Exploring the Impact of Antihyperglycemic Agents on Diabetoporosis: An Updated Perspective
Received: 05-Nov-2023 / Manuscript No. CPB-24-119262 / Editor assigned: 07-Nov-2023 / PreQC No. CPB-24-119262 / Reviewed: 21-Nov-2023 / QC No. CPB-24-119262 / Revised: 03-Feb-2025 / Manuscript No. CPB-24-119262 / Published Date: 10-Feb-2025
Abstract
Diabetes mellitus is an enduring ailment that is swiftly spreading worldwide. Its repercussions are profound, significantly diminishing the quality of life by giving rise to numerous associated afflictions like kidney disease, cardiovascular conditions, obesity, and neurological disorders. Furthermore, it disrupts the metabolism of bones. Among the myriad complications accompanying diabetes, osteoporosis stands out as a prominent concern, often manifesting as a consequence of the disease. Both type-1 and type-2 diabetes bear a linkage to bone anomalies and an elevated susceptibility to fractures, making diabetes a recognized risk factor for osteoporosis. Consequently, there arises a need to scrutinize the potential therapeutic influence of hypoglycemic agents in addressing osteoporosis. The Glucagon-Like Peptide-1 (GLP-1) agonist, a class of hypoglycemic agents, emerges as a novel and promising candidate for osteoporosis management due to its additional involvement in the intricate process of bone remodeling. Studies suggest that GLP-1 agonists can bolster bone mineral density, enhance bone quality, and avert fractures in diabetic individuals. This comprehensive review spotlights recent discoveries concerning the utilization of antidiabetic medications in mitigating diabetes-induced osteoporosis. Additionally, it offers insights into the pathophysiology of this condition and the potential mechanisms that play a role in treating osteoporosis resulting from diabetes. Nevertheless, further research is imperative to gain a complete understanding of the therapeutic potential and distinctive mechanisms associated with GLP-1 agonists in the context of bone remodeling.
Keywords: Antidiabetic medications; GLP-1; Antihyperglycemic; Diabetoporosis; Kidney disease
Introduction
Osteoporosis, a metabolic bone disorder, is characterized by diminished bone mass, increased bone fragility, and an imbalance between bone resorption and formation [1]. This imbalance, in turn, results in reduced bone density or irreversible loss of bone mass [2,3]. Notably, postmenopausal women are more prone to developing this condition due to decreased estrogen secretion [4,5]. Numerous risk factors contribute to the onset of osteoporosis, including genetic factors, chronic alcohol and tobacco consumption, abnormal parathyroid hormone levels, a sedentary lifestyle, and prolonged use of certain drugs such as corticosteroids [6-8].
Diabetes, a chronic metabolic disorder driven by insulin resistance and resulting in elevated blood glucose levels, poses a substantial risk for a range of other diseases, including cardiovascular conditions, vision impairment, kidney dysfunction, osteoporosis, and even mortality [9]. Diabetes and osteoporosis often co-occur. Considering prior research, it has been observed that diabetes may lead to a reduction in bone mass, thus increasing the risk of osteoporosis development [10,11]. Nevertheless, the impact of diabetes on bone metabolism remains a subject of debate within the research community. Some studies suggest that diabetes is associated with decreased bone density, while others indicate it is linked to normal bone density, and still, others propose a connection with elevated bone density [12-14].
Literature Review
The origin OD osteoporosis triggered by diabetes mellitus
Diabetes, a persistent metabolic disorder, is characterized by sustained hyperglycemia resulting from impaired insulin secretion and/or response. This condition leads to malfunction and deterioration of various organs, particularly the eyes, heart, nerves, kidneys, and blood vessels. Furthermore, diabetes is associated with metabolic disorders affecting the skeletal system, including osteoporosis, osteoarthritis, and osteopenia [15].
