Fibromyalgia in the Context of Rheumatoid Arthritis: A Review
Received: 02-Jan-2016 / Accepted Date: 27-Jan-2016 / Published Date: 01-Feb-2016
Abstract
Fibromyalgia syndrome (FMS) is the second most common rheumatologic disorder affecting 2%-8% of the population; it can also occur secondary to many inflammatory conditions, in particular prevalence is estimated at 15%-20% in rheumatoid arthritis (RA). The underlying mechanism for primary and secondary FMS has previously been poorly understood, however evidence in support of central and peripheral sensitization theories alongside impaired pain inhibition are becoming progressively recognised to contribute towards allodynia and hyperalgesia; the abnormal pain responses confirmed to occur in FMS in neuroimaging studies.
A diagnosis of secondary FMS poses many challenges to physicians, hence, alongside the American College of Rheumatology 2010 FMS criteria; a joint count difference scoring methods has been proposed to assist in diagnosing concomitant FMS in RA. However, cognitive and somatic elements of FMS must also be taken into consideration when diagnosing the condition. Literature demonstrates that it is of paramount importance to identifying secondary FMS in RA because unrecognized FMS can result in heightened disease activity scores and thus over treatment of RA or, in some cases, the cessation of therapies due to a perceived lack of efficacy.
Furthermore, it is imperative to diagnose coexisting FMS in patients with RA as treatments for RA alone will not target the symptoms of FMS, additional pharmacological and non-pharmacological treatments such as antidepressants and a graded exercise program respectively, are of proven benefit and should be encouraged for all patients. The intricate relationship between RA and FMS is also entwined with underlying depression; the combination of all three has vast implications for quality of life and is known to impede the chances of obtaining RA disease remission.
Keywords: Hyperalgesia; Fibromyalgia syndrome; Allodynia
4727Introduction
Fibromyalgia syndrome (FMS) is a chronic pain disorder characterized by widespread musculoskeletal pain and concomitant somatic symptoms such as fatigue, cognitive disturbance, disruption of sleep and psychiatric symptoms, for which no other cause is identified [1]. It is the second most common “rheumatic” disorder after osteoarthritis with prevalence varying from 2% to 8% of the population [1-3]. It has been shown to be more prevalent amongst females with a female to male ratio of 2:1 according to current diagnostic criteria; this statistic reflects what is known regarding other chronic pain conditions [3].
FMS can develop in any person at any age, including, in rare cases, young children; however it is most common in middle age. Interestingly it is recognized worldwide with similar prevalence’s amongst differing countries, cultures, and ethnic groups with no evidence of increased prevalence in industrialized countries and cultures [2]. Individuals with strong family history of FMS are 8.5 times more likely to develop this condition [4] and several studies have indicated an underlying genetic predisposition for this. Whole exome sequencing has even identified potential gene variants; although further investigation is still required [5-7].
There are several factors, which increase susceptibility to FMS including infections, such as Epstein-Barr virus, Lyme disease, Q fever and viral hepatitis [8] alongside environmental factors, such as physical trauma and psychosocial stressors [9]. Multiple studies have demonstrated an increased prevalence of FMS in patients with chronic inflammatory arthritis and systemic autoimmune rheumatic diseases compared to the general population; over recent years this has been termed ‘Secondary FMS”.
Among inflammatory rheumatological disorders, approximately 15%-30% of individuals meet FMS criteria however even within the field of Rheumatology the prevalence can vary according to the underlying condition. FMS is estimated to have the highest prevalence when associated with vasculitic conditions such as Granulomatous Polyangiitis or Takayasau’s arteritis with an estimated prevalence of 23% and 28.5% respectively [10,11]. FMS secondary to Systemic Lupus Erythematous can vary between 6%-22% [12,13], whereas the prevalence in Sjogren’s syndrome has been shown to be 18% [14]. Within rheumatoid arthritis (RA) it is estimated that 15%-20% of patient will suffer from FMS [15,16].
Pathophysiology of pain in fibromyalgia
In FMS, functional neuroimaging confirms that patients experience hyperalgesia (increased pain in response to normally painful stimuli) and allodynia (pain in response to normally painless stimuli) [17,18]. This altered response is thought secondary to the process of central sensitization (CS), peripheral sensitization and change in the descending pathways [17].
