: 318-324.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 12622
- [From(publication date):
December-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11647
- PDF downloads : 975