Feasibility and Safety of Combining Therapeutic Hypothermia with Magnesium Sulfate Administration, in the Management of Neonates with Hypoxic Ischemic Encephalopathy - Randomized Control Trial
Received: 12-Aug-2018 / Accepted Date: 04-Sep-2018 / Published Date: 13-Sep-2018 DOI: 10.4172/2572-4983.1000165
Keywords: Newborn; Asphyxia; Hypoxic ischemic encephalopathy; Magnesium sulfate; Therapeutic hypothermia
Abbreviations
aEEG: Amplitude-integrated Electroencephalogram; BE: Base Excess; HIE: Hypoxic-Ischemic encephalopathy; TH: Therapeutic Hypothermia; SEM, Standard Error of the Mean; SHC: Selective Head Cooling; WBC: Whole Body Cooling; HIES: Hypoxic- Ischemic Encephalopathy Score
Introduction
In the first decade of the 21st century therapeutic hypothermia became broadly used in the management of the newborns with hypoxic ischemic encephalopathy [1]. The neonatal resuscitation guidelines since 2010 recommend that therapeutic hypothermia be used as a standard treatment method in asphyxiated newborns diagnosed with moderate or severe hypoxic ischemic encephalopathy [2]. Although the efficacy of therapeutic hypothermia has been shown in several randomized trials its beneficial effect is in many cases viewed as modest only. Therefore most new research efforts tend to focus on finding the ways to enhance the effect that therapeutic hypothermia produces. The currently considered potential new treatment modalities include: modifications of the cooling process, combining therapeutic hypothermia with potentially neuroprotective drugs, and stem cells therapy. These new potentially therapeutic drugs include, but are not limited to: topiramate, erythropoietin, xenon, melatonin, magnesium sulfate and cannabinoids [3]. Magnesium plays an essential role in numerous biochemical pathways, including the reactions which may protect the developing brain. This molecule is also an irreplaceable co factor in glycolysis reaction, oxidative phosphorylation, protein synthesis, DNA, and RNA aggregation as well as in the maintenance of plasmatic membrane integrity [4-6].
Magnesium is a physiological calcium channel blocker and calcium antagonist. It has been reported that magnesium defends tissues against free radicals and magnesium deficiency increases the risk of lipid peroxidation.
When administered peripherally, it has the ability to readily pass through to central nervous system and finally block NMDA receptors. The increase in extracellular level of Mg2+ from 0.5 to 1.0 mmol/L is adequate to induce synaptic and neuronal response. Numerous papers have already published on the neuroprotective efficacy of NMDA receptor antagonists in disorders resulting from toxic glutamate impact. Potential-dependent blocking by magnesium ions is one of the most significant features of NMDA receptor (Mg2+). NMDA receptor inhibition by Mg2+ ions increases along with postnatal age [7,8]. Magnesium sulfate administered antenatally to the mothers has recently been used as potentially neuroprotective management in preterm neonates [9]. In some centers, including ours, neuroprotecti ve properties of magnesium sulfate are also exploited in term, asphyxiated newborns [10,11]. Taking into account our previous experience with magnesium sulfate administered to asphyxiated term neonates and the outcomes of several other clinical trials, we’ve designed a randomized controlled trial to study the effect of therapeutic hypothermia combined with magnesium sulfate [12-15]. The research project was titled “Hypothermia Enhanced by Magnesium in Encephalopathy of Newborn” (acronym “HEMEN).
Aim of the Study
The aim of our study was to investigate the safety of therapeutic hypothermia combined with magnesium sulfate and its effect on the clinical course (circulatory stability, level of respiratory support, inotropes use, anti-seizure medications, use of blood components) and short term outcomes (death, neurological assessment, time to achieve full enteral nutrition, duration of hospitalization) in the group of neonates with HIE.
Material and Methods
Prospective randomized controlled trial was conducted at three centers: two Neonatal Intensive Care Units located in level III perinatal centers and one Pediatric Intensive Care Unit. The RCT protocol has been approved by the local bioethical board - PMM Hospital - Research Institute Ethical Committee. Written informed consent was obtained from all the parents of the study subjects.
The eligibility criteria were similar to previously reported in other RCTs studying the effects of therapeutic hypothermia in newborns: gestational age above 36 weeks of gestation, acute perinatal sentinel event (perinatal asphyxia) confirmed by Apgar score of ≤5 at 10 minutes of life and/or umbilical cord blood or any blood during the first hour after birth with pH <7.1 and/or base deficit ≥16, need of mechanical ventilation for at least 10 minutes after birth, and admission within 6 hrs of birth. Neonates with major congenital malformations and extremely poor prognosis (Apgar score 0 at 15 minutes of life or later) were not included in the study.
All infants meeting the above listed criteria underwent the standardized neurological evaluation (Sarnat grading scale). Newborns which have fulfilled the clinical criteria of moderate or severe hypoxic ischemic encephalopathy were further screened with aEEG using Infant aEEG cerebral function monitor Olympic CFM™ 6000. Patients with moderate or severe abnormalities on aEEG were randomized to either the control (therapeutic hypothermia - TH) or study group (therapeutic hypothermia with magnesium sulfate - TH+MgSO4). Each of the participating centers received an individually designed randomization table prepared by the central coordinating board. The long-term evaluation of psychomotor development according to the scale BAYLEY III is still being conducted and will be the subject of further publications.