In Insulin-Dependent Diabetes Mellitus (IDDM), there is a decrease in bone mass and an elevated risk of fractures, meeting the criteria for osteoporosis. However, in Non-Insulin-Dependent Diabetes Mellitus (NIDDM), bone mass may be normal or increased, but the risk of fractures is heightened [16]. Despite having stronger bone density, NIDDM individuals are susceptible to fractures, potentially linked to factors like hypoglycemia or an increased risk of falls resulting from neuropathy. Nonetheless, earlier studies have not provided substantial explanations for the heightened fracture risk but have suggested potential associations with renal complications [17].
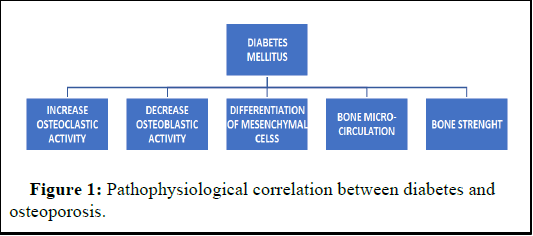
Renal impairment in diabetes can have numerous adverse effects, including reduced synthesis of active vitamin D, hyperparathyroidism, and chronic mineral and bone disorders [18]. These effects, in turn, result in altered bone turnover, changes in bone density, and diminished bone biomechanical strength. Consequently, hyperglycemia emerges as a pivotal factor that directly or indirectly influences the bone remodeling process, producing detrimental effects as depicted in Figure 1.
Pathophysiology
The pathophysiological intricacies of osteoporosis in individuals with diabetes involve a multifaceted interplay of elements. This includes oxidative stress, elevated blood glucose levels, and the accumulation of Advanced Glycation End products (AGEs). AGEs affect collagen properties, trigger the release of inflammatory mediators, promote the deposition of fat within the marrow cavity, and induce significant alterations in the functionality of osteocytes [19,20].
Additional contributing factors encompass drug-induced hypoglycemia, with medications like thiazolidinediones directly impacting bone metabolism and elevating the risk of falls. The combined influence of these factors amplifies the susceptibility to fractures among diabetic patients (Figure 1).
Figure 1: Pathophysiological correlation between diabetes and osteoporosis.
Pathophysiological mechanisms in bone remodeling in insulin-dependent diabetes mellitus
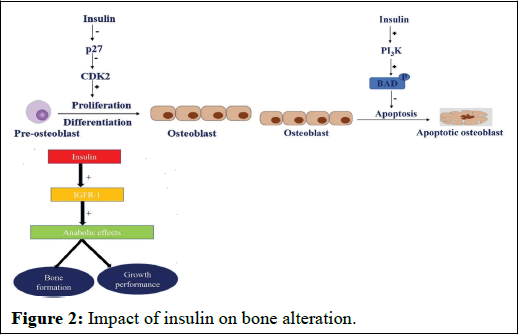
Insulin-Dependent Diabetes Mellitus (IDDM) is characterized by a complete absence of insulin, a vital metabolic hormone presents in the liver, adipose tissue, muscle cells, and even bone-forming cells. Insulin plays a crucial role in bone-forming cells by promoting cell division, inhibiting cell death, and counteracting the adverse effects of elevated glucose levels on bone formation.
Research has elucidated that insulin's influence on bone-forming cells is mediated through three distinct signaling pathways:
• Firstly, insulin signaling obstructs the enzyme p27, an inhibitor of cyclin-dependent kinase enzyme. By inhibiting p27, it de-represses cyclin-dependent kinase enzyme activity, stimulating cell cycle progression, leading to the multiplication and differentiation of preosteoblasts.
• Secondly, insulin activates the phosphatidylinositol 3-kinase enzyme, which, in turn, phosphorylates the BAD promoter, effectively blocking the apoptotic impact on osteoblasts (Figure 2).
• Thirdly, insulin triggers the insulin-like growth factor-1 receptor, fostering anabolic effects such as bone formation and growth.
Figure 2: Impact of insulin on bone alteration.