CS can result from peripheral stimulation causing excessive release of neurotransmitters, glutamate and substance P, at the spinal level. These cause neuron excitability and thus, when in excessive quantity, heightened transmission of pain signal resulting in reduced pain threshold, diffuse pain and increased pain following stimuli [17,19]. This theory led to the misconception that constant noxious peripheral stimuli, was required to maintain this response. Lately, this has been reconsidered with reports of ‘innate’ CS influenced by genetics, chemical exposure, neurotransmitter or endocrine abnormalities, past trauma and sleep deprivation. Thus, conventional peripheral stimuli are not involved and we can surmise that in FMS, CS pathophysiology is multifactorial [19].
In RA peripheral sensitization results from the initial systemic inflammatory chemical environment stimulating peripheral nociceptors (pain receptors). Overtime, heightened stimulation of nociceptors leads to abnormal receptor sensitivity and altered peripheral nerve endings. The net result is an increase in basal sensitivity and stimulus induced hypersensitivity. Thus there is evidence for hyperalgesia and allodynia occurring via peripheral sensitization in inflammatory conditions [20]. Peripheral sensitization has also been proposed in FMS, although the mechanism is less certain. Similarly to the chemical milieu formed in RA, skin biopsies of patients with FM have also exhibited increased expression of many cytokines implying immune driven nociceptor sensitization [17,20].
Descending pathways ordinarily inhibit pain. In FMS neurotransmitters, such as serotonin and norepinephrine, which act on the descending serotonergic-noradrenergic system to inhibit pain, have been found to be of low levels in the cerebrospinal fluid; thus resulting in impaired pain inhibition and increased pain perception. In contrast the opioid pathway has heightened activity and therefore supports the knowledge that opioids are ineffective analgesic agents in FM [17].
Primary and secondary fibromyalgia: Assessment and influence on underlying disease
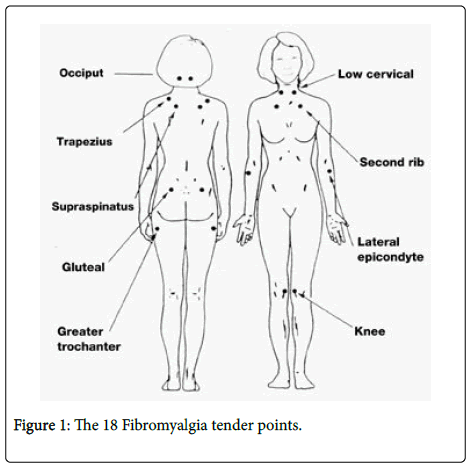
The first American College of Rheumatology (ACR) classification criteria for FMS were published in 1990 detailing chronic (>3 months) generalized pain (in at least 3 out of the 4 body quadrants), with ≥ 11/18 specific tender points (Figure 1). Consequently FM was defined as a strictly musculoskeletal disease and systemic features such as sleep disturbance and cognitive disturbance were not taken into account [21].
The revised ACR 2010 criteria (Figure 2) consist of the widespread pain index (WPI), which produces a score between 0 and 19, and the symptom severity score (SS-score), which produces a score from 0 to 12. Fulfilment of the criteria correctly classifies 83% of cases but unfortunately is not validated in FMS secondary to inflammatory disease [22,23]. Advantages of the 2010 criteria are that FMS is formally recognized as a systemic disease consisting of widespread pain, fatigue and somatic symptoms. The requirement of specialized tender point assessment is removed lending to increased recognition and diagnosis of FMS in primary care. In addition their use has been proposed for objective monitoring of disease severity [23].
Figure 2: ACR 2010 Fibromyalgia diagnostic criteria [22].
In 2011, a modified 2010 ACR criteria combined WPI and SS-score to produce a total score out of 31 and instead of a physician assessment; the newly termed ‘FMS symptoms scale’ was completed solely by the patient. A score ≥ 13 was considered positive and classified 93% of patients correctly, providing a validated self-directed patient assessment [23].
As mentioned, the 2010 ACR criteria are not validated for the assessment of secondary FMS; however, FMS affects 15%-20% of patients with RA resulting in high pain levels, fatigue and disability. Pollard et al. proposed that FMS could be diagnosed in RA when the difference between the tender and swollen joint count was ≥ 7, this method predicts fulfilment of ACR 1990 criteria with 72% sensitivity and 98% specificity [24]. However, unfortunately, it does not take into account systemic symptoms, highlighting an area for development.