Therapeutic hypothermia protocol
Therapeutic hypothermia was induced and maintained for 72 hours. The studied neonates underwent either selective head cooling (SHC) or whole body cooling (WBC). Natus/Olympic Medical Cool Cap System or Inspiration Healthcare/Tecotherm Neo was used for the cooling process.
Rectal temperature was maintained at 34.0-35.0°C in the SHC group, and slightly lower at 33.0-34.0 C in the whole body cooling group. The cooling period was followed by a rewarming phase at the rate of 0.5°C/ hr.
Administration of MgSO4 protocol
Neonates who were randomized to the study group (TH+Mg) received three 250 mg/kg doses of magnesium sulfate given as onehour continuous infusion spaced 24 hours apart on three consecutive days. 20% Magnesium Sulfuricum (Polpharma), 2 g/10 ml were used.
Serum Mg level expressed as mmol/dL, was assessed on study day 1 just before and one hour after infusion of the drug was completed, and then on day 2, 3, and 4 randomly with other routine labs. Serum magnesium level was measured with dry chemistry system - colorimetric method using a formazon dye (Analyser: Vitros 5.1/350 Ortho Clinical Diagnostics or Roche Diagnostics, Cobas 6000 analyzer series).
Throughout the duration of the cooling and rewarming process, vital signs (heart rate, arterial blood pressure, rectal temperature) and parameters of respiratory support (FiO2, MAP, level of respiratory support) were monitored and recorded every hour for the first 6 hours, then every three hours, and again every hour during rewarming. Total of 36 measurements were taken. The vital signs were monitored using Philips IntelliVue MP70 Neonatal Monitors or Drager Infinity® Delta Monitors.
The use of inotropes, anticonvulsants, volume expanders as well as the number of blood components (platelets, fresh frozen plasma, red blood cells) transfusions during first 7 days of life were also recorded. Parameters of coagulation were followed closely. Additionally clinical data such as number of seizure episodes, incidence of IVH detected by cranial USG, rate of PPHN confirmed by ECHO exam and feeding difficulties were analyzed.
Assessment of the neurological status of the studied infants was performed by trained personnel daily during the cooling and rewarming phase using the Hypoxic-Ischemic Encephalopathy Score described by Thompson-HIES [16].
Statistical analysis
Chi-square or Fisher exact test were used to compare the percent accuracy between groups. The difference between the groups and over time changes for continues data were analyzed with one-way or twoway analysis of variance (ANOVA). Fishers LSD (least squares difference) post hoc comparison tests were applied to examine if significant differences between means exist. Differences in mean ranks values between groups were analyzed with the Student's t-test or Mann-Whitney U test. Statistical analysis was carried out using STATISTICA (StatSoft, Tulsa, OK, USA, version 6.0 PL). The p-value <0.05 was considered statistically significant.
Results
Study population demographic data and clinical characteristics are presented in Table 1.
|
|
Control group (TH) 37 |
Study group (TH+Mg) 38 |
p-value |
|---|---|---|---|
| Gestational age (weeks); mean (±SD) | 38.58 (±1.9) | 38.73 (±1.7) | 0.71 |
| Female gender, number (%) | 28/14 (43) | 20/16 (36) | 0.48 |
| Birth body weight (g); mean (±SD) | 3173.8 (644.7) | 3321.9 (524.0) | 0.28 |
| Cesarean section (%) | 28 (75.7) | 24 (64.2) | 0.25 |
| Apgar score: | |||
| @ 5 minutes | 2.52 (±1.71) | 3.08 (±1.74) | 0.16 |
| @ 10 minutes | 3.08 (±1.71) | 3.71 (±1.79) | 0.13 |
| Umbilical blood pHa (±SD) | 6.87 (0.148) | 6.89 (0.157) | 0.47 |
| Outborns n (%) | 29 (78.4) | 30 (78.6) | 0.87 |
| Base deficit | -20.50 (±5.8) | 19.8 (±5.6) | 0.83 |
| Core temperature at admission (°C) | 33.9 (±1.69) | 33.8 (±1.85) | 0.83 |
| Sarnat grading scale at <6 | |||
| -Stage II | 22 | 24 | |
| -Stage III | 15 (42.9) | 14 (37.8) | 0.67 |
| Seizures on admission (n; %) | 13 (35.1) | 15 (44.1) | 0.45 |
| Time to initiation of hypothermia (minutes of life) | 33.79 (±108.2) | 305.0 (±134.2) | 0.21 |
| Infusion start time of MgSO4 | - | 227.8 (±143.2) | - |
The p-value <0.05 was considered statistically significant. aumbilical cord blood or any blood sampling during the first hour after birth
Table 1: Demographic data and clinical characteristic of the study subjects.