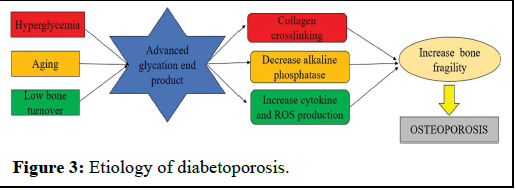
Hence, the deficiency of insulin and the presence of hyperglycemia may negatively affect bone quality. Hyperglycemia leads to the formation of Advanced Glycation End products (AGEs) when proteins, nucleic acids, and phospholipids undergo nonenzymatic glycation. The accumulation of AGEs results in the nonenzymatic cross-linking of collagen, disrupting osteoblasts' adherence to the extracellular matrix, which in turn compromises bone strength. These alterations in the extracellular matrix diminish mature osteoblasts' ability to produce alkaline phosphatase, significantly impacting bone mineralization.
Furthermore, AGE receptors prompt an increased production of Reactive Oxygen Species (ROS) and cytokines, contributing to decreased osteoblast formation and heightened osteoclast formation. Consequently, the buildup of these glycation end products amplifies bone resorption and chronic inflammation in individuals with diabetes (Figure 3).
Figure 3: Etiology of diabetoporosis.
Pathophysiological mechanisms in bone remodeling in noninsulin- dependent diabetes mellitus
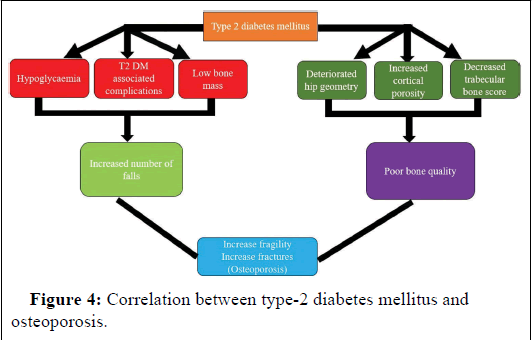
Non-Insulin Dependent Diabetes Mellitus (NIDDM) is typified by normal to elevated bone mass density, yet it carries an augmented risk of fracture occurrence. The mechanisms underlying this heightened fracture risk involve various factors: Alteration in bone's mechanical properties due to nonenzymatic glycation, microdamage within bone structure, and disturbances in the mineralization process.
Impaired kidney function bears adverse consequences, including reduced synthesis of active vitamin D, aplastic bone disorders, excessive parathyroid hormone release, visual impairment, and neuropathy. These complications may contribute to diminished mobility.
Furthermore, hypoglycemia alone stands out as a significant contributor to the increased fracture risk. In conclusion, factors such as hypoglycemia, comorbidities, low bone mass, compromised hip geometry, elevated cortical porosity, and reduced bone trabecular score collectively contribute to the development of osteoporosis in Type-2 Diabetes Mellitus (T2DM), as depicted in Figure 4.
Figure 4: Correlation between type-2 diabetes mellitus and osteoporosis.
Discussion
Impacts of anti-diabetic drugs on bone metabolism
Osteoporosis and diabetes mellitus exhibit a strong interconnection influenced by a multitude of factors. The focus has increasingly shifted towards understanding how antidiabetic medications impact bone metabolism. As a consequence of Type 2 Diabetes Mellitus (T2DM), individuals often experience bone fragility, an increased risk of fractures, inadequate bone regeneration, and various bone-related ailments. Therefore, the clinical approach to treating diabetes mellitus must encompass not only blood glucose management but also the mitigation of bone-related complications (Table 1).
Metformin: This widely-used first-line treatment for T2DM has garnered attention for its effect on bone health. In vitro studies suggest that it induces osteoblast differentiation and enhances the expression of osteogenic markers like alkaline phosphatase, osteopontin, and bone morphogenic protein-2. Previous findings indicate neutral to mildly beneficial effects, positioning it as an effective option in the management of "diabetoporosis."
Thiazolidinediones: These peroxisome proliferator-activated receptor agonists modify gene expression, improving glucose uptake, insulin sensitivity, and beta-cell functions. Clinical use of thiazolidinediones for T2DM treatment, however, has raised concerns about reduced bone density and increased fracture risk, making them contraindicated for individuals with bone disorders.