Our previous work compared joint count difference against a diagnosis according to ≥ 11/18 specific tender points and found that the tender point score may significantly underestimate the presence of FMS in RA, thus further supporting a preference to assess for FMS in RA according to joint count [15].
The effect of concomitant FMS on disease activity assessment in RA results in a raised Disease Activity Score (DAS-28) in comparison to the level of inflammation, thus if unrecognized FMS can result in the over treatment of RA or the discontinuation of therapies due to a perceived lack of efficacy. In addition symptoms of FMS will not be addressed and potentially beneficial treatment omitted, this is particularly important as FMS has been shown to be related to depression, rather than joint damage when assessed according to mechanical joint score and on ultrasound [15,25].
Given the complexity of FMS we would recommend taking into consideration both difference in joint count and the presence of any cognitive or somatic symptoms to diagnose FMS in RA.
Depression, FMS and RA
The relationship between RA, FMS and depression is complex with each impacting upon the other. A study published in 2010 estimated depression to be present in 15% of patients with RA and 34%-39% of patients with FMS [26], however estimated prevalence of depression in RA varies widely between sources with some reports as high as 42% [27].
The effect that depression has on RA remission has recently been studied, Leblanc-Trudeau et al. found that clinical depression, as defined by the Centre for Epidemiologic Studies Questionnaire Depression scale (CES-D) ≥ 19/60, correlates to a longer period of time to achieve remission. If depression persists 12 months after diagnosis this period of time is prolonged further than if depression has resolved [28].
The study also found that depression as an independent variable exceeds other traditional variables, such as age, gender, tender or swollen joint count, inflammatory markers or antibody status, when predicting future disease activity and morbidity [28].
Conversely, increased pain levels in patients with RA are also related to both higher Health Assessment Questionnaire (HAQ) and Depression scores, which appear to be independent to the amount of joint damage [15]. Therefore it is possible to deduce that increased pain experienced, either from RA or secondary FMS leads to increased levels of depression but this in turn has a negative impact on the chances of achieving disease remission; hence a vicious circle is formed.
In general, depression scores are higher among patients with FMS who perceive higher levels of pain than patients with RA. Scheidt et al. even demonstrated correlation between the two with depression scores predicting the level of pain experienced in FMS. This relationship, however, was not observed in RA inferring that correlation between depression and RA associated pain is not as strong [29].
When studying health related quality of life in patients with FMS or RA against the general population, patients with FMS reported high levels of impairment in terms of mental health, social functioning, vitality, pain and general health. On the contrary, patients with RA were found to have impaired physical functioning and role limitation due to physical function [30].
In summary high depression and/or anxiety scores in patients with FMS or RA translates to a significantly reduced quality of life and diminished chances of obtaining disease remission, therefore it is important to assess patients from a multidisciplinary team approach, addressing not only the physical but also the psychosocial aspects of patient care [31].
Management of FMS
Management of primary and secondary FMS does not differ significantly so long as the physician can be sure that any underlying disease process is quiescent. A multi-modal approach is required, involving a combination of non-pharmacological and pharmacological therapies to achieve a synergistic beneficial outcome and ultimately, reduction in the burden of FMS. Treatment focuses upon controlling disease activity, maintenance of function and individualized management of somatic symptoms [32].
The three most widely studied non-pharmacological approaches for FMS are graded exercise therapy, patient education and cognitive therapies, which are also beneficial in RA. Low-impact aerobic exercise has been shown to enhance general well-being, reduce overall pain and depressive symptoms and lead to an improvement in somatic symptoms [32]. The variety and intensity of exercise should be personalized and take into account patient preference and comorbidities.
Patient education includes explaining the nature of the condition, rationale behind management and setting of treatment goals. Education has been shown to be particularly beneficial in FMS when combined with exercise [33]. Cognitive Behavioural Therapy programs significantly reduce pain, depressive symptoms and healthcare-seeking behaviour [34]. Complementary and alternative medicine therapies, such as tai chi, yoga, chiropractic manipulation, balneotherapy and acupuncture can be useful adjuncts [35].