There were no statistically significant differences between the groups according to birth body weight, gestational age and Apgar score. Umbilical cord blood pH, BE, core temperature at admission and neurological assessment at the time of initiation of the cooling were also not different between the groups. 29 (78.4%) of patients in the control group and 30 (78.6%) patients in the study group were outborn and transferred to the one of the three study centers by specialized regional neonatal transport. The rate of cesarean sections, which were performed mainly for the emergency reasons, was not different between the groups. Time to initiation of cooling procedure didn’t exceed 6th hours of life and was (mean, ± SD) 337.9 (±108.2) and 305.0 (±134.2) minutes in the control and study group respectively. The difference was not statistically significant.
We did not consider the outcome with regard to the cooling method. There were 49 subjects in the SHC and 26 in the WBC group. Due to randomization conducted separately for each center the groups were equal according to magnesium administration: WBC (TH-13; TH +Mg-13) and SHC (TH-24; TH+Mg-25).
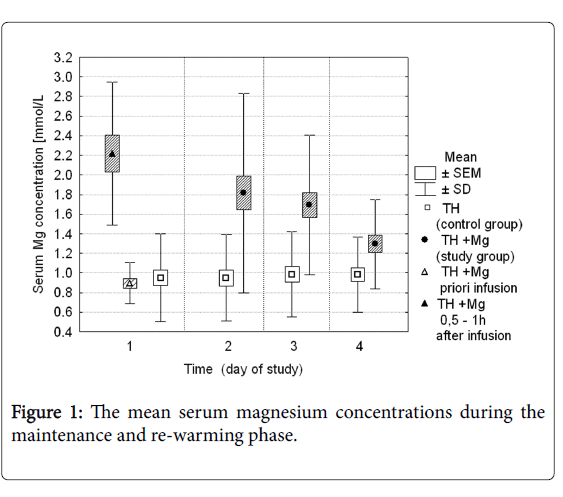
Serum magnesium concentration in both groups during the first 4 days of the study is presented in Figure 1. At the study entry (time 0), mean serum Mg concentration was 0.89 mmol/L in the study group, and 0.95 mmol/L in the control group. Values were not significantly different. After the first infusion magnesium sulfate serum magnesium concentration increased to mean 2.21 mmol/L (ranged 1.43 to 3.77 mmol/L). In the next two days the average Mg concentrations ranged between 1.82 and 1.69 mmol/L, and then decreased to the mean values of 1.32 mmol/L on the study day 4. During the same time period in the control group serum magnesium concentration remained stable and ranged from 0.94 to 0.98 mmol/L. The difference between the groups was statistically significant (p-values <0.05).
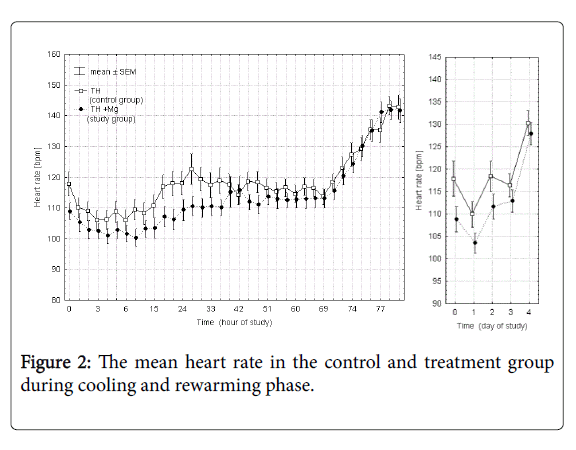
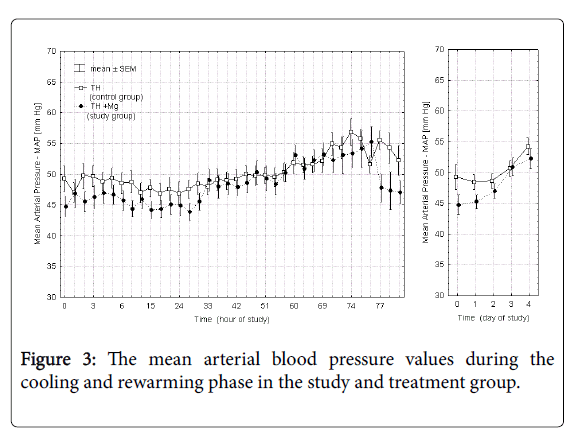
Figures 2 and 3 presents the mean heart rate and mean arterial blood pressure values in each of the 36 points of measurement in the control and study groups during cooling and rewarming phase and additionally the averaged values over each day of the study. Time 0 in these and subsequent figures marks the initiation of the cooling procedure. In the study group (TH+Mg) there was a slight decrease in heart rate (by 7.6%) and mean arterial pressure (by 7.2%) on the first day of the study, but the difference between the groups was not statistically significant and of minor clinical significance.