Sulphonylureas: These agonists of sulfonylurea receptor-1 stimulate insulin secretion by inhibiting adenosine triphosphatesensitive K+ channels. Most clinical studies suggest positive or neutral effects on bone metabolism, except for the "MrOS (the osteoporotic fractures in men) trial," which links them to an increased fracture risk in elderly men with T2DM. They are rarely prescribed for diabetics with osteoporosis due to potential hypoglycemic side effects.
DPP-4 (Dipeptidyl peptidase 4) inhibitors: These medications inhibit the DPP-4 enzyme, which deactivates bioactive peptides that regulate glucose homeostasis. They also inhibit osteoclast development, making them beneficial in managing osteoporosis. Although some studies show a neutral effect on fracture risk, meta-analyses suggest their positive role in fracture prevention for T2DM patients.
SGLT-2 inhibitors: These drugs inhibit a kidney glucose reabsorption transporter and may have detrimental effects on bone mass density, bone resorption, and hip fracture risk, particularly the drug canagliflozin. Most drugs in this class exhibit a relatively neutral effect on bone metabolism.
GLP-1 agonists: This class of drugs has gained prominence in diabetes treatment due to their involvement in various metabolic pathways. Research suggests they may prevent osteoporosis by maintaining blood glucose levels, promoting bone formation, and inhibiting bone resorption. Recent clinical data supports their effectiveness in managing glucose levels and promoting weight loss without increasing fracture risk.
Insulin: No specific randomized clinical studies have been designed to investigate the effect of insulin treatment on bone health. Observational studies, however, point to a higher fracture risk in patients receiving insulin therapy, making it an undesirable option for osteoporosis management.
| Drug | Mechanism | Indication | Impact on bone health |
| Metformin | It blocks the respiratory chain complex I which results in an increased AMP: ATP ratio and ultimately activates 5 AMP‑Activated Protein Kinase (AMPK). This activated AMPK thus reduces reactive oxygen species, advanced glycation end product, and insulin‑like growth factor‑1. | Diabetes, osteoporosis | Beneficial effects |
| Thiazolidinediones | They are peroxisome proliferator‑activated receptor agonists and induction of adipogenesis requires peroxisome proliferator‑activated receptor G. This induction of adipogenesis results in the reduction of osteoblast differentiation and expression. | Diabetes | Negative effect |
| Sulphonyl urea’s | They block the ATP‑sensitive potassium channels which in turn close the potassium channels and open the calcium channels, leading to the release of insulin. | Diabetes | Neutral effect |
| DPP4‑I | They act by inhibiting AT inflammation, and AGE‑receptor gene expression and activate vit D absorption and hence increase bone growth and remodeling. It also inhibits the signaling of p38 MAPK (Mitogen‑Activated P‑Kinase) phosphorylation and thus reduces osteoclast development. | Diabetes, rarely used in osteoporosis | Neutral/ beneficial effects |
| SGLT‑2 inhibitors | It produces detrimental effects but the mechanism is unclear | Diabetes | Negative impacts |
| GLP‑1 agonists | It binds to the GLP‑1 receptor and reduces AGEs which is responsible for increased bone resorption by decreasing osteoclast activity and enhancing C‑terminal telopeptide (CTX, a biological marker to measure bone turnover). | Diabetes, osteoporosis | Beneficial effects |
| Insulin | Due to its hypoglycemic effect, it increases the risk of fractures | Type‑1 diabetes and type‑2 diabetes | Negative impacts |
Table 1: Impact of the antihyperglycemic agents on bone disorders.