Pharmacological management of FMS is mainly centered on the central nervous system. In particular there is robust evidence for the use of tricyclic antidepressants (e.g., amitriptyline), anti-convulsants such as gabapentin or pregabalin and agents from the serotonin norepinephrine reuptake inhibitor (SNRI) family such as duloxetine [36,37]. Not all antidepressants are effective though, those with mixed evidence for efficacy include selective SNRI (venlafaxine) [38] and selective serotonin reuptake inhibitors (SSRIs) with higher noradrenergic properties, such as fluoxetine or citalopram [39], thus these are not recommended in the management of FMS and so care must be taken when prescribing antidepressants for FMS with simultaneous depression. In the treatment of depression and FMS it is important to remember that cognitive behavioural therapy and exercise is beneficial in both conditions and so is highly recommended.
Unfortunately when treating FMS standard analgesic agents frequently prescribed have been found to be non-efficacious, these include Non-Steroidal anti-inflammatory drugs, glucocorticoids and analgesics such as paracetamol and opioids [40-43].
In conclusion concomitant FMS is increasingly recognized in patients with RA; studies report that up to 1 in 5 patients with RA will satisfy the diagnostic criteria of FMS at some point. The pathophysiology of secondary FMS is multifactorial with intricate links amongst RA, FMS and depression and it is now also becoming clear that pain in FMS has prominent biological underpinnings.
Given the complexity of the different phenotypes of secondary FMS, its diagnosis poses significant challenges to physicians. The revised ACR criterion for FMS considers it as a systemic disease with a constellation of cognitive and somatic symptoms. Furthermore, this article proposes both difference in joint count and the presence of any cognitive or somatic symptoms can be considered to diagnose FMS in RA. We also recognize the importance of making a timely diagnosis to help initiate appropriate treatment and assist in improving quality of life.
References
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38: 19-28.
- McBeth J, Jones K (2007) Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 21: 403-425.
- Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, et al. (2013) Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken) 65: 786-792.
- Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, et al. (2004) Family study of fibromyalgia. Arthritis Rheum 50: 944-952.
- Inanir A, Karakus N, Ates O, Sezer S, Bozkurt N, et al. (2014) Clinical symptoms in fibromyalgia are associated to catechol-O-methyltransferase (COMT) gene Val158Met polymorphism. Xenobiotica 44: 952-956.
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, et al. (2013) The fibromyalgia family study: a genome-wide linkage scan study. Arthritis Rheum 65: 1122-1128.
- Feng J, Zhang Z, Wu X, Mao A, Chang F, et al. (2013) Discovery of potential new gene variants and inflammatory cytokine associations with fibromyalgia syndrome by whole exome sequencing. PLoS One 8: e65033.
- Buskila D, Atzeni F, Sarzi-Puttini P (2008) Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun Rev 8: 41-43.
- Harkness EF, Macfarlane GJ, Nahit E, Silman AJ, McBeth J (2004) Mechanical injury and psychosocial factors in the work place predict the onset of widespread body pain: a two-year prospective study among cohorts of newly employed workers. Arthritis Rheum 50: 1655-1664.
- Alibaz-Oner F, Can M, lhan B, Polat O, Mumcu G, et al. (2013) Presence of fibromyalgia in patients with Takayasu's arteritis. Intern Med 52: 2739-2742.
- Hajj-Ali RA, Wilke WS, Calabrese LH, Hoffman GS, Liu X, et al. (2011) Pilot study to assess the frequency of fibromyalgia, depression, and sleep disorders in patients with granulomatosis with polyangiitis (Wegener's). Arthritis Care Res (Hoboken) 63: 827-833.
- Torrente-Segarra V, Salman-Monte TC, Rúa-Figueroa, Pérez-Vicente S, López-Longo FJ, et al. (2015) Fibromyalgia prevalence and related factors in a large registry of patients with systemic lupus erythematosus. Clin Exp Rheumatol.
- Wolfe F, Petri M, Alarcón GS, Goldman J, Chakravarty EF, et al. (2009) Fibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activity. J Rheumatol 36: 82-88.
- Iannuccelli C, Spinelli FR, Guzzo MP, Priori R, Conti F, et al. (2012) Fatigue and widespread pain in systemic lupus erythematosus and Sjögren's syndrome: symptoms of the inflammatory disease or associated fibromyalgia? Clin Exp Rheumatol 30: 117-121.
- Kapoor SR, Hider SL, Brownfield A, Mattey DL, Packham JC (2011) Fibromyalgia in patients with rheumatoid arthritis: driven by depression or joint damage? Clin Exp Rheumatol 29: S88-91.
- Wolfe F, Häuser W, Hassett AL, Katz RS, Walitt BT (2011) The development of fibromyalgia--I: examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 152: 291-299.