Selected neonatal outcomes in the control and study group are presented in Table 2. Time to full oral feedings (bottle feeding or breastfeeding) was significantly reduced in the treatment group (16.5 vs. 14.5 days). Furthermore, 33 of 34 (97%) patients in the treatment group were discharged from the hospital on the full oral feedings compared with only 27 of 36 (75%) in the control group. Also the duration of hospitalization time was shorter in the group neonates receiving magnesium sulfate (27.7 vs. 23.5 days). Number of patients requiring anticonvulsants were not different between the groups, but number of doses per patient was significantly lower in the study group.
| Control group (TH) 37 | Study group (TH+Mg) 38 | Statistical significance | |
|---|---|---|---|
| Respiratory support [days] mean (±SD)a | 5.3 (±4.0) | 4.67 (±4.8) | NS |
| Noninvasive support [days] mean (±SD) aa | 0.63 (±1.1) | 1.85 (±4.1) | 0.08 |
| Oxygen supplementation [days] mean (±SD) | 4.67 (±4.8) | 4.51 (±6.1) | NS |
| Diagnosis of PPHN | 6 (16.2) | 3 (7.9) | 0.049 |
| iNO treatment | 3 (8.1) | 2 (5.3) | 0.21 |
| RBC transfusion (No of patients) | 5 | 5 | NS |
| No/patient | 0.17 | 0.24 | |
| FFP transfusion | 13 | 11 | NS |
| No/patient | 0.72 | 0.4 | |
| PLT transfusion | 4 | 3 | NS |
| No/patient | 0.14 | 0.08 | |
| Catecholamine use: | |||
| - None | 18 | 11 | |
| - 1 | 19 | 27 | 0.08 |
| - =2 | 15 | 15 | NS |
| Use of anticonvulsant (No of patients) | 31 | 28 | NS |
| No of doses/patient | 4.4 | 2.6 | 0.0025 |
| Intracranial hemorrhage (grade) | I-3 | I-0 | NS |
| II-2 | II-2 | ||
| Antibiotic therapy [days] mean (±SD) | 14.81 (±7.6) | 13.63 (±6.8) | NS |
| Full enteral feeding | 14.5 (±5.9) | 14.2 (±5.1) | NS |
| Oral feedings (sucking) [days] mean (±SD) | 16.5 (±9.4) | 14.5 (±5.5) | 0.0057 |
| Age at discharge/DOL mean (±SD) | 27.77 (±14.1) | 23.55 (±8.0) | 0.0026 |
| Death during hospital stay n (%) | 2 (5.4) | 3 (7.9) | NS |
The p value of < 0.05 was considered statistically significant
aany type of mechanical ventilation (SIMV, PC, HFOV), aaany type of noninvasive respiratory support (Noninvasive ventilation, nasal CPAP).
Table 2: Neonatal outcomes in the study population.
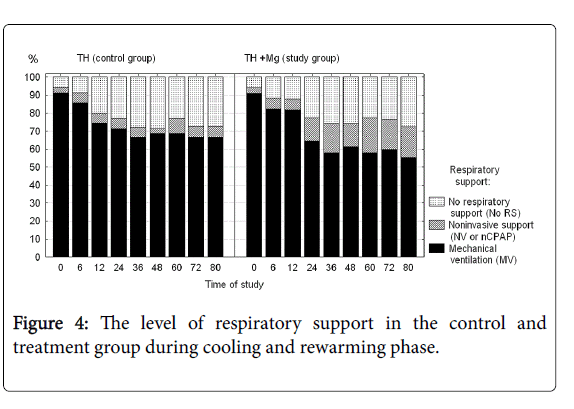
Number of days on mechanical ventilation and number of days on oxygen were not significantly different between the groups. During the cooling phase neonates receiving magnesium required a somewhat longer support with non-invasive ventilation (Figure 4), but again the difference did not reach statistical significance.
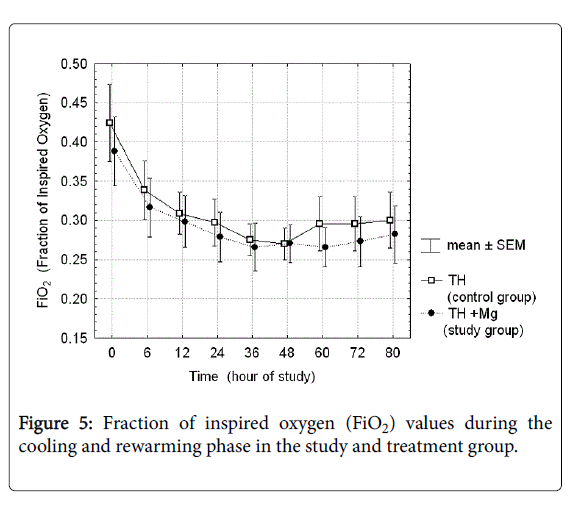
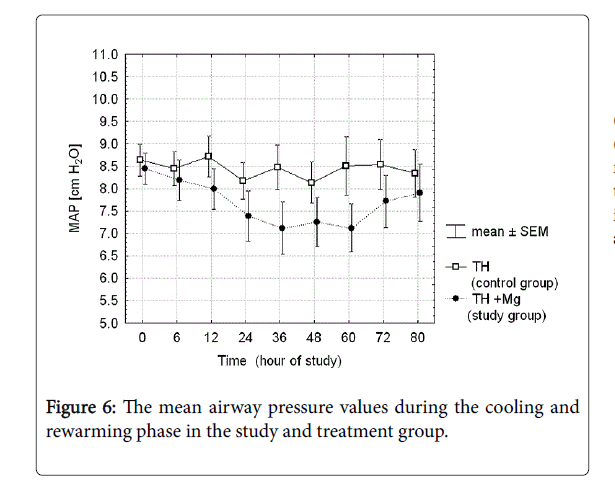
There were analyzed together of any type of mechanical ventilation (SIMV, PC, HFOV) and any type of noninvasive respiratory support (noninvasive ventilation, nasal CPAP). There was a three-fold reduction of PPHN rate and need for INO in the treatment group, but the differences did not reach the statistical significance. Fraction of inspired oxygen (Figure 5) and mean airway pressure (Figure 6) were also not different between the groups.