Potential mechanisms of GLP-1 agonists in diabetoporosis management
cAMP/PI3K/AKT (Phosphoinositide-3-kinase-protein kinase B/Akt) signaling pathway: The PI3K/AKT signaling pathway is vital for the regulation of apoptosis, proliferation, as well as osteoblast and osteoclast differentiation. Previous research indicates that GLP-1 agonists play a significant role in modulating the PI3K/AKT signaling pathway, thereby reducing cell apoptosis. This modulation results in elevated cAMP levels and AKT phosphorylation, promoting bone growth and osteogenic differentiation. Hence, they play a pivotal role in osteoporosis management by influencing bone metabolic pathways
MAPK (Mitogen-Activated Protein Kinase) signaling pathway: MAPK is essential for cell differentiation and is categorized into various classes, including ERKs (Extracellular Signal-Regulated Kinases), Stress-Activated Protein Kinases (SAPKs) or c-Jun Nterminal kinases, and p38 MAPKs. ERKs govern cell proliferation, SAPKs respond to stress, and p38 MAPKs regulate various cellular processes, such as inflammation, apoptosis, and cell cycle control. Recent evidence suggests that p38 MAPK plays a pivotal role in the differentiation of various tissues, including adipose tissue, neural tissue, and myocytes. It has also been observed that GLP-1 receptor agonists bind to GLP-1Rs, regulating the MAPK signaling pathway and exerting beneficial effects in the treatment of conditions like diabetes, tumors, and atherosclerosis. Moreover, GLP-1RA demonstrates similar effects in mitigating osteoporosis and reducing fracture risk.
Wnt/β-catenin (wingless-related integration site/β-catenin) signaling pathway: The Wnt/β-catenin pathway is indispensable for governing cell division, tissue repair, and apoptosis. Activation of this pathway promotes the differentiation and proliferation of mesenchymal stem cells, osteoblasts, and other cellular activities related to bone formation. Activation of the GLP-1 receptor enhances bone formation and inhibits fat cell formation by controlling the PKA/ β-catenin signaling pathway. Consequently, GLP-1 agonists are a promising class for managing osteoporosis through their regulation of the Wnt/β-catenin signaling pathway.
OPG/RANKL (Osteoprotegerin ligand/Receptor Activator of Nuclear factor Kappa-Β Ligand) signaling pathway: Osteoprotegerin, released by stromal stem cells and bone-forming cells, safeguards the skeletal system against bone resorption by binding with RANK ligands, preventing their interaction with RANK receptors. The RANKL/OPG ratio within the medullary cavity plays a pivotal role in determining bone mass under normal and abnormal conditions. This signaling pathway is thus central to the pathophysiology of bone-related disorders.
Influence of anti-osteoporotic medication on glucose metabolism
Bisphosphonates: These are primary medications for bone disorders. They readily adsorb hydroxyapatite, enabling radiolabeling for bone scanning. Bisphosphonates function by interacting with boneresorbing cells, gradually increasing bone mass and strength. They achieve this by reducing osteoclast activity, thereby decreasing bone turnover. Consequently, they aid in preserving bone density and strength and are commonly used in osteoporosis treatment.
As diabetes and osteoporosis often coexist, ongoing research assesses bisphosphonates' role in glucose metabolism. Multiple studies suggest that bisphosphonates either decrease glycemic levels or have neutral effects, making them a safer choice for diabetoporosis management.
Estrogen-Related Therapy (ERT): Raloxifene is a selective estrogen receptor modulator, approved by the FDA for preventing and treating osteoporosis in postmenopausal women.
Research continues to explore ERT's impact on glucose metabolism, and findings indicate neutral effects on glucose metabolism, making it a safe therapy for diabetoporosis management.
Parathyroid hormone analog: Teriparatide is approved for treating glucocorticoid-induced osteoporosis in individuals at high fracture risk, regardless of gender. However, it does not significantly affect glucose metabolism and, in some studies, even elevates glucose levels. Therefore, it is not recommended for osteoporotic patients with diabetes.
RANKL inhibitor: Denosumab, a fully human monoclonal antibody against RANKL, is FDA-approved for individuals at high fracture risk, irrespective of gender. While this class does not show promising results in glucose metabolism, clinical trials indicate neutral effects on glucose metabolism, allowing it to be prescribed to diabetic patients.
Sclerostin inhibitor: Romosozumab, a fully human monoclonal antibody to sclerostin, is FDA-approved for treating osteoporosis in postmenopausal women at high fracture risk. Similar to the aforementioned drugs, it does not produce significant effects on glucose metabolism and is not recommended for osteoporotic patients with diabetes.