- Lee YC, Nassikas NJ, Clauw DJ (2011) The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther 13: 211.
- Gracely RH, Petzke F, Wolf JM, Clauw DJ (2002) Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 46: 1333-1343.
- Yunus MB (2012) The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat 2012: 584573.
- Cazzola M, Atzeni F, Boccassini L, Cassisi G, Sarzi-Puttini P (2014) Physiopathology of pain in rheumatology. Reumatismo 66: 4-13.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 33: 160-172.
- Garg N, Deodhar A (2012) New and modified fibromyalgia diagnostic criteria: Ambiguity, uncertainty, and difficulties complicate diagnosis and management. J Musculoskel Med 29: 1-5.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62: 600-610.
- Pollard LC, Kingsley GH, Choy EH, Scott DL (2010) Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 49: 924-928.
- Meenagh G, Sakellariou G, Iagnocco A, Delle Sedie A, Riente L, et al. 2012 Ultrasound imaging for the rheumatologist XXXIX. Sonographic assessment of the hip in fibromyalgia patients. Clin Exp Rheumatol 30: 319-321.
- Wolfe F, Michaud K, Li T, Katz RS (2010) Chronic conditions and health problems in rheumatic diseases: Comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol 37: 305-315.
- Kojima M, Kojima T, Suzuki S, Oguchi T, Oba M, et al. (2009) Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum 61: 1018-1024.
- Leblanc-Trudeau C, Dobkin PL, Carrier N, Cossette P, de Brum-Fernandes AJ, et al. 2015 Depressive symptoms predict future simple disease activity index scores and simple disease activity index remission in a prospective cohort of patients with early inflammatory polyarthritis. Rheumatology (Oxford) 54: 2205-2214.
- Scheidt CE, Mueller-Becsangèle J, Hiller K, Hartmann A, Goldacker S, et al. (2014) Self-reported symptoms of pain and depression in primary fibromyalgia syndrome and rheumatoid arthritis. Nord J Psychiatry 68: 88-92.
- Salaffi F, Sarzi-Puttini P, Girolimetti R, Atzeni F, Gasparini S (2009) Health-related quality of life in fibromyalgia patients: A comparison with rheumatoid arthritis patients and the general population using the SF-36 health survey. Clin Exp Rheumatol 27: S67-74.
- Ozcetin A, Ataoglu S, Kocer E, Yazici S, Yildiz O, et al. (2007) Effects of depression and anxiety on quality of life of patients with rheumatoid arthritis, knee osteoarthritis and fibromyalgia syndrome. West Indian Med J 56: 122-129.
- Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M (2009) Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum 61: 216-224.
- Rooks DS, Gautam S, Romeling M, Cross ML, Stratigakis D, et al. (2007) Group exercise, education, and combination self-management in women with fibromyalgia: a randomized trial. Arch Intern Med 167: 2192-2200.
- Bernardy K, Füber N, Köllner V, Häuser W (2010) Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 37: 1991-2005.
- Porter NS, Jason LA, Boulton A, Bothne N, Coleman B (2010) Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med 16: 235-249.
- Häuser W, Bernardy K, Uçeyler N, Sommer C (2009) Treatment of fibromyalgia syndrome with gabapentin and pregabalin--a meta-analysis of randomized controlled trials. Pain 145: 69-81.
- Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B (2013) Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev 1: CD010292.
- Sayar K, Aksu G, Ak I, Tosun M (2003) Venlafaxine treatment of fibromyalgia. Ann Pharmacother 37: 1561-1565.
- Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W (2015) Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev 6: CD011735.
- Goldenberg DL, Felson DT, Dinerman H (1986) A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis Rheum 29: 1371-1377.
- Goldenberg DL, Burckhardt C, Crofford L (2004) Management of fibromyalgia syndrome. JAMA 292: 2388-2395.
- Bennett RM, Kamin M, Karim R, Rosenthal N (2003) Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: A double-blind, randomized, placebo-controlled study. The American Journal of Medicine 114: 537-545.
- Gaskell H, Moore RA, Derry S, Stannard C (2014) Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 6: CD010692.
Citation: Dolan L, Tung LLD, Raizada SR (2016) Fibromyalgia in the Context of Rheumatoid Arthritis: A Review. Fibrom Open Access 1:103.
Copyright: © 2016 Dolan L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 16733
- [From(publication date): 1-2016 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 15672
- PDF downloads: 1061