Duration of antibiotic therapy was not affected by the treatment with magnesium sulfate. Inotropes use was slightly higher in the group of patients receiving magnesium sulfate, but the difference was not statistically significant. The overall death rate was 6.7%. The mortality did not differ between the groups.
Coagulation parameters were evaluated as clinical indicated. Although more often controlled in the study group (34 vs. 25 results,) the values were similar in both groups (Table 3).
| Control group (TH) | Study group (TH+Mg) | p-value | |
|---|---|---|---|
| No of results | 25 | 34 | |
| Prothrombin time | 27.6 (±18.6) | 23.3 (±15.6) | 0.334 |
| INR time | 60.5 (±20.9) | 67.4 (±21.5) | 0.351 |
| aPTT | 54.9 (±27.1) | 54.7 (±29.7) | 0.981 |
The p-value of <0.05 was considered statistically significant.
Table 3: Coagulation parameters in the study and treatment group.
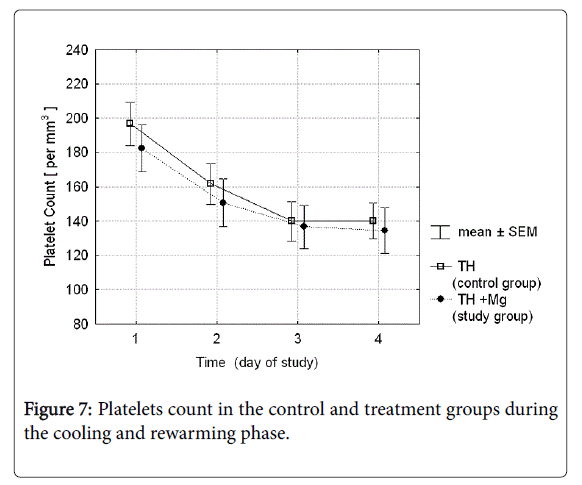
The mean platelet count in the group receiving magnesium sulfate was slightly lower than in controls, but the difference was not statistically significant (Figure 7). The rate of thrombocytopenia ≤100,000/mm3 was 40.5 vs. 50.0% in TH vs. TH+Mg group respectively; p-value 0.336. Number of blood components transfusions needed was not significantly different either (Table 2).
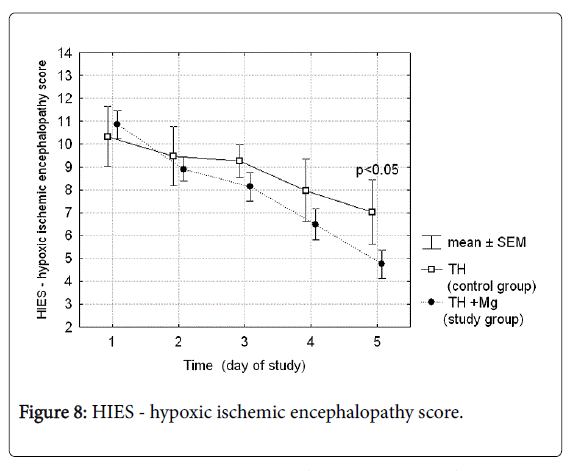
Hypoxic-ischemic encephalopathy scores (Thompson scale) performed daily during the cooling and rewarming procedure in both groups of patients are presented in Figure 8. On day 5 of the study after the cooling procedure was completed the score was significantly lower in the treatment group (4.75 vs. 7.03; p-value 0.03).