Calcitonin salmon: Calcitonin is FDA-approved for postmenopausal women with osteoporosis at least 5 years after menopause. However, calcitonin produces hyperglycemic effects by reducing glycogen content and enhancing glucose-6-phosphate dehydrogenase activity. Consequently, this drug is not recommended for diabetoporosis treatment.
Supervision of medication regimen for osteoporosis
Clinical trials have proven least useful in determining the effectiveness and safety of the strategies that we can use to manage osteoporosis. However, they have proven to be far more challenging in determining the best follow-up methods for osteoporosis. How frequently should bone turnover indicators or bone mineral density measurements be taken? What level of change indicates acceptable antifracture effectiveness? What more steps are necessary if adequate changes are not seen? Because of the difficulty in answering these concerns through randomized controlled trials, clinical practice has relied more on "expert opinion" than on reliable data. Therefore, in spite of doing trials, epidemiological studies are going on to collect the appropriate data.
The best way to evaluate the effectiveness of pharmacological therapy is to assess the risk of fracture before and after treatment or to directly monitor alterations in bone strength. Dual X-ray absorptiometry and bone turnover markers are the two methods most frequently used in clinical settings to evaluate osteoporosis medication.
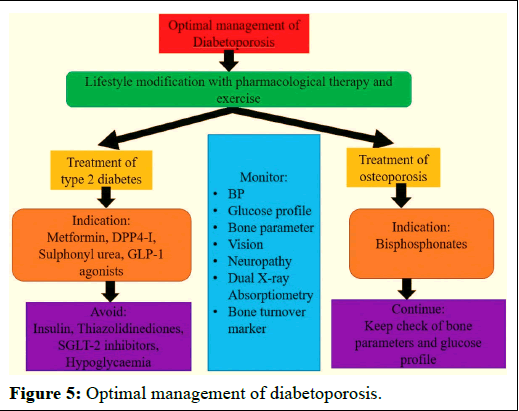
Successful osteoporosis care includes monitoring anti-osteoporotic therapy with the aim of identifying noncompliant patients (Figure 5). To date, there are not any official, widely accepted clinical practice guidelines for monitoring anti-osteoporosis medications. Future research is required to design monitoring therapy for osteoporosis and to determine how to use them economically.
Figure 5: Optimal management of diabetoporosis.
Conclusion
Osteoporosis is the most prevalent yet frequently overlooked issue in the diabetic population. Both T1DM and T2DM bring about pathological changes that drive the progression of osteoporosis, creating a mutual influence. Moreover, diabetes-related complications, such as impaired vision, kidney function, and balance, heighten the risk of fractures by increasing the likelihood of falls.
Furthermore, elevated blood sugar levels weaken bone strength and quality, rendering bones more prone to fractures. Hence, the cornerstone of diabetoporosis management lies in maintaining optimal glucose control. This is achieved through antidiabetic medications, herbal remedies, recombinant insulin, and/or lifestyle adjustments, all of which enhance osteoblast function, bone perfusion, and reduce medullary fat accumulation, ultimately improving bone quality and strength.
Future research is essential to uncover the mechanisms underpinning bone-related changes resulting from hyperglycemia and to gain a deeper understanding of how GLP-1 agonists are utilized in diabetoporosis management. This involves delving into the mechanisms and advantageous effects of GLP-1 agonists, a novel antihyperglycemic agent in osteoporosis management. Further exploration is needed to design and apply innovative antihyperglycemic agents for diabetoporosis management.
Conflict of Interest
There is no conflict of interests.
References
- Zarowitz BJ, Cheng LI, Allen C, O’Shea T, Stolshek B (2015) Osteoporosis prevalence and characteristics of treated and untreated nursing home residents with osteoporosis. J Am Med Dir Assoc 16: 341-348.
[Crossref] [Google Scholar] [PubMed]
- Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13: 791-801.