Discussion
Since the therapeutic hypothermia is considered a standard of care for the patients with HIE, clinical trials investigating the effects of new treatment options for this group of patients, especially drugs with potential neuroprotective effects will have to include hypothermia as a part of a study protocol. Until now there are several published studies evaluating the effectiveness of magnesium sulfate in the group of asphyxiated neonates, including two randomized controlled trials [17,18]. Results are promising. However, all of these studies were conducted before the era of therapeutic hypothermia. Furthermore, irrespective of the potential benefits, safety of using magnesium sulfate during therapeutic hypothermia in the group of term and late preterm neonates was not studied. It is particularly important in the light of the results presented by Mittendorf, et al. They studied the effects of prenatal aggressive treatment with magnesium sulfate on the outcome of the neonates born with very low birth weight and showed that patients exposed to high doses of magnesium were at higher risk for developing severe intracranial bleeding [19,20]. Other known side effects of high serum magnesium levels are: vasodilatation, hypotension, cardiac arrhythmias, coagulopathy, and gastrointestinal disturbances. Some of the potential side effects of magnesium administration are similar to the symptoms observed in the newborns with asphyxia or side effects of therapeutic hypothermia: bradycardia, hypotension, thrombocytopenia [21].
Although the therapeutic window for magnesium sulfate to act as a neuroprotective agent is not known it seems reasonable to start magnesium therapy as soon as possible after the hypoxic insult. In our study, the magnesium treatment was started on average 75 minutes before the initiation of hypothermia. In the study group expected therapeutic range of serum magnesium level was reached with the first dose of MgSO4 and then maintained for 3 days of treatment [7,22,23]. According to Levene and co-workers serum magnesium concentration in the range between 1.5-2.8 mmol/dL should have the neuroprotective effect. Previously published studies have also shown that the target concentration range can be achieved with magnesium sulfate administered at the dose of 250 mg/kg.
We have observed that magnesium sulfate given to hypoxic newborns undergoing hypothermia does not significantly increase the occurrence of bradycardia or hypotension. The small drop in mean blood pressure in the group receiving magnesium was not associated with an increased need for vasopressors. This observation is consistent with the results reported by other authors using magnesium sulfate in similar dosage but without hypothermia [24]. Since, before the era of inhaled nitric oxide, magnesium sulfate was used for the treatment of persistent pulmonary hypertension of newborn observed in our study mild vasodilation may be perceived as an additional beneficial effect of magnesium administration [25]. It may also explain observed threefold reduction of PPHN rate and need for iNO in our treatment group.
Because of its platelets inhibiting properties, antenatal exposure to high doses of magnesium sulfate may be associated with increased risk of intracranial bleeding. In our study magnesium sulfate administration was not associated with the increased risk of intracranial bleeding, and had no significant effect on the number of blood products transfusion or coagulation test results [19,20,26-28].
Side effects might depend on the dose of the drug. Optimal dose of magnesium sulfate for the neonates with hypoxic-ischemic encephalopathy is yet not determined. Levene and Blennow evaluated two different doses of magnesium sulfate (MgSO4) in a group of full term infants with Apgar scores of <6 at 10 minutes [22]. With the larger dose (400 mg/kg) they observed hypotension at one hour post infusion and respiratory depression lasting three to six hours. EEG readings and heart rate were not affected. Mean serum Mg2+ increased from 0.79 to 3.6 mmol/L at one hour after infusion. After administration of the lower dose of MgSO4 (250 mg/kg) mean serum level Mg2+ increased from 0.71 to 2.42 mmol/L at one hour and blood pressure, heart rate, muscle tone and EEG were unchanged. The authors concluded that dose of 250 mg/kg MgSO4 was not associated with hypotension although respiratory depression can occur. Because of the potential risk of hypotension, some experts suggest staring dopamine infusion with magnesium sulfate administration [23]. Others, however, didn’t observe any significant effect on blood pressure, heart rate or respiratory rate. Respiratory depression was previously reported by some authors especially when using higher doses of MgSO4 [21]. In this study, magnesium administration did not have a significant effect on the duration and level of respiratory support or oxygen requirement. Ichiba, et al conducted randomized control trial studying the effect of postnatal administration of MgSO4 (250 mg/kg, 3 doses administered daily as continuous infusion) [23,24]. Analyzing the clinical outcomes they’ve noticed significantly higher survival rate with normal results of cranial computed tomography, electroencephalography and establishment of oral feeding by 14 days of age in the treated group compared with the control group. They’ve concluded that magnesium sulfate administered postnatally may improve neurodevelopmental outcome in infants with severe birth asphyxia. Those studies however were performed before the era of therapeutic hypothermia.