[Crossref] [Google Scholar] [PubMed]
- Sealand R, Razavi C, Adler RA (2013) Diabetes mellitus and osteoporosis. Curr Diab Rep 13: 411-418.
[Crossref] [Google Scholar] [PubMed]
- Li H, Xiao Z, Quarles LD, Li W (2021) Osteoporosis: Mechanism, molecular target, and current status on drug development. Curr Med Chem 28: 1489-1507.
[Crossref] [Google Scholar] [PubMed]
- Fistarol M, Rezende CR, Figueiredo Campos AL, Kakehasi AM, Geber S (2019) Time since menopause, but not age, is associated with increased risk of osteoporosis. Climacteric 22: 523-526.
[Crossref] [Google Scholar] [PubMed]
- Gao ST, Lv ZT, Zhou CK, Mao C, Sheng WB (2018) Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: A metaanalysis. BMC Musculoskelet Disord 19: 141.
[Crossref] [Google Scholar] [PubMed]
- Fu SC, Wang P, Qi MX, Peng JP, Lin XQ, et al. (2019) The associations of TNF-alpha gene polymorphisms with bone mineral density and risk of osteoporosis: A meta-analysis. Int J Rheum Dis 22: 1619-1629.
[Crossref] [Google Scholar] [PubMed]
- Chen YW, Ramsook AH, Coxson HO, Bon J, Reid WD (2019) Prevalence and risk factors for osteoporosis in individuals with COPD: A systematic review and meta-analysis. Chest 156: 1092-1110.
[Crossref] [Google Scholar] [PubMed]
- Albright FRE (1948) Bone development in diabetic children: A roentgen study. Am J Med Sci 12: 313-319.
- Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, et al. (2011) Increasing duration of type 1 diabetes perturbs the strength–structure relationship and increases brittleness of bone. Bone 48: 733-740.
[Crossref] [Google Scholar] [PubMed]
- Goswami R, Nair A (2019) Diabetes mellitus, vitamin D and osteoporosis: Insights. Indian J Med Res 150: 425-428.
[Crossref] [Google Scholar] [PubMed]
- Leidig-Bruckner G, Ziegler R (2001) Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 109: S493-S514.
[Crossref] [Google Scholar] [PubMed]
- Schwartz AV (2003) Diabetes mellitus: Does it affect bone? Calcif Tissue Int 73: 515-519.
[Crossref] [Google Scholar] [PubMed]
- Thrailkill KM, Lumpkin CK, Jr Bunn RC, Kemp SF, Fowlkes JL (2009) Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289: E735-E745.
[Crossref] [Google Scholar] [PubMed]
- Brown SA, Sharpless JL (2004) Osteoporosis: An under-appreciated complication of diabetes. Clin Diabetes 22: 10-20.
- Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int 18: 427-444.
[Crossref] [Google Scholar] [PubMed]
- Vestergaard P, Rejnmark L, Mosekilde L (2009) Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int 84: 45-55.
[Crossref] [Google Scholar] [PubMed]
- Melton LJ, Leibson CL, Achenbach SJ, Therneau TM, Khosla S (2008) Fracture risk in type 2 diabetes: Update of a population-based study. J Bone Miner Res 23: 1334-1342.
[Crossref] [Google Scholar] [PubMed]
- Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, et al. (2017) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 13: 208-219.
[Crossref] [Google Scholar] [PubMed]
- Lorenzati B, Zucco C, Miglietta S, Lamberti F, Bruno G (2010) Oral hypoglycemic drugs: Pathophysiological basis of their mechanism of action. Pharmaceuticals 3: 3005-3020.
[Crossref] [Google Scholar] [PubMed]
Citation: Fawad A, Sarwar MH, Ahmar Z (2025) Exploring the Impact of Antihyperglycemic Agents on Diabetoporosis: An Updated Perspective. Clin Pharmacol Biopharm 14: 540.
Copyright: © 2025 Fawad A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 255
- [From(publication date): 0-0 - Nov 10, 2025]
- Breakdown by view type
- HTML page views: 171
- PDF downloads: 84