The presented research was carried out in the first three hypothermia centers established in the country. The studied neonates underwent either selective head cooling (SHC) or whole body cooling (WBC) depending on the availability. So far we have a several studies comparing the two aforementioned methods of therapeutic hypothermia of newborns [29]. The stability of vital parameters: ABP and HR at hourly intervals were previously assessed by Hoque et al. [30]. The authors did not find differences in mean arterial blood pressure or mean heart rate between groups (SHC vs. WBC) during the maintenance phase of cooling. The risk of adverse effects and early outcome depending on the therapeutic hypothermia method used were assessed by another group of researchers [31]. The authors did not reveal differences in terms of hypotension, bradycardia, thrombocytopenia, abnormality in the coagulation tests, renal dysfunction, need for mechanical ventilation and weaning from respiratory support while cooling or PPHN. Likewise, research conducted at University of Michigan confirmed that important components of multi-organ system dysfunction and hypotension treated with inotropes for >24 h, commonly required in newborns with HIE, were similar for both methods of TH [32]. The requirement for invasive mechanical ventilation, ability to perform extubation during the period of cooling and risk of PPHN were analyzed in two studies [31,33]. No differences were found between groups in regard to the need of mechanical ventilation during cooling or ability to be extubated during the cooling. Ventilatory parameters during the cooling including maximum peak inspiratory pressure (PIP), maximum positive end-expiratory pressure (PEEP), maximum FiO2 (fraction of inspired oxygen), and highest PaCO2 (partial pressure of carbon dioxide) were not different between whole body and selective head cooling either [33]. Finally, also Sarkar et al. who conducted an observational study examining organ function in whole body cooling and selective head cooling, stated that indicators of organ dysfunction were similar between group including coagulopathy treated with fresh frozen plasma (FFP), thrombocytopenia treated with platelet transfusion, hypotension treated with vasopressors for >24 hours [32]. The above mentioned group of authors published results of long term follow-up in prospective randomized, small-scale pilot study, when the rate of death, severe disability, composite outcome of death or neuromotor development delay was evaluated. The authors did not reach statically significant differences [34]. Thus, neither SHC nor WBC has been proven superior in terms of safety and efficacy. At present, both methods are considered as appropriate and equivalent [35] and therefore in this study we did not consider the outcome with regard to the cooling method.
Easy availability makes magnesium sulfate a very attractive option as a neuroprotective drug. It can be administered to the patient in the birth hospital while neonate is being prepared for the transport to the center with therapeutic hypothermia. Timing of the intervention is very important in the management of the neonates suffering from perinatal asphyxia. Both, therapeutic hypothermia, as well as administration of potentially neuroprotective drug should be started during so called “therapeutic window”. It is the initial potentially reversible phase of hypoxic insult lasting about 6 hours followed by the irreversible phase of apoptosis and destruction of neurons [36]. If the long terms follow up shows that magnesium sulfate has an additive neuroprotective effect and no significant side effects in the group of asphyxiated neonates treated with therapeutic hypothermia this relatively simple and not expensive intervention may be introduced into clinical practice.
Conclusion
• Combining therapeutic hypothermia with MgSO4 administration is not associated with significant adverse effects. Coagulation parameters, number of blood components transfusions and incidence of intracranial bleedings were not affected by the magnesium sulfate administration.
• Magnesium sulfate administration improved the neurological status at 5 days of age and shortened hospitalization time. The study group was also more likely to be on full oral feedings at the time of discharge.
• Potential beneficial effects of the magnesium sulfate on the neurological status need tbe confirmed by a long term follow-up.
Funding
NN407547538 form Polish Ministry of Education and Science
Clinical Trials Gov. ID: NCT02499393.
Acknowledgements
The authors wish to thank the patients’ parents for their consent to include the newborns in this trial. The authors also express their gratitude to the regional hospitals for their cooperation with the hypothermia therapeutic centers engaged in this study.
Competing Interests
None declared
Ethics Approval
The RCT protocol has been approved by the local bioethical board – PMMH-Research Institute Ethical Committee.
References
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, et al. (2013) Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 1: CD003311.
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, et al. (2010) Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122: 516-538
- Buonocore G, Perrone S, Turrisi G, Kramer BW, Balduini W (2012) New pharmacological approaches in infants with hypoxic-ischemic encephalopathy. Curr Pharm Des 18: 3086-3100.
- Berger R, Garnier Y (1999) Pathophysiology of perinatal brain damage. Brain Res Rev 30: 107-134.
- Bachelard HS, Clark AG, Thompson MF (1971) Cerebral-cortex hexokinase. Elucidation of reaction mechanisms by substrate and dead-end inhibitor kinetic analysis. Biochem J 123: 707-715.
- Schuchmann S, Buchheim K, Meierkord H, Heinemann U (1999) A relative energy failure is associated with low-Mg2+ but not with 4-aminopyridine induced seizure-like events in entorhinal cortex. J Neurophysiol 81: 399-403.
- Gathwala G, Khera A, Singh J, Balhara B (2010) Magnesium for neuroprotection in birth asphyxia. J Pediatr Neurosci 5: 102-104.
- Chahal H, D'Souza SW, Barson AJ, Slater P (1998) Modulation by magnesium of N-methyl-D-aspartate receptors in developing human brain. Arch Dis Child Fetal Neonatal Ed 78: 116-120.
- Crowther CA, Brown J, McKinlay CJ, Middleton P (2014) Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev 8: CD001060.
- Whitelaw A (2000) Systematic review of therapy after hypoxic-ischaemic brain injury in the perinatal period. Semin Neonatol 5: 33-40.
- Perlman JM (2006) Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther 28: 1353-1365.
- Szemraj J, Sobolewska B, Gulczynska E, Wilczynski J, Zylinska L (2005) Magnesium sulfate effect on erythrocyte membranes of asphyxiated newborns. Clin Biochem 38: 457-464.
- Zylinska L, Gulczynska E, Kozaczuk A (2008) Changes in erythrocyte glutathione and plasma membrane calcium pump in preterm newborns treated antenatally with MgSO4. Neonatology 94: 272-278.
- Gulczynska E, Gadzinowski J, Wilczynski J, Zylinska L (2006) Prenatal MgSO4 treatment modifies the erythrocyte band 3 in preterm neonates. Pharmacol Res 53: 347-352.
- Gulczynska E (2007) The effect of antenatal magnesium sulphate administration on erythrocytes membrane structure and function and on neonatal outcome of very low birth weight neonates. PAN, Poznan, Poland.
- Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, et al. (1997) The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 86: 757-761.
- Bhat MA, Charoo BA, Bhat JI, Ahmad SM, Ali SW, et al. (2009) Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics 123: 764-769.
- Hossain MM, Mannan MA, Yeasmin F, Shaha CK, Rahman MH, et al. (2013) Short-term outcome of magnesium sulfate infusion in perinatal asphyxia. Mymensingh Med J 22: 727-735.
- Mittendorf R, Dambrosia J, Dammann O, Pryde PG, et al. (2002) Association between maternal serum ionized magnesium levels at delivery and neonatal intraventricular hemorrhage. J Pediatr 140: 540-546.
- Mittendorf R, Dammann O, Lee KS (2006) Brain lesions in newborns exposed to high-dose magnesium sulfate during preterm labor. J Perinatol 26: 57-63.
- Boutaybi N, Razenberg F, Smits-Wintjens VE, van Zwet EW, Rijken M, et al. (2014) Neonatal thrombocytopenia after perinatal asphyxia treated with hypothermia: a retrospective case control study. Int J Pediatr 2014: 760654.
- Levene M, Blennow M, Whitelaw A, Hanko E, Fellman V, et al. (1995) Acute effects of two different doses of magnesium sulphate in infants with birth asphyxia. Arch Dis Child Fetal Neonatal Ed 73: F174-177.
- Ichiba H, Yokoi T, Tamai H, Ueda T, Kim TJ, et al. (2006) Neurodevelopmental outcome of infants with birth asphyxia treated with magnesium sulfate. Pediatr Int 48: 70-75.
- Ichiba H, Tamai H, Negishi H, Ueda T, Kim TJ, et al. (2002) Randomized controlled trial of magnesium sulfate infusion for severe birth asphyxia. Pediatr Int 44: 505-509.
- Abu-Osba YK, Galal O, Manasra K, Rejjal A (1992) Treatment of severe persistent pulmonary hypertension of the newborn with magnesium sulphate. Arch Dis Child 67: 31-35.
- Ho JJ, Rasa G (2007) Magnesium sulfate for persistent pulmonary hypertension of the newborn. Cochrane Database Syst Rev 3: CD005588.
- Leaphart WL, Meyer MC, Capeless EL, Tracy PB (1998) Adenosine diphosphate-induced platelet activation inhibited by magnesium in a dose-dependent manner. Obstet Gynecol 91: 421-425.
- Ravn HB, Kristensen SD, Vissinger H, Husted SE (1996) Magnesium inhibits human platelets. Blood Coagul Fibrinolysis 7: 241-244.
- Allen KA (2014) Moderate hypothermia: Is selective head cooling or whole body cooling better? Adv. Neonatal Care 14: 113-118.
- Hoque N, Chakkarapani E, Liu X, Thoresen M (2010) A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics 126: e124-130.
- Atıcı A, Çelik Y, Gülaşı S, Turhan AH, Okuyaz C, et al. (2015) Comparison of selective head cooling therapy and whole body cooling therapy in newborns with hypoxic ischemic encephalopathy: short term results. Turk Pediatri Ars 50: 27-36.
- Sarkar S, Barks JD, Bhagat I, Donn SM (2009) Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: Whole-body cooling versus selective head cooling. J Perinatol 29: 558-563.
- Sarkar S, Barks JD, Bhagat I, Dechert R, Donn SM (2008) Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns: Whole body versus selective head cooling. Am. J Perinatol 26: 265-270.
- Celik Y, Atıcı A, Gulası S, Okuyaz C, Makharoblidze K, et al. (2016) Comparison of selective head cooling versus whole-body cooling. Pediatr Int 58: 27-33.
- Takenouchi T, Iwata O, Nabetani M, Tamura M (2012) Therapeutic hypothermia for neonatal encephalopathy. Brain Dev 34: 165-170.
- Scher M (2001) Perinatal asphyxia: timing and mechanisms of injury in neonatal encephalopathy. Curr Neurol Neurosci Rep 1: 175-184.
Citation: Gulczynska E, Gadzinowski J, Nowiczewski M, Sobolewska B, Caputa J, et al. (2018) Feasibility and Safety of Combining Therapeutic Hypothermia with Magnesium Sulfate Administration, in the Management of Neonates with Hypoxic Ischemic Encephalopathy - Randomized Control Trial. Neonat Pediatr Med 4: 165. DOI: 10.4172/2572-4983.1000165
Copyright: © 2018 Gulczynska E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5187
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4276
- PDF downloads: